Abstract
BACKGROUND AND OBJECTIVE:
Despite a thorough history and comprehensive testing, many children who present with recurrent symptoms consistent with allergic reactions elude diagnosis. Recent research has identified a novel cause for “idiopathic” allergic reactions; immunoglobulin E (IgE) antibody specific for the carbohydrate galactose-α-1,3-galactose (α-Gal) has been associated with delayed urticaria and anaphylaxis that occurs 3 to 6 hours after eating beef, pork, or lamb. We sought to determine whether IgE antibody to α-Gal was present in sera of pediatric patients who reported idiopathic anaphylaxis or urticaria.
METHODS:
Patients aged 4 to 17 were enrolled in an institutional review board–approved protocol at the University of Virginia and private practice allergy offices in Lynchburg, VA. Sera was obtained and analyzed by ImmunoCAP for total IgE and specific IgE to α-Gal, beef, pork, cat epithelium and dander, Fel d 1, dog dander, and milk.
RESULTS:
Forty-five pediatric patients were identified who had both clinical histories supporting delayed anaphylaxis or urticaria to mammalian meat and IgE antibody specific for α-Gal. In addition, most of these cases had a history of tick bites within the past year, which itched and persisted.
CONCLUSIONS:
A novel form of anaphylaxis and urticaria that occurs 3 to 6 hours after eating mammalian meat is not uncommon among children in our area. Identification of these cases may not be straightforward and diagnosis is best confirmed by specific testing, which should certainly be considered for children living in the area where the Lone Star tick is common.
Keywords: α-Gal; galactose-α-1,3-galactose; delayed anaphylaxis; pediatric urticaria
What’s Known on This Subject:
Delayed anaphylaxis, urticaria, and angioedema to mammalian meat products were first described in the adult population in 2009. Patients with this syndrome who consume mammalian meat typically develop symptoms 4 to 6 hours after ingestion.
What This Study Adds:
Specific diagnoses for children who develop urticaria, angioedema, and idiopathic anaphylaxis are few and far between. We have now shown delayed anaphylaxis, urticaria, and angioedema due to mammalian meat products in the pediatric population.
In studies in which the etiology of anaphylaxis has been established, foods or venoms cause most reactions,1 and, classically, these immunoglobulin E (IgE)-mediated reactions are thought to occur within 5 to 30 minutes after ingestion or injection of an offending agent.2 Numerous epitopes responsible for IgE-mediated food allergy have been described and are primarily protein-based. Although it is well known that the carbohydrate moieties present on many plant foods can induce antiglycan IgE responses, the clinical significance of these cross-reactive carbohydrate determinants is unclear.3–5 In contrast, recent work has shown that IgE antibodies specific for the carbohydrate, galactose-α-1,3-galactose (α-Gal), are capable of eliciting serious, even fatal, delayed reactions that occur 3 or more hours after eating red meat.6,7
An IgG/IgM immune response to α-Gal has been well described, and this mediates hyperacute rejection of pig-to-primate xenotransplantation.8 Work by Chung et al9 demonstrated that in adults, an IgE response to α-Gal was responsible for immediate hypersensitivity reactions that occurred during infusion of the monoclonal antibody cetuximab, an anti–epidermal growth factor receptor cancer therapeutic. The α-Gal carbohydrate moiety is known to be present on multiple tissues (notably thyroglobulin) from nonprimate mammals,10,11 and more recently, IgE to α-Gal has been associated with delayed urticaria and even anaphylaxis.6,7 The development of IgE antibody to α-Gal has been linked to bites from ecto-parasitic ticks, especially those of the Lone Star tick, Amblyomma americanum.12 Patients with IgE antibody to α-Gal report symptoms of urticaria, angioedema, or even anaphylaxis starting 3 to 6 hours after the ingestion of mammalian meat products.7 The symptoms can be severe, and many patients have required epinephrine injections for their reactions as well as care in emergency departments.7,13 Because the timing of ingestion occurs much earlier than the actual symptoms, diagnosis and recognition of this food allergy has been challenging. In fact, we have seen many children who had been diagnosed with idiopathic urticaria/anaphylaxis, or who had been specifically told that the reactions were not a result of food allergy, who had IgE antibodies to α-Gal and, in retrospect, a history consistent with delayed reactions to mammalian meat (A.P.S., P.W.H., S.P.C., unpublished observations). Immediate hypersensitivity to meat in children has been reported by multiple investigators14–18 and the role of beef allergens in children with atopic dermatitis and milk sensitization has also been well established.19,20
Because α-Gal has been found to be an important cause of urticaria, angioedema, and anaphylaxis in the adult population, we investigated whether IgE antibodies to α-Gal were present in the sera of pediatric patients with a clinical history suggestive of delayed urticaria, angioedema, or anaphylaxis to mammalian meat products. Here we report 45 pediatric patients, aged 4 to 17, who were found to have IgE antibodies to α-Gal. To our knowledge, this is the first report of delayed reactions to mammalian meat in the pediatric population.
Methods
Patients and Control Subjects
The University of Virginia Human Investigation Committee has approved these studies. Our patients were enrolled as subjects from the University of Virginia Allergy and Immunology Clinic, as well as from private practice allergy clinics in Lynchburg, VA, because each had a history suggestive of delayed anaphylaxis, urticaria, or angioedema. A total of 51 subjects were enrolled from September 2011 to May 2012 on the basis of clinical history and answers to questions regarding tick bites and bite site characteristics. Specific questions included (1) did episodes occur before or after midnight, (2) did episodes follow having eaten mammalian meat at the meal before the reaction (even if 4 to 5 hours prior), and (3) was there a history of tick or seed tick bites. Additional subjects aged 4 to 18 were enrolled (n = 142) from the University of Virginia Hospital where they presented with (or without) wheeze.21
ImmunoCAP IgE Assays
Total and specific IgE antibodies were measured by using either commercially available ImmunoCAP (Phadia US, Portage, MI) or a modification of the assay with streptavidin on the solid phase (α-Gal, Fel d 1).7,22 The assays were performed with the ImmunoCAP 250 instrument and the results expressed as IU/mL. For specific assays, the cutoff used for a positive reaction was 0.35 IU/mL. The sera were tested with commercially available assays for IgE antibodies to dust mite (Dermatophagoides pteronyssinus), cat (dander and epithelium in addition to Fel d 1), dog dander, Timothy grass, Alternaria alternata, oak, beef, pork, chicken, codfish, cow’s milk and milk components (Bos d 4, Bos d 5, Bos d 8), boiled milk, goat’s milk, peanut, egg, and total IgE.
Statistical Analysis
We compared the specific IgE antibody results between α-Gal, beef, and pork to fish, chicken, peanut, and egg by using the Mann-Whitney test. We correlated quantitative measures of IgE antibodies between α-Gal and other specific IgE antibodies by using the Spearman rank-order correlation. A P < .05 was considered to indicate statistical significance. Statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA).
Results
Our population included children (n = 51) with a history of recurrent urticaria, idiopathic anaphylaxis, or angioedema suggestive of a delayed response to mammalian meat, of which 45 tested positive for IgE antibody to α-Gal (Table 1). Some patients were referred as cases of chronic urticaria; however, on careful questioning, a more appropriate diagnosis would have been acute, recurrent urticaria. Many of the patients had used an emergency department for their symptoms (5/51 had been to the emergency department ≥4 times before diagnosis), and they required the use of epinephrine, antihistamines, and/or injected steroids. There were also several patients who had required admission to the hospital for observation (Table 1). All of these patients had a clear history of tick exposure before our evaluation of IgE to α-Gal, and 39 had histories of itching, redness, and swelling for several weeks after the tick bite (Table 1). Of the 51 children, 6 subjects were enrolled with similar histories, yet were found to be negative for IgE antibody to α-Gal.
TABLE 1.
Patient Demographics
| n = 51 | |
|---|---|
| Gender, % male | 69 |
| Mean age at presentation (range) | 12 (4–17) |
| Total IgE, geometric mean (95% confidence interval) | 147 IU/mL (105–206 IU/mL) |
| No. of subjects testing positive for IgE antibody to α-Gal (%) | 45 (88) |
| Symptoms at presentation, % | |
| Anaphylaxisa | 44 |
| Gastrointestinal/Oral | 64 |
| Urticaria | 92 |
| Angioedema | 31 |
| Average time to symptoms (range) | 4.68 h (10 min to 24 h) |
| Tick exposure, % | 100 |
| Redness and itching at site of tick bite | 87 |
| Tick-borne illnessb | 10 |
| Emergency department visits, % | 46c |
| Medications administered in the emergency department, % | |
| Epinephrine | 19 |
| Antihistamines | 35 |
| Oral steroids | 19 |
| Parenteral steroids | 17 |
| Intravenous fluids | 17 |
| Hospital admissions, % | 8 |
Anaphylaxis was defined as hypotension and/or respiratory symptoms including laryngeal edema and wheezing.
All patients with positive answers reported a history of Lyme disease.
Five of the 45 subjects required ≥4 visits to the emergency department for symptoms.
As previously reported in adults, our pediatric subjects had positive immunoassays to mammalian meat products, including beef and pork (Fig 1). The specific IgE levels for these tests were significantly higher than those for fish (P < .05), chicken (P < .001), egg (P < .05), and peanut (P < .001) by Mann-Whitney analysis. There was a close correlation (r = 0.99) between beef- and pork-specific IgE, supporting the view that these assays were measuring IgE antibodies to a single component: α-Gal (Fig 2A). There was also a strong correlation between a positive immunoassay for α-Gal and a positive test for beef and pork (r = 0.87 and r = 0.89, respectively; Table 2, Fig 2 B and C). The symptoms reported by these children included urticaria, angioedema, and anaphylaxis, and in nearly every case these symptoms were delayed 3 to 6 hours, much like those of their adult counterparts (Table 1). Milk-specific IgE was also elevated in these patients, as reported in previous studies.7 However, tests for IgE to milk components, including α-lactalbumin (Bos d 4), β-lactoglobulin (Bos d 5), and casein (Bos d 8), were negative in most of the patients who had a positive immunoassay to milk. Boiled milk immunoassays were also negative in this same population (Fig 3). To confirm that α-Gal–specific IgE antibody were responsible for the positive cow’s milk IgE test, absorption studies were carried out on 3 sera, which showed that removing IgE antibody to α-Gal also removed the positive milk IgE result (Supplemental Table 3).
FIGURE 1.
Specific IgE antibody binding to allergens in serum samples from 45 patients with IgE antibodies to α-Gal. The horizontal lines indicate geometric mean values. Numbers below the limit of detection indicate the number of negative values for each allergen. * Complete panel of immunoassays was performed for those sera positive for IgE antibody to α-Gal (n = 45). ** Cat allergen includes epithelium and dander. # The values for chicken, egg, peanut, and fish have significantly lower titers (P < .05) compared with α-Gal, beef, and pork by means of a Mann-Whitney analysis.
FIGURE 2.
Correlations of IgE to α-Gal and specific allergens. A, Correlation of IgE antibody to pork and IgE antibody to beef (r = 0.99), suggesting that these tests are actually measuring the amount of specific IgE to α-Gal in the serum. B, Correlation of IgE antibody to α-Gal and beef (r = 0.89; P < .001) in patients with IgE antibody to α-Gal. C, Correlation of IgE antibody to α-Gal and pork (r = 0.87; P < .001) in patients with IgE antibody to α-Gal. D, Correlation of IgE antibody to α-Gal and total IgE (r = 0.18; P = not significant) in patients with IgE antibody to α-Gal.
TABLE 2.
Correlations Between the IgE Specific for α-Gal (IU/mL) and Other Specific Allergens (IU/mL)
| Specific IgE Antibody | No. of Positives/No. Tested | Spearman Correlation (r) With α-Gal | P Value |
|---|---|---|---|
| α-Gal | 45/45 | 1 | |
| Dog dander | 33/45 | 0.71 | <.001 |
| Cata | 39/45 | 0.73 | <.001 |
| Fel d 1b | 9/45 | 0.07 | .66 |
| Porkc | 39/45 | 0.87 | <.001 |
| Beefc | 38/45 | 0.89 | <.001 |
| Milk | 34/45 | 0.79 | <.001 |
| Peanut | 10/45 | −0.07 | .67 |
| Dust mited | 13/45 | 0.25 | .1 |
Cat immunoassay includes epithelium and dander.
Fel d 1 is the major cat allergen.
Pork- and beef-specific IgE antibody were significantly correlated with each other (r = 0.99 and P < .001).
Dust mite = D pteronyssinus immunoassay.
FIGURE 3.
Component analysis of milk-allergic children with α-Gal. Patients with α-Gal–specific IgE who were positive for specific IgE to milk (n = 34) were tested for milk components, including α-lactalbumin, β-lactoglobulin, and casein-specific IgE. Interestingly, there does not appear to be any reactivity to the milk components (α-lactalbumin, β-lactoglobulin, and casein), suggesting that there is no α-Gal in these immunoassays. Similar results are present for boiled milk and goat’s milk.
In keeping with the known distribution of α-Gal, positive immunoassay responses were seen to cat dander and epithelium and dog dander in our patients with α-Gal allergy (Fig 1). Despite a positive test for cat and dog, only 9 of 32 subjects reported rhinitis symptoms on exposure to cat or dog. The immunoassay test for these mammals is known to contain α-Gal because of the presence of proteins, such as cat IgA (Fel d 5).23 Sensitization to dust mite and to the major cat allergen (Fel d 1) were similar to the general population (Fig 1) and are not associated with the α-Gal syndrome.
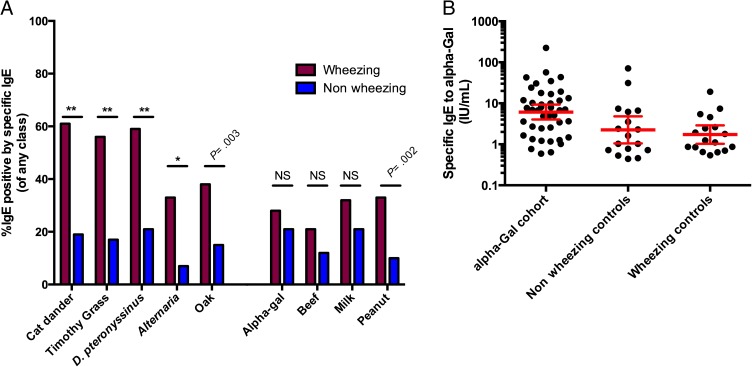
To further characterize the IgE antibody to α-Gal response in the pediatric population, an assessment of the prevalence of this antibody response in a geographically similar but distinct cohort was performed. Sera from subjects (n = 142) presenting to the University of Virginia Hospital with and without wheeze were assayed for indoor, outdoor, and food-specific IgE antibody responses (Fig 4A). In this group of 142 subjects, the percentage of sera positive for IgE antibody to α-Gal overall was 24%. Because this cohort was enrolled to investigate asthma, it included patients with and without wheezing.21 As might be expected, patients with wheezing (surrogate for asthma) had higher overall IgE percent positive for many allergens but notably percent α-Gal sensitization was not significantly different between wheezing and nonwheezing subjects (P = .43; Fig 4 A and B). Analysis of the 3 different cohorts showed that the IgE antibody titer to α-Gal was significantly higher in patients reporting delayed reactions after consuming mammalian meat as compared with those subjects enrolled with and without wheeze (P < .001; Fig 4B). A more detailed analysis of the IgE antibody to α-Gal response shows that among those subjects with wheeze, IgE to α-Gal comprised <1% of the total IgE in most cases (Supplemental Fig 5). On the contrary, those subjects enrolled specifically because the clinical history supported delayed reactions to mammalian meat had IgE to α-Gal responses that constituted >1% of total IgE, and in many instances >5% of total IgE (Supplemental Fig 5).
FIGURE 4.
A, Percentage of children (aged 4–18) positive for specific IgE antibodies in wheezing (n = 61; red) and nonwheezing (n = 81; blue) control groups. There were significant differences between the wheezing and control population with regard to aeroallergen sensitization (**P < .001; *P = .001), whereas there were no differences in sensitization patterns to α-Gal, beef, or milk (P = .43, P = .15, and P = .21, respectively). B, Comparison of positive tests for α-Gal among an age-matched pediatric cohort with symptoms of delayed mammalian meat allergy (n = 45) and a cohort of wheezing (n = 17) and nonwheezing (n = 16) control subjects in the hospital. The pediatric cohort with symptoms of delayed mammalian meat allergy has significantly higher levels of specific IgE to α-Gal (P < .001). NS, not significant.
Discussion
The α-Gal syndrome, in children and adults, is unlike any other known IgE-mediated food allergy. Despite high titers of IgE antibodies to beef and pork, these cases consistently report a delay of 3 to 6 hours after eating mammalian meat.7 Furthermore, the symptoms often become severe, including significant episodes of hives and hypotension. In fact, >45% of the subjects used an emergency department at least once for their symptoms and 8% required admission to the hospital for observation (Table 1). Thus, it is our general practice to prescribe an epinephrine autoinjector and instruct patients in its proper use. Not only the serious nature of the reactions but also the rising frequency of idiopathic angioedema and urticaria across all age groups24–29 underscore the importance of identifying a cause for these cases if possible. Our results show clearly that physicians should keep this diagnosis in mind even in the pediatric population, especially if the history is consistent with the disease syndrome, including delayed symptoms after ingestion of beef, pork, lamb, or even milk.
It is important to note, however, that patients with IgE antibody to α-Gal may not experience reactions with every ingestion of mammalian meat. The explanations for such an observation are several-fold. First, α-Gal is a carbohydrate and this “inconsistency” may simply be a result of the inherent properties of digestion, processing, and absorption of glycans. Second, the amount of α-Gal that actually reaches the bloodstream in an antigenic form (which we believe to be that of a glycolipid) may be significantly less than is ingested. Moreover, the food itself (ie, hamburger versus cow’s milk) may offer more or less antigen. Fourth, the dose of meat appears to be important, and in some instances children are able to consume a small amount of mammalian meat or products without adverse reactions. Fifth, it may well be that preparation (mechanical, thermal, or freezing) is a significant factor in contributing to whether foods retain enough of the appropriate antigen to cause a reaction. Finally, it is also important to keep in mind that the natural history of this IgE antibody response appears to be one that decreases over time. Thus, as the IgE antibody titer decreases, children could experience fewer or inconsistent reactions.
The incidence of food allergy is increasing across the population, with almost 6% of children and 4% of adults in North America now allergic to 1 or more foods.30,31 Children who develop IgE antibody to α-Gal may have positive skin, intradermal, or immunoassay testing to milk, beef, pork, cat, or dog.32 It is important to understand that many children suffer from milk allergy, but IgE to α-Gal is distinct from the more traditional, protein-based cow’s milk allergy. α-Gal–related reactions present in older children, many of whom have no previous history of either food allergy or any allergic disease.7 Clinicians should recognize that the carbohydrate moiety α-Gal is found in mammalian milk, as evidenced by the positive immunoassay results to cow’s milk and goat’s milk. Therefore, in a patient older than 5 who has an apparent new-onset milk allergy, IgE antibody to α-Gal should be considered as an alternative diagnosis to a protein-based milk allergy, a cross-reactivity between beef allergy and cow’s milk,19 or even a distinct mammalian protein cross-reactivity.18
Interestingly, we were unable to show positive tests for α-Gal on the individual components of milk as tested in this study. Children with IgE antibody to α-Gal (and, therefore, “milk allergic”) had negative immunoassays to α-lactalbumin, β-lactoglobulin, and casein in 32, 31, and 33 of 34 instances, respectively, leading us to surmise that these milk protein antigens are not significant sites of α-Gal–based glycosylation. Similarly, one might anticipate that the allergens bovine immunoglobulin (Bos d 7) or bovine serum albumin (Bos d 6) could contain glycosylation with α-Gal, but the published evidence that has assessed this possibility for Bos d 6 suggests α-Gal is not present, and our unpublished data have also been in keeping with a lack of α-Gal on bovine serum albumin.11 The negative results for milk allergens could also be explained by the processing of these components for the immunoassay, which might change the structure or alter the galactose linkages. The latter theory is supported by our finding that the boiled milk immunoassay was negative in most of the patients with a positive α-Gal–specific IgE, whereas another mammalian milk (goat) was positive in those sera that had the highest titer of IgE antibody to cow’s milk. Taken together, the data suggest that the goat’s milk ImmunoCAP has fewer α-Gal epitopes than does the cow’s milk assay, not that α-Gal is absent from goat’s milk or that goat’s milk may be a safe alternative for these children. In fact, we have not a priori recommended removal of milk or dairy products from the diet of adults with this syndrome if they have previously tolerated these products. We have continued a similar approach in the pediatric population, unless the allergic episodes persist, at which time we would suggest performing an oral milk challenge.
Skin testing for beef, pork, or lamb (mammalian meat) in both adult and pediatric patients has been challenging. Many patients have only small reactions (2–5 mm) to these allergens by skin-prick testing, and intradermal tests have been used in adults to clarify the intermediate results.7 We have, on occasion, also performed intradermal testing in older teenagers, and these results mirrored those seen in adults. Overall, we are less likely to perform intradermal testing in children and, therefore, recommend use of in vitro assays. Although we have performed mammalian meat challenges in adult subjects to document the delayed appearance of clinical symptoms, these food challenges have produced significant symptoms beyond what the subject had reported after natural exposure. In protein-based food allergies in which symptoms arise in 5 to 30 minutes, food challenges use small amounts of allergen and proceed incrementally, such that the procedure is stopped when patients begin to react. Because of the time course to symptoms, incremental dosing is not possible in the case of delayed reactions to mammalian meat and the entire dose must be given at the start of the challenge. Because of the (significant) reactions observed during mammalian meat challenges with adult subjects and the inability to incrementally dose, we do not plan to perform food challenges in pediatric subjects and acknowledge the lack of food challenges as a limitation in our study.
The pediatric population seems to follow the trend seen in adult subjects with regard to the geographic distribution of this disease. Screening serum samples from multiple geographic locales reveals a distinct regional pattern of disease in the southeastern United States, a pattern that roughly correlates with the higher incidence of cetuximab hypersensitivity in adults.33 In fact, we have been made aware of children presenting with IgE antibody to α-Gal in numerous centers throughout the eastern and now central United States. Colleagues at Duke University (Dr Michael Land and Dr Moira Breslin), Kansas City Children’s (Dr Paul Dowling and Dr Tara Federly), and in East Hampton, NY (Dr Erin McGintee) have diagnosed pediatric patients with IgE antibody to α-Gal and the characteristic delayed reactions to mammalian meat. Based on our assessment of sera from children enrolled in studies in central Virginia, the prevalence of specific IgE (sIgE) antibody to α-Gal can be as high as 15% in some areas. Interestingly, this area overlaps with the known distribution of the Lone Star tick, A americanum.34 As suggested in our recent publication, we believe that there is a causal relationship between tick bites and sensitization to α-Gal.12 In the current study, >90% of patients with this syndrome reported tick bites in the previous year. For patients with IgE antibody to α-Gal, tick bites cause significantly pruritic reactions at the site of the bite(s) which often persist. Thus, 2 clinically relevant questions that can assist in formulating a diagnosis are to inquire about a history of tick or seed tick bites, and further, whether the site(s) of a bite(s) had persistent (ie, 2–3 weeks) itching, erythema, or swelling.12 Of note, in our experience, if patients are able to avoid subsequent tick bites, the level of α-Gal–specific IgE tends to decrease over time. In fact, some adult patients with this form of allergy have been able to tolerate mammalian meat again after avoiding additional tick bites for 1 to 2 years (S.P.C., T.A.E.P-M., and J.L.K., unpublished data, 2010–2013).
Although there are multiple potential causes for both acute and chronic urticaria, as well as angioedema and idiopathic anaphylaxis, we report here 45 pediatric patients who fit the syndrome of delayed reactions to red meat. This study not only further broadens the differential for evaluating “idiopathic” allergic reactions but informs of an expanded population at risk for developing this unique allergy. In keeping with the known distribution of α-Gal, we have found that restriction of mammalian meat can lead to complete remission of previous symptoms. Most children and adults are able to continue to drink milk products, although a few patients may have symptoms with dairy ingestion. Importantly, we believe that this research provides clear evidence that the α-Gal syndrome is important in the pediatric population, and it should be diagnostically considered in children with a history suggestive of delayed responses to red meat and acute, recurrent urticaria, angioedema, or idiopathic anaphylaxis, particularly in those patients living in areas where the Lone Star tick is common.
Supplementary Material
Glossary
- α-Gal
galactose-α-1,3-galactose
- Ig
immunoglobulin
Footnotes
Dr Kennedy performed data analysis and patient enrollment, and drafted the manuscript; Drs Stallings, Lane, and Matos were instrumental in patient enrollment; Dr Platts-Mills conceptualized and designed the study, and was involved in drafting the manuscript; Mr Oliveira performed specific immunoglobulin E (IgE) measurements; Ms Workman collected and performed all specific IgE measurements; Ms James performed data analysis and assisted in the specific IgE measurements; Dr Tripathi helped with the draft of the manuscript; Dr Heymann was involved in enrolling patients as well as in drafting the manuscript; Dr Commins conceptualized and designed the study, and was instrumental in patient enrollment, data analysis, and drafting the manuscript; and all authors approved the final manuscript as written.
FINANCIAL DISCLOSURE: Dr Platts-Mills is a consultant to Viracor/IBT and has a patent on the use of streptavidin solid phase to evaluate immunoglobulin E antibodies to recombinant molecules; Dr Lane is a consultant for Mylan Specialty, which markets the EpiPen epinephrine autoinjector, used for life-threatening anaphylaxis/food allergy; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by National Institutes of Health grants AI-20565, U19-AI-070364, R21-AI-087985, and K08-AI-1085190. Funded by the National Institutes of Health (NIH).
References
- 1.Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122(6):1161–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May CD. Objective clinical and laboratory studies of immediate hypersensitivity reactions to foods in asthmatic children. J Allergy Clin Immunol. 1976;58(4):500–515 [DOI] [PubMed] [Google Scholar]
- 3.Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68(5):356–364 [DOI] [PubMed] [Google Scholar]
- 4.Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129(4):286–295 [DOI] [PubMed] [Google Scholar]
- 5.Commins SP, Platts-Mills TA. Allergenicity of carbohydrates and their role in anaphylactic events. Curr Allergy Asthma Rep. 2010;10(1):29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commins SP, Platts-Mills TA. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J Allergy Clin Immunol. 2009;124(4):652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon PM, Neethling FA, Taniguchi S, et al. Intravenous infusion of Galalpha1-3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation. 1998;65(3):346–353 [DOI] [PubMed] [Google Scholar]
- 9.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiro RG, Bhoyroo VD. Occurrence of alpha-D-galactosyl residues in the thyroglobulins from several species. Localization in the saccharide chains of the complex carbohydrate units. J Biol Chem. 1984;259(15):9858–9866 [PubMed] [Google Scholar]
- 11.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780(2):75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–1293.e1286 [DOI] [PMC free article] [PubMed]
- 13.Commins SP, Kelly LA, Rönmark E, et al. Galactose-α-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am J Respir Crit Care Med. 2012;185(7):723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayuso R, Lehrer SB, Tanaka L, et al. IgE antibody response to vertebrate meat proteins including tropomyosin. Ann Allergy Asthma Immunol. 1999;83(5):399–405 [DOI] [PubMed] [Google Scholar]
- 15.Fiocchi A, Restani P, Riva E, et al. Meat allergy: I—Specific IgE to BSA and OSA in atopic, beef sensitive children. J Am Coll Nutr. 1995;14(3):239–244 [DOI] [PubMed] [Google Scholar]
- 16.Fiocchi A, Restani P. Adverse reactions to bovine proteins—then and now. Ann Allergy Asthma Immunol. 2002;89(6 suppl 1):1–2 [DOI] [PubMed] [Google Scholar]
- 17.Restani P, Fiocchi A, Beretta B, Velonà T, Giovannini M, Galli CL. Meat allergy: III—Proteins involved and cross-reactivity between different animal species. J Am Coll Nutr. 1997;16(4):383–389 [DOI] [PubMed] [Google Scholar]
- 18.Restani P, Beretta B, Fiocchi A, Ballabio C, Galli CL. Cross-reactivity between mammalian proteins. Ann Allergy Asthma Immunol. 2002;89(6 suppl 1):11–15 [DOI] [PubMed] [Google Scholar]
- 19.Martelli A, De Chiara A, Corvo M, Restani P, Fiocchi A. Beef allergy in children with cow’s milk allergy; cow’s milk allergy in children with beef allergy. Ann Allergy Asthma Immunol. 2002;89(6 suppl 1):38–43 [DOI] [PubMed] [Google Scholar]
- 20.Werfel SJ, Cooke SK, Sampson HA. Clinical reactivity to beef in children allergic to cow’s milk. J Allergy Clin Immunol. 1997;99(3):293–300 [DOI] [PubMed] [Google Scholar]
- 21.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114(2):239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erwin EA, Custis NJ, Satinover SM, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115(5):1029–1035 [DOI] [PubMed] [Google Scholar]
- 23.Grönlund H, Adédoyin J, Commins SP, Platts-Mills TA, van Hage M. The carbohydrate galactose-alpha-1,3-galactose is a major IgE-binding epitope on cat IgA. J Allergy Clin Immunol. 2009;123(5):1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey E, Shaker M. An update on childhood urticaria and angioedema. Curr Opin Pediatr. 2008;20(4):425–430 [DOI] [PubMed] [Google Scholar]
- 25.Kaplan AP. Clinical practice. Chronic urticaria and angioedema. N Engl J Med. 2002;346(3):175–179 [DOI] [PubMed] [Google Scholar]
- 26.Kauppinen K, Juntunen K, Lanki H. Urticaria in children. Retrospective evaluation and follow-up. Allergy. 1984;39(6):469–472 [DOI] [PubMed] [Google Scholar]
- 27.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol. 2007;120(4):878–884 [DOI] [PubMed] [Google Scholar]
- 28.Volonakis M, Katsarou-Katsari A, Stratigos J. Etiologic factors in childhood chronic urticaria. Ann Allergy. 1992;69(1):61–65 [PubMed] [Google Scholar]
- 29.Zitelli KB, Cordoro KM. Evidence-based evaluation and management of chronic urticaria in children. Pediatr Dermatol. 2011;28(6):629–639 [DOI] [PubMed] [Google Scholar]
- 30.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113(5):805–819, quiz 820 [DOI] [PubMed] [Google Scholar]
- 31.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116–S125 [DOI] [PubMed] [Google Scholar]
- 32.Sampson HA, Rosen JP, Selcow JE, et al. Intradermal skin tests in the diagnostic evaluation of food allergy. J Allergy Clin Immunol. 1996;98(3):714–715 [DOI] [PubMed] [Google Scholar]
- 33.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644–3648 [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Available at: www.cdc.gov/ticks/maps/lone_star_tick.html. Accessed August 2012
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.