Abstract
OBJECTIVE:
In August 2011, the Pediatric Infectious Disease Society and Infectious Disease Society of America published an evidence-based guideline for the management of community-acquired pneumonia (CAP) in children ≥3 months. Our objective was to evaluate if quality improvement (QI) methods could improve appropriate antibiotic prescribing in a setting without a formal antimicrobial stewardship program.
METHODS:
At a tertiary children’s hospital, QI methods were used to rapidly implement the Pediatric Infectious Disease Society/Infectious Disease Society of America guideline recommendations for appropriate first-line antibiotic therapy in children with CAP. QI interventions focused on 4 key drivers and were tested separately in the emergency department and on the hospital medicine resident teams, using multiple plan-do-study-act cycles. Medical records of eligible patients were reviewed weekly to determine the success of prescribing recommended antibiotic therapy. The impact of these interventions on our outcome was tracked over time on run charts.
RESULTS:
Appropriate first-line antibiotic prescribing for children admitted with the diagnosis of CAP increased in the emergency department from a median baseline of 0% to 100% and on the hospital medicine resident teams from 30% to 100% within 6 months of introducing the guidelines locally at Cincinnati Children’s Hospital Medical Center and has been sustained for 3 months.
CONCLUSIONS:
Our study demonstrates that QI methods can rapidly improve adherence to national guidelines even in settings without a formal antimicrobial stewardship program to encourage judicious antibiotic prescribing for CAP.
Keywords: pneumonia, antibiotic use, pediatric
Community-acquired pneumonia (CAP) is a common and serious infection in children. In the United States, >3 million children are diagnosed with CAP each year, and >150 000 of these children require hospitalization.1,2 In August 2011, the first US national evidence-based guideline for the management of uncomplicated CAP in children was published by the Pediatric Infectious Disease Society (PIDS) and the Infectious Disease Society of America (IDSA). This guideline recommends ampicillin as first-line antibiotic therapy for the fully immunized child without underlying medical conditions and with uncomplicated CAP who requires hospital admission.3 Additionally, the guideline recommends empirical combination therapy with a macrolide and β-lactam antibiotic when atypical pneumonia is a diagnostic consideration.
Before the publication of this guideline, several studies documented variability in the management of CAP in hospitalized children.4,5 A multicenter study of children’s hospitals showed that cephalosporins accounted for 45% of all empirical therapy for patients diagnosed with CAP but penicillins and aminopenicillins were rarely used.5 A Cincinnati Children’s Hospital Medical Center (CCHMC) evidence-based guideline for CAP, last updated in 2006, discussed oral and intramuscular antibiotic recommendations but did not discuss any intravenous therapy options for admitted patients. High-dose amoxicillin for children aged 60 days to 5 years of age, and macrolide monotherapy in children ≥5 years was recommended. Cephalosporins (second or third generation) were recommended for children with a penicillin allergy or as an intramuscular injection on day 1 of therapy.6
Although antimicrobial stewardship programs (ASPs) have been shown to improve antibiotic prescribing patterns; CCHMC does not currently have one.7,8 There is, however, quality improvement (QI) infrastructure at CCHMC, which we hypothesized could be used in the place of a formal ASP to introduce this new guideline. Thus, a multidisciplinary improvement team was formed to design, test, and implement interventions aimed at reducing unwarranted variation in initial antibiotic therapy for children admitted with the diagnosis of CAP. We targeted interventions on both the emergency department (ED) and, if missed in the ED or directly admitted, on the hospital medicine (HM) resident teams. We aimed to increase the percent of otherwise healthy patients admitted to the HM resident teams with a diagnosis of uncomplicated CAP who received appropriate evidence-based first-line antibiotic therapy, as defined by the PIDS/IDSA guideline, to 80% from a baseline of 0% in the ED and a baseline of 30% on the HM resident teams.
Methods
Setting
CCHMC is a free-standing, tertiary-care, pediatric academic medical center with 523 licensed inpatient beds at 2 locations that serves southwestern Ohio, southeastern Indiana, and northern Kentucky. It has ∼90 000 ED visits per year and >7000 admissions to the HM resident teams per year at the main campus, the site of this study. There are ∼300 residents, who are the main prescribers at the institution, that rotate each year in the ED and on the HM resident teams. This study was approved by the institutional review board as exempt from human subject research.
Planning the Intervention
The improvement team consisted of 3 pediatric hospital medicine physicians, 2 pediatric emergency medicine physicians, 2 pediatric infectious diseases physicians, 1 community pediatrician, 2 hospital medicine fellows, 1 pharmacist, 1 pediatric chief resident, and 1 research assistant. The team created a process map depicting the current antibiotic prescribing for a patient initially treated in the ED and then admitted to an HM resident team. This also included patients admitted to HM resident teams with a community pediatrician serving as the attending physician (n = 7). From this map (Fig 1), the team conducted a failure mode and effects analysis9,10 and developed key drivers to accomplish the improvement aim (Fig 2).
FIGURE 1.
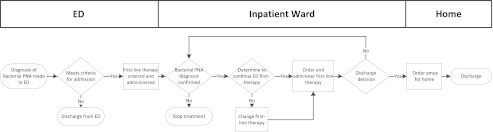
Process map of antibiotic prescribing for a patient being admitted for CAP. Amox amoxicillin; PNA, pneuomonia.
FIGURE 2.
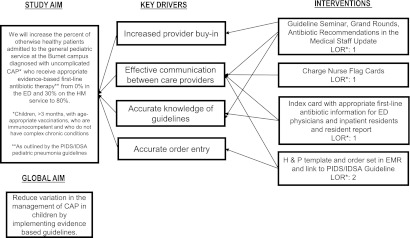
Key driver diagram summarizing the project aim and interventions implemented to achieve the study aim. H&P, history and physical examination note; LOR, level of reliability.
Data Collection
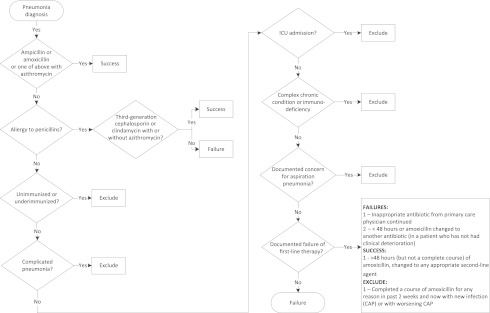
Patients were eligible for inclusion if they were admitted to the hospital with a primary International Classification of Disease, Ninth Revision (ICD-9) discharge diagnosis code of pneumonia (480–483, 485–486, 510, 511.0–1, or 511.9) or a primary ICD-9 discharge diagnosis code of a pneumonia-related symptom such as wheezing or tachypnea (780.6, 786.00, 786.05, 786.06, 786.07, 786.2, 786.3, 786.4, 786.5, 786.51, 786.52, or 786.7) and a secondary ICD-9 discharge diagnosis code of pneumonia.11–14 Included patients were between 3 months and 19 years of age and had, by parental report, received age-appropriate vaccinations. We excluded patients with complicated pneumonia, patients who had immunodeficiency or complex chronic conditions, and patients admitted to the ICU on the first day of admission.15 Appropriate first-line therapy was defined as empirical receipt of (1) amoxicillin (dosing: 90 mg/kg/day orally) or ampicillin (200 mg/kg/day intravenously); or (2) ceftriaxone (50–100 mg/kg/day intravenously or intramuscularly) or oral equivalent cefdinir (14 mg/kg/day orally) or clindamycin (40 mg/kg/day orally or intravenously) for children with penicillin allergy; or (3) azithromycin (10 mg/kg/day orally or intravenously) on day 1 in combination with either aminopenicillins or, in the case of allergy, other appropriate antibiotics. To determine a baseline proportion of appropriate first-line therapy prescribing in the ED and on the HM resident teams, data were collected retrospectively from May 1, 2011, to October 23, 2011. Although the PIDS/IDSA guideline was first available electronically on August 30, 2011, our interventions did not begin until the week of October 24, 2011, with a seminar introducing the guideline. All medical records of eligible patients were reviewed from the previous week on an ongoing weekly basis to determine appropriateness of first-line antibiotic prescribing. These charts were reviewed independently by 2 members of the improvement team (physician or pharmacist) using an algorithm that verified eligibility and determined success or failure of first-line antibiotic treatment prescription according to the guideline recommendations (Appendix A).
Interventions
The interventions were designed as part of a campaign message to increase appropriate evidence-based antibiotic prescribing for children with CAP. Changes were tested through multiple plan-do-study-act cycles16 initiated by the improvement team after the initial intervention in mid-October. These interventions were developed to address our key drivers (Fig 2).
In October 2011, a seminar was given to introduce the management of childhood CAP as detailed in the PIDS/IDSA guideline to the attending physicians and clinical fellows of the Divisions of HM, Pulmonary Medicine, Infectious Diseases, and ED. The seminar provided an overview of the guidelines, discussed the rationale for the guideline recommendations, and identified situations where local practice or opinion diverged from guideline recommendations. Following the seminar, a 1-page summary sheet for the management of childhood CAP for hospitalized patients was provided to all participants of the seminar and to their divisions to distribute to faculty who were unable to attend. In January, 42 pediatric residents were informed about the PIDS/IDSA guideline at morning report, a 30-minute educational conference held daily for resident physicians.
To improve the accessibility of the guideline recommendations to all providers at the point of care, a 4×6 inch index card with the recommended antibiotics in bullet point form was first tested with a subset of prescribers in both the ED and on the HM resident teams in March 2012. Feedback on the composition of the index card was provided by each of these groups after 1 week. The index card was subsequently modified and distributed to all clinical staff (eg, attending physicians, fellows, residents, nurse practitioners) in the ED and on the HM resident teams. This modified index card was then used to update a pocket-sized reference book created by the CCHMC residency program and distributed to all residents within the program at the beginning of the new academic year (July 1, 2012).
A highly reliable intervention indicates minimal to no failures in the system over time.17 A level of reliability of 1 indicates an intervention that will only allow 1 or 2 failures out of every 10 opportunities. A level of reliability of 2 allows for <5 failures out of every 100 opportunities. In April 2012, the team incorporated higher reliability interventions.17,18 The team worked with a specialist for our electronic medical record (EMR) system (EPIC Systems Corporation, Verona, WI) to update the existing CAP order set to include a hyperlink to the PIDS/IDSA guideline and defaulted orders to appropriate doses of the recommended first-line antibiotics. Before this change, the default antibiotic choice in the order set was ceftriaxone. Additionally, the history and physical examination note template in the electronic medical record was updated to reflect the recommendations in the national guidelines within the assessment and plan section. To begin the process of spreading to other divisions within the hospital, the guidelines were disseminated by electronic mail to the entire medical staff in May. The guidelines and the improvement work to date were presented at Pediatric Grand Rounds in the first week of July.
Analysis
The primary outcome was measured as the proportion of eligible patients admitted with CAP receiving appropriate first-line antibiotic therapy. Run charts depicting weekly proportions were used to measure the effect of interventions over time. Patients who were admitted to the HM resident teams with a diagnosis of CAP from the ED are represented on both run charts as antibiotic therapy is decided while the patient is in the ED and then again when the patient is transferred to the floor. Patients who were admitted through the ED with a diagnosis other than CAP (eg, bronchiolitis) but subsequently were diagnosed with CAP on the HM resident team service were only included in the HM resident team run charts (n = 32). Special causes were identified by using established statistical rules for run chart interpretation.19 Patient length of stay in the hospital and patient age is presented as median and interquartile range. Statistical significance was determined a priori to be <.05 by using a Mann-Whitney U test. Statistical significance was determined by using a χ2 analysis for the difference in proportion of the most common ICD-9 code.
Results
There were 217 patients who were discharged with a diagnosis of pneumonia and met the eligibility criteria during the study period. Of these, 67 patients were discharged between May 1, 2011, and October 23, 2011, and 150 patients were discharged between October 24, 2011, and July 21, 2012. The median length of stay for the total cohort was <1 day (interquartile range [IQR]: 0–2 days), and the median age was 3 years (IQR: 1–6 years). The baseline group, with a median age of 4 years (IQR: 2–7 years), was significantly older than the intervention group, with a median age of 2 years (IQR: 1–5 years; P value <.001). The baseline group also had a significantly shorter length of stay (median LOS: <1 day, IQR: 0–1 day) compared with the intervention group (median LOS: 1 day, IQR: 0–2 days; P value <.001). The most common primary ICD-9 discharge diagnosis code which occurred in 180 patients (83%) was 486, “pneumonia; organism nos [no organism specified].” There was no statistically significant change in ICD-9 discharge coding between the baseline and intervention groups indicating the age and length of stay differences between groups was most likely not due to primary diagnosis.
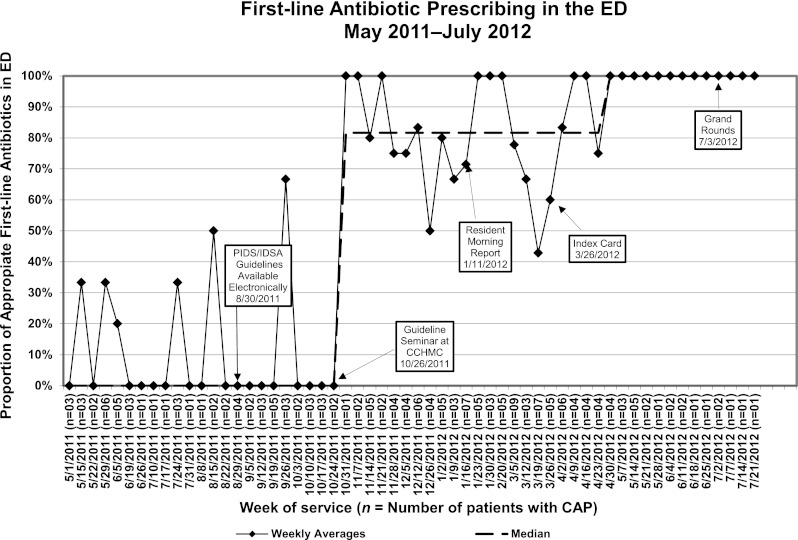
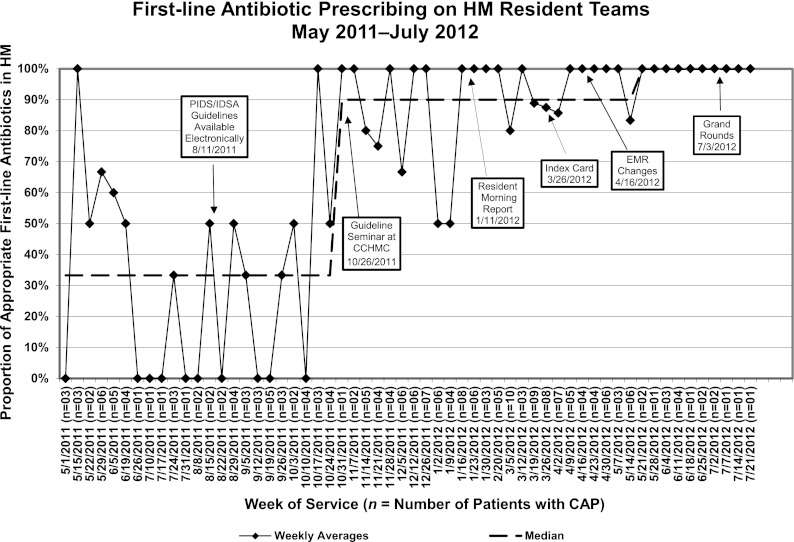
A significant improvement in appropriate first-line antibiotic prescribing was seen in the ED and on the HM resident teams after the first educational intervention, the guideline seminar (Figs 3 and 4). After the seminar on October 26, 2011, appropriate prescribing increased in the ED from 0% to 82% and from 30% to 90% on the HM service. The additional interventions allowed us to meet our goal of >80% of all patients, both in the ED and on the HM resident teams, receiving appropriate first-line antibiotic therapy. Currently, these results have been sustained at 100% prescribing of appropriate first-line antibiotic therapy for 3 months on both services.
FIGURE 3.
Run chart for appropriate first-line antibiotic prescribing for CAP in the ED.
FIGURE 4.
Run chart for appropriate first-line antibiotic prescribing for CAP on the HM resident teams.
Discussion
In this study, we used QI methodology to rapidly adopt a new national guideline for appropriate first-line antibiotic therapy in children hospitalized with CAP. Our initial education interventions improved prescribing reliability to ∼80% as would be expected with a level 1 reliability intervention. The addition of level 2 reliability interventions provided additional performance gains. Over a 6-month period, the ED increased the proportion of antibiotic prescribing as recommended by the guideline from 0% to 100% and the HM resident teams improved prescribing practices from 30% to 100%. Both of these services now prescribe according to the national guidelines in 100% of eligible cases and have done so consistently for the past 3 months.
Previous literature has used prospective-audit with feedback ASPs and an antimicrobial stewardship task force (ASTF) to implement institution-specific clinical practice guidelines (CPG) for CAP.7,8 Importantly Newman et al and Smith et al were able to increase and sustain prescribing of ampicillin from 13% to 63% and from 2% to 44%, respectively, after the introduction of their CPG.7,8 Newman et al used a combination of an institution-specific CPG and a prospective audit with feedback ASP to increase the use of ampicillin for children hospitalized with CAP.7 Smith et al highlighted the success of their CPG for management of pediatric CAP in conjunction with forming an ASTF despite not using prospective audit with feedback or a requirement for previous approval.8 Smith et al also introduced system-level change in the form of a preprinted CAP paper order set.
Although physicians may be more likely to accept guidelines created inhouse and can participate in modifying the institutional guidelines if deemed necessary, institutional guidelines may not be comparable between institutions.20 We chose not to modify any aspect of the guidelines provided by the PIDS/IDSA because our goal was to implement national guidelines and standardize the management of care within our institution and in alignment with other children’s hospitals. Additionally, the effects of guideline implementation are difficult to separate from the effects of the ASTF/ASP in previous studies.7,8 The improvements attained in our study can likely be attributed to guideline implementation and the culture of change that exist within our institution. Furthermore, we addressed system-level concerns with higher reliability interventions, modifications in our EMR to both the order set and the template used by resident physicians for the history and physical examination note. Our work highlights the effectiveness of QI methodology to increase antimicrobial prescribing to 100% in agreement with a national guideline in an institution with an integrated EMR but without an existing ASTF/ASP.
Because pneumonia is a difficult disease to diagnose, the process of antibiotic prescribing at different stages of the admitting process was evaluated. Previous studies documented the antibiotic that was administered within 24 hours of their admission, regardless of the service that prescribed the antibiotic.8 We were able to differentiate patients who were not diagnosed with pneumonia in the ED but were subsequently diagnosed with pneumonia following hospital admission to the HM resident teams. If these patients were not prescribed an antibiotic in the ED, because of suspicion of an alternate diagnosis but received an appropriate antibiotic once admitted, we were able to classify them a success only on the HM resident team service as the decision of appropriate antibiotic treatment was aligned with the clinical diagnosis at the time. This approach allowed us to have a broader spectrum of disease at presentation represented in our study population, which minimized our chances for missing potential patients with pneumonia when they initially presented to the ED.
There are several limitations to this study. First, the main interventions were implemented in the peak respiratory season, between late October and beginning of April, but sustainability has only been documented from April to July when relatively few children are hospitalized with pneumonia. Although it may be more difficult to remember the appropriate antibiotic to prescribe when in any given week a physician may see only 1 patient with pneumonia, we believe sustaining at 100% of appropriate prescribing during this period is a potential strength of the study. We also anticipate that sustainability of our overall improvement in prescribing will be easier during the respiratory season when appropriate prescribing practices will be reinforced daily. In addition the significant differences in age and LOS between our baseline and intervention groups are likely due to seasonal differences and are unrelated to our primary outcome, age-appropriate antibiotic prescribing.
Second, only 2 clinical services from our hospital were actively involved in this QI study. Our study design included only the ED and HM resident teams, as they care for most patients diagnosed with uncomplicated pneumonia in our hospital. We did not include patients who were evaluated in the ED and then discharged to home because this represents a different population. To increase appropriate prescribing practices in this latter population, we will need to implement interventions within the ED and in the surrounding community of urgent care centers and primary care providers.
Third, CCHMC is a hospital with a robust QI program and a culture responsive to change that facilitates rapid adoption of new evidence-based recommendations. Our infrastructure and level of support may limit the generalizability of our intervention to other institutions; however, most of the simple methods used by our team, such as education programs and EMR adjustments, can be replicated at other institutions. Also, our institution is a large, teaching facility with an EMR, which makes it difficult to generalize these results to a smaller community hospital or other facility without resident physicians. Similarly, our results cannot be generalized to hospitals without an EMR.
Lastly, we did not investigate readmission rates or cost of care in this study. Although these are important measures to consider, the current study focused on the potential success of using QI methodology to implement a new national guideline which other institutions have integrated using ASPs. Future work should evaluate whether the adherence to the PIDS/IDSA guidelines is cost-effective and results in equivalent or improved outcomes for the patient.
Conclusions
ASPs are effective in promoting the judicious use of antibiotics but can be timely and costly to implement at a children’s hospital.21 We have found that QI methods can be used to instill appropriate stewardship of antibiotics in the absence of a formal ASP. These methods may also be useful in improving antibiotic stewardship even in hospitals that have an established ASP. We have also shown that rapid adoption of national guidelines is possible within 6 months of the publication.
Acknowledgments
We are grateful for the contributions of Susan Ginter, RN, to our improvement work, as well as all the clinical staff in the HM and ED who provide a welcoming environment for change. We also thank Leslie Horn and Lisa Ulland for extracting the data for this study from the EMRs at the hospital. We are extremely appreciative for the support and assistance in the delivering of interventions that Dr Denise White provided and the content expertise that Dr Rebecca Brady contributed to our study.
Glossary
- ASP
antimicrobial stewardship program
- ASTF
antimicrobial stewardship task force
- CAP
community-acquired pneumonia
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CPG
clinical practice guideline
- ED
emergency department
- EMR
electronic medical record
- HM
hospital medicine
- ICD-9
International Classification of Disease, Ninth Revision
- IDSA
Infectious Disease Society of America
- IQR
interquartile range
- PIDS
pediatric infectious disease society
- QI
quality improvement
APPENDIX A. Algorithm of Eligibility
Footnotes
Dr Ambroggio participated in the design of the study, developed the data collection criteria, carried out the statistical analysis, executed interventions, drafted the initial manuscript, and approved the final manuscript for submission; Dr Thomson participated in the design of the study, designed the chart review algorithm, performed chart reviews, designed and executed interventions, drafted the introduction for the initial draft of the manuscript, reviewed all subsequent drafts of the manuscript, and approved the final manuscript for submission; Dr Murtagh Kurowski participated in the design of the study, performed chart reviews, designed and executed interventions, reviewed all drafts of the manuscript, and approved the final manuscript for submission; Dr Courter performed chart reviews, reviewed all drafts of the manuscript, and approved the final manuscript for submission; Dr Statile participated in the design of the study, designed the chart review algorithm, performed chart reviews, designed and executed interventions, reviewed all drafts of the manuscript, and approved the final manuscript for submission; Dr Graham participated in the design of the study, performed chart reviews, designed and executed interventions, reviewed all drafts of the manuscript, and approved the final manuscript for submission; Ms Sheehan participated in the design of the study, designed and executed interventions, carried out statistical analysis, reviewed all drafts of the manuscript, and approved the final manuscript for submission; Dr Iyer participated in the design of the study, participated in the design of the interventions, supervised the statistical analysis, reviewed all drafts of the manuscript, and approved the final manuscript for submission; Dr Shah participated in the design of the study, participated in the design of the interventions, developed the data collection criteria, reviewed all drafts of the manuscript, and approved the final manuscript for submission; and Dr White participated in the design of the study, participated in the design of the interventions, developed the data collection criteria, reviewed all drafts of the manuscript, and approved the final manuscript for submission.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr. Ambroggio and Dr. Thomson were supported by funds from the NRSA T32HP10027-14 and Dr. Shah was supported by funds from NIAID K01A173729. Funded by the National Institutes of Health (NIH).
References
- 1.Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994–2007. Pediatrics. 2011;127(3):411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126(2):204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley JS, Byington CL, Shah SS, et al. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America . The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambroggio L, Tabb LP, O’Meara T, Sheffler-Collins S, McGowan KL, Shah SS. Influence of antibiotic susceptibility patterns on empiric antibiotic prescribing for children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(4):331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Community-Acquired Pneumonia Guideline Team. Evidence-based care guideline for medical management of community acquired pneumonia in children 60 days to 17 years of age. Cincinnati, OH: Cincinnati Children’s Hospital Medical Center; December 22, 2005
- 7.Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/e597 [DOI] [PubMed] [Google Scholar]
- 8.Smith MJ, Kong M, Cambon A, Woods CR. Effectiveness of antimicrobial guidelines for community-acquired pneumonia in children. Pediatrics. 2012;129(5). Available at: www.pediatrics.org/cgi/content/full/129/5/e1326 [DOI] [PubMed] [Google Scholar]
- 9.Cohen MR, Senders J, Davis NM. Failure mode and effects analysis: a novel approach to avoiding dangerous medication errors and accidents. Hosp Pharm. 1994;29(4):319–330 [PubMed] [Google Scholar]
- 10.DeRosier J, Stalhandske E, Bagian JP, Nudell T. Using health care failure mode and effect analysis: The VA National Center for Patient Safety’s prospective risk analysis system. Jt Comm J Qual Improv 2002;28:248–267, 209 [DOI] [PubMed]
- 11.van de Garde EM, Oosterheert JJ, Bonten M, Kaplan RC, Leufkens HG. International classification of diseases codes showed modest sensitivity for detecting community-acquired pneumonia. J Clin Epidemiol. 2007;60(8):834–838 [DOI] [PubMed] [Google Scholar]
- 12.Whittle J, Fine MJ, Joyce DZ, et al. Community-acquired pneumonia: can it be defined with claims data? Am J Med Qual. 1997;12(4):187–193 [DOI] [PubMed] [Google Scholar]
- 13.Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM, Jr, Breiman RF. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol. 1999;149(3):282–289 [DOI] [PubMed] [Google Scholar]
- 14.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328 [DOI] [PubMed] [Google Scholar]
- 15.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/E99 [DOI] [PubMed] [Google Scholar]
- 16.Langley G, Nolan K, Nolan T, Norman C, Provost L. The Improvement Guide. A Practical Approach to Enhancing Organizational Performance. San Francisco, CA: Jossey-Boss; 1996 [Google Scholar]
- 17.Nolan T, Resar R, Haraden C, Griffin FA. Improving the Reliability of Health Care (IHI Innovation Series White Paper). Boston, MA: Institute for Healthcare Improvement; 2004: 1–16
- 18.Luria JW, Muething SE, Schoettker PJ, Kotagal UR. Reliability science and patient safety. Pediatr Clin North Am. 2006;53(6):1121–1133 [DOI] [PubMed] [Google Scholar]
- 19.Provost L, Murray S. The Health Care Data Guide: Learning from Data for Improvement. San Francisco, CA: Jossey-Bass; 2011 [Google Scholar]
- 20.Neuman MI, Hall M, Hersh AL, et al. Influence of hospital guidelines on management of children hospitalized with pneumonia. Pediatrics. 2012;130(5). Available at: www.pediatrics.org/cgi/content/full/130/5/e823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hersh AL, Beekmann SE, Polgreen PM, Zaoutis TE, Newland JG. Antimicrobial stewardship programs in pediatrics. Infect Control Hosp Epidemiol. 2009;30(12):1211–1217 [DOI] [PubMed] [Google Scholar]