Abstract
BACKGROUND:
The factors that drive overtreatment of gastroesophageal reflux disease (GERD) are not well understood, but it has been proposed that the use of the “GERD” disease label could perpetuate use of medication in otherwise healthy infants.
METHODS:
To determine if use of the disease label GERD influences parents’ perceived need to medicate an infant, we surveyed parents in a general pediatric clinic. Parents were given a hypothetical clinical scenario describing an infant who cries and spits up excessively but is otherwise healthy. Using a 2 × 2 factorial design, parents were randomized to receive a scenario in which the doctor either gave a diagnosis of GERD or did not provide a disease label; additionally, half of parents were told that existing medications are probably ineffective, whereas the rest were not given any effectiveness information. We measured parent interest in medication, perception of illness severity, and appreciation of medication offer.
RESULTS:
Parents who received a GERD diagnosis were interested in medicating their infant, even when they were told that the medications are likely ineffective. However, parents not given a disease label were interested in medication only when medication effectiveness was not discussed (and hence likely assumed).
CONCLUSIONS:
Labeling an otherwise healthy infant as having a “disease” increased parents’ interest in medicating their infant when they were told that medications are ineffective. These findings suggest that use of disease labels may promote overtreatment by causing people to believe that ineffective medications are both useful and necessary.
Keywords: Gastroesophageal reflux disease, GERD, disease labels, overtreatment
What’s Known on This Subject:
Medications used to treat gastroesophageal reflux disease (GERD) are some of the most widely used medications in children younger than 1 year. There are strong indications that GERD is overdiagnosed and overtreated.
What This Study Adds:
The factors that drive overtreatment of GERD are not well understood, but it has been proposed that the use of the GERD disease label could perpetuate use of medication. In this study we find evidence for this possibility.
There is growing concern that gastroesophageal reflux disease, or GERD, is overdiagnosed and overtreated in infants.1 Studies have documented rapid increases in the numbers of infants in the United States diagnosed with GERD and treated with prescription medications, such as proton pump inhibitors.2 Specifically, from 1999 to 2004, there was a sevenfold increase in the use of prescription medications to treat GERD in infants younger than 1 year.3 Although acid-reducing medications may be effective in cases in which GERD is confirmed using endoscopy, clinical trials have shown that acid-reducing medications are no better than placebo in treating behavioral symptoms frequently diagnosed as GERD, such as excessive crying and regurgitation.4–7
Many factors likely perpetuate the medicalization and treatment of these symptoms as GERD in the face of evidence that treatment is ineffective. Specific hypotheses focus on physician-patient communication factors, such as (1) the relative ease of prescribing medicines rather than providing behavioral or lifestyle interventions, (2) parent demand fueled by Web sites and advocacy groups, and (3) physician labeling of regurgitation and irritability as “GERD,” making parents erroneously believe that acid-reducing medications are appropriate and necessary.1 With regard to the latter hypothesis, it has been proposed that physicians’ use of labels, such as GERD, “subvert rational thinking and subliminally encourage the prescription of acid-suppressant medication even when acid or reflux is unlikely to be the problem.”1(p195)
Although there may be multiple factors at work in medicalizing excessive crying and regurgitation in infants, we hypothesized that the disease label may indeed encourage the use of medications, and therefore help fuel overtreatment of these symptoms. To our knowledge, there has been no empirical test of how the use of disease labels, such as GERD, influence parents’ perceived need to medicate their child (which may, in turn, influence the physician to write the prescription). In the current study, we tested whether use of the label “GERD” can foster parental interest in using acid-reducing medication to treat infants’ excessive irritability and reflux.
Methods
Participants and Setting
Participants were parents (≥18 years old) of children presenting to a primary care pediatrics clinic between May 2011 and February 2012 at the University of Michigan for an appointment (health maintenance or acute visit). Participants were surveyed in the waiting room before their child’s appointment with the health care provider. Those who were not able to finish the survey in the waiting room completed it in the examination room. Parents were not compensated for their participation. This study was reviewed and approved by the University of Michigan Institutional Review Board.
Design
The intervention was a randomized trial using a written self-administered survey instrument. Participants were randomly assigned to receive 1 of 4 written vignettes. The experiment varied 2 factors within the vignette in a between-participant design. We varied (1) disease label: the presence versus absence of a GERD label, and (2) medication ineffectiveness: stated ineffective versus no information (Table 1).
TABLE 1.
Study Design
| GERD Diagnosis | No GERD Diagnosis | |
|---|---|---|
| Medication effectiveness: no information | GERD diagnosis + No information about medication effectiveness | No GERD diagnosis + No information about medication effectiveness |
| Medication effectiveness: Medications are ineffective | GERD diagnosis + Medication ineffective | No GERD diagnosis + Medication ineffective |
Materials and Intervention
The study materials included vignettes in which an infant with excessive crying and regurgitation is treated as a possible case of GERD, a common clinical situation faced by primary care pediatricians, given recent studies on GERD diagnosis and management.4 The vignette was written so as to be consistent with current North American infant GERD clinical care guidelines.8 Although current guidelines state that “the available evidence does not support an empiric trial of acid suppression in infants with unexplained crying, irritability, or sleep disturbance”(p 521), the guidelines also state that “if irritability persists with no explanation other than suspected GERD…a time-limited (2-week) trial of antisecretory therapy may be considered” (p 501).8 Given these somewhat conflicting directives, we sought to ensure that the vignette represented a plausible clinical situation. Hence, the vignette was pilot tested for plausibility with 6 senior and junior pediatric clinicians at the University of Michigan. All clinicians confirmed that the vignette represented a plausible and familiar clinical scenario.
In the first section of each vignette, all participants read the same background information about a 1-month-old infant’s symptoms, which included excessive crying and regurgitation (see Appendix for full text). For example, participants were told “Your infant spits up a lot…. After spitting-up, your infant cries a lot. The crying and spitting seems especially bad after eating. But sometimes it seems like she is uncomfortable most of the time.” Participants were also told that behavioral interventions, such as keeping the infant upright after feeding, had been tried with no alleviation of symptoms. Apart from these symptoms, the infant was described as appearing healthy and having gained an appropriate amount of weight. Next, each vignette described an appointment with the infant’s pediatrician (Figure 1). This part of the vignette was written to represent a situation in which a physician chooses to treat excessive crying and regurgitation as evidence of potential GERD and offers a trial of acid-reducing medication. Moreover, in recognition of current GERD clinical care guidelines and research, the physician in the vignette indicated that medication is optional and that the infant will likely grow out of these symptoms without intervention. All participants received the same description of the physiologic mechanism (acid reflux) thought to cause symptoms in cases of GERD. By holding the physiologic description constant, we were able to specifically test the influence of our experimental factors on judgments. Those experimental factors are described as follows.
FIGURE 1.
Doctor Appointment Scenario.
Experimental Factor 1: Disease Label
Half of participants received a scenario in which the physician provided the disease label “gastroesophageal reflux disease (GERD),” and GERD was mentioned throughout the narrative. For the rest of the participants, the physician provided the same explanation of the symptoms, but referred to the symptoms as “this problem,” and made no mention of a specific diagnosis or disease label.
Experimental Factor 2: Medication Ineffectiveness
Half of participants were told, “studies have shown that this medicine probably doesn’t do anything to help improve symptoms in infants with GERD.” The rest of the participants were not provided with any information about the effectiveness of the medication, and therefore likely assumed that the treatment was effective. The purpose of this experimental factor was to test whether a GERD disease label would increase interest in medication even when participants are explicitly informed of the potential ineffectiveness of these medications.
Outcome Measures
Perception of Child’s Health
Parents responded to 3 items indicating their perception of the child’s health: How worried are you about your infant’s health? (0 = not at all worried, 5 = very worried), How serious do you think your infant’s condition is? (0 = not at all serious, 5 = very serious), and My infant is sick (0 = strongly disagree, 5 = strongly agree).
Beliefs About Medicating Child
Next, parents answered 3 questions indicating their degree of interest in giving medication to their infant: Will you give your infant this medicine? Do you think your infant needs the medicine that the doctor offered? Do you think that the medicine will help your infant get better? (0 = no definitely not, 5 = yes, definitely). Parents also rated whether they appreciated the physician’s offer of medications using the same scale.
Demographics
At the end of the survey, parents were asked to report their age, gender, race, ethnicity, education level, and age of youngest child in the home. Parents were also asked if any of their children had been diagnosed with GERD, and, if so, whether they had given their child medication for GERD.
Data Analysis
To analyze the effect of the experimental factors on parents’ judgments, we conducted three 2 (disease label: GERD diagnosis versus no diagnosis) × 2 (medication information: ineffective versus no information) between-participant analyses of variance (ANOVAs). Outcome variables included (1) perceptions of illness, (2) interest in medication, and (3) appreciation of medication offer.
Results
A total of 175 parents participated. The average parent age was 35.27 years (SD = 8.61) and the average age of the youngest child in the household was 4.5 years (SD = 4.34). Most participants were women (82.4%) and identified as being white (58.9%). Overall, the sample was highly educated, with 66% of the sample having received a bachelor’s degree or more (Table 2).
TABLE 2.
Participant Characteristics
| Characteristic | % (n) |
|---|---|
| Age of parent, range: 18–56 | Mean = 35.27 (SD = 8.61) |
| Age of child, range: <1 wk–17 y | Mean = 4.5 (SD = 4.34) |
| Gender | |
| Men | 16.6 (29) |
| Women | 82.9 (145) |
| Not reported | .05 (1) |
| Race | |
| White | 58.9 (103) |
| African American | 8.0 (14) |
| Native American | 0.6 (1) |
| Asian | 16.0 (28) |
| Other/mixed race | 5.6 (10) |
| Not reported | 10.9 (19) |
| Hispanic ethnicity, any race | 2.3 (4) |
| Middle Eastern ethnicity, any race | 2.9 (5) |
| Education | |
| High school diploma or less | 6.3 (11) |
| Some college/trade school | 16.6 (29) |
| Bachelor’s/Associate’s degree | 33.1 (58) |
| Master’s degree or more | 33.1 (58) |
| Not reported | 10.8 (19) |
Additionally, 37 parents (21.1%) indicated that 1 or more of their children had been diagnosed with GERD in the past, and 26 said that they had medicated their child as a result of that diagnosis. To provide a complete report of the data, we included the presence/absence of a previous GERD diagnosis as a factor in all of the following analyses, and we report all results involving effects of previous GERD diagnosis.
Perceptions of Infant’s Health
Parents reported (using a 0–5 scale for each question) being somewhat worried about the infant’s health (mean = 2.28, SD = 1.30), thought that the condition was somewhat serious (mean = 2.12, SD = 1.19), and were relatively unlikely to describe the infant as “sick” (mean = 1.87, SD = 1.45). Parents’ answers to these questions were not influenced by the presence or absence of the GERD label in the scenario, information about medication efficacy, or by having a child with prior GERD diagnosis (all P > .12).
Beliefs About Medicating Infant
The three questions involving parent interest in medication were highly intercorrelated (Cronbach’s α = 0.92), and so we created a single variable by taking the average of responses to these questions. The resultant variable was submitted to a 2 (GERD Label: present versus absent) × 2 (Medicine Ineffectiveness: present versus absent) × 2 (Previous GERD diagnosis: present versus absent) between-participant ANOVA.
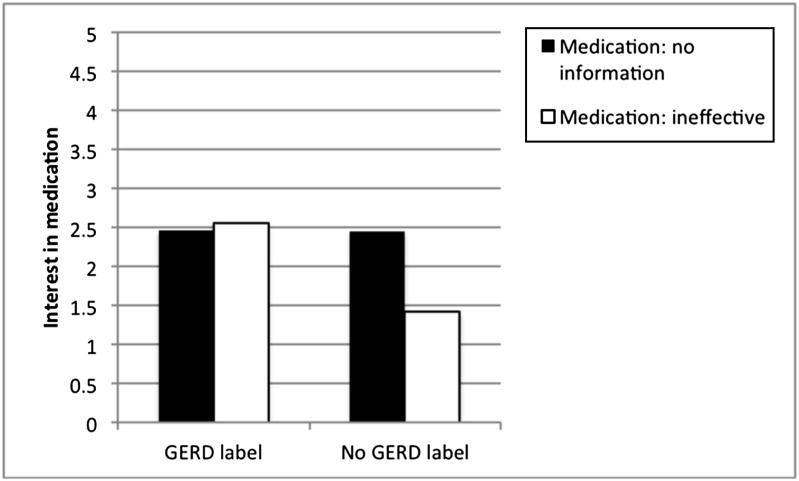
Results revealed 3 significant effects. First, as predicted, parents who received the GERD label in the scenario were more interested in medication than parents who did not receive that label, F(1,165) = 6.95, P < .01. Second, parents with children who had been diagnosed with GERD were more interested in using medications than parents who did not have a child with GERD, F(1,165) = 10.62, P < .01. Third, there was an interaction between GERD Label and Medicine Ineffectiveness, F(1,165) = 4.52, P < .05 (Fig 2). Figure 2 reveals that parents who received a GERD diagnosis were interested in medicating the infant, even when they were told that the medications are likely ineffective; however, parents not given a diagnosis were interested in medication only when they were allowed to assume that the medications are effective (ie, when the physician did not indicate that medications were ineffective). No other effects were significant (all P > .10).
FIGURE 2.
Parent interest in medication. Higher numbers indicate greater interest in medicating infants.
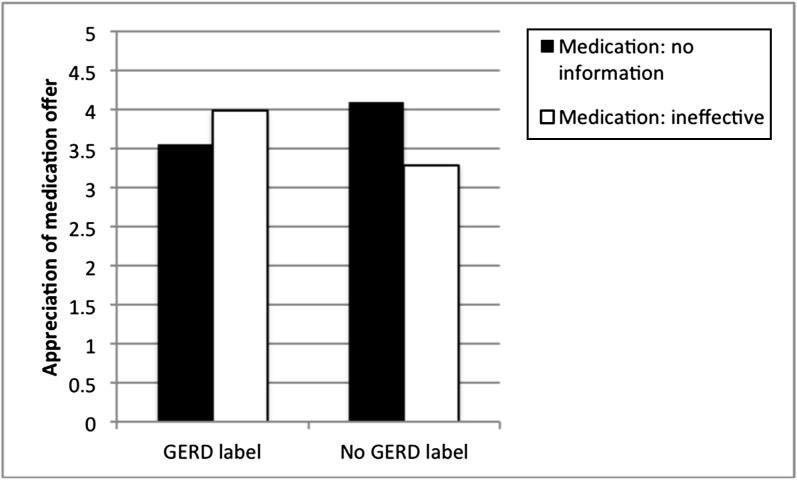
Furthermore, all parents were asked whether they appreciated the doctor’s offer of medication. An analogous ANOVA revealed only 1 significant effect, which was an interaction between GERD Label and Medicine Ineffectiveness (F[1,165] = 7.16, P < .01; all other P > .20). Figure 3 displays this interaction, and reveals that parents were least appreciative of medication when they were (1) told that the medication is likely ineffective and (2) not given a GERD disease label. Moreover, parents who were told that the medication was ineffective but also given the GERD label were among those most appreciative of the medication offer.
FIGURE 3.
Parents’ appreciation of medication offer. Higher numbers indicate greater appreciation.
Comment
The present findings demonstrate that disease labels, such as GERD, and information about medication ineffectiveness can strongly influence parents’ interest in medications. Not surprisingly, parents were less likely to be interested in using medication when they were told that the medication was likely ineffective. More surprising, however, was the fact that this was only true of parents who received no GERD disease label. When the GERD label was used, parents were interested in using medication even when they were explicitly told that these medications were probably ineffective. Together, these findings lend support for our hypothesis that the use of the GERD label can influence interest in using potentially ineffective medical interventions.
One possible explanation of these effects is that the disease label made the symptoms seem more serious, which in turn amplified the perceived need for medical intervention. However, our data do not support this explanation. The GERD label had no impact on parents’ perceptions of illness severity, both in terms of reported amount of worry, and perceived seriousness. This suggests that the GERD label influenced parents’ judgments not by changing their perceptions of the illness itself, but rather by changing their assumptions about what kinds of interventions would be most appropriate.
Given the current concerns about the overtreatment of GERD,1 our findings suggest that physicians can reduce interest in medications by (1) not labeling the symptoms as GERD, and (2) explaining to parents that acid reflux medications are not effective. The present data also suggest that this course of action leads to a significant reduction in appreciation of the physician’s offer of medication. By contrast, in the presence of a disease label, that same information about medication ineffectiveness led to significantly increased level of appreciation. One possible explanation is that in the context of a disease label, information about medication ineffectiveness might be seen as an act of candidness and honesty. However, in the absence of a disease label, offering ineffective medication might create confusion because it seems as if the physician is medicalizing a normal condition.
On Clinical Guidelines, Overdiagnosis, and the Influence of Medical Labels
In our vignette, we described a clinical scenario in which a physician can follow a number of courses of action, according to clinical care guidelines. When dealing with an infant with excessive crying and regurgitation, the clinician can (1) order an invasive diagnostic test, (2) continue trying behavioral and lifestyle interventions, or (3) prescribe medication for a trial period.8 Given that testing is invasive, and behavioral/lifestyle changes often produce no apparent result, the path of least resistance may often be a trial period of medication. However, the potential for spontaneous recovery and placebo effects means that this approach is also the most likely to lead to overdiagnosis and overtreatment. Moreover, in the absence of a clear directive from clinical guidelines (eg, do not use proton pump inhibitors to treat infants with irritability and reflux under any circumstances), we can expect that infants with these symptoms will continue to be treated for possible GERD. Our data suggest that use of a disease label may fuel such behaviors, because once the GERD label is used, information about medication ineffectiveness does not dissuade parents from wanting to try medications.
GERD is certainly not the first or only condition to be suspected of widespread overdiagnosis and overtreatment. In recent years there has been considerable attention to these issues,9–12 and through the recent Choosing Wisely initiative, a number of specialty areas have presented lists of tests and procedures that should be questioned by both physicians and patients.9 Hence, it is unlikely that GERD is the only clinical case in which a disease label can influence perceived need for unnecessary treatment. The possibility that diagnostic labels affect patient judgments could have wide-reaching implications for medical communication and health care utilization.13,14 The present data suggest that physicians’ language can play a role in the process of medicalization and overtreatment.15
Limitations
The current study has limitations that merit discussion. First, we sampled parents from 1 clinic, and although we had some ethnic diversity, most of our participants were highly educated women. However, this sample provided a strong test of our hypothesis, given that a highly educated sample may be among those most likely to question the legitimacy of diagnoses provided by a physician.
Second, the physiologic explanation that was provided in the scenario may conflict with data-driven studies, or some experts’ opinions about the appropriateness of attributing excessive crying and regurgitation to GERD or acid reflux. We share others’ concerns that physicians might ignore the recent randomized controlled trials for GERD, and that guidelines allow for empirical trials of medications that are not indicated by randomized controlled trials and that may frequently lead to overdiagnosis.1 Unfortunately, it has been established that physicians do not always practice evidence-based medicine,16 and we often propagate medical ideas and causal mechanisms for which we do not have substantial evidence.16 As a result, our scenario likely represents the way physicians care for such infants in real-world settings. Moreover, the physiologic explanation provided in the current study was consistent with offering acid-reducing medications. That is, there is no reason to offer acid-reducing medications, even for a trial period, if acid is not the suspected problem.
A third potential weakness of our study is that the physiologic explanation that was provided in the scenario was presented to all participants, not just those in the GERD label condition. As a result, one may worry that all participants were misled by the description of acid reflux. However, this feature of our design allowed us to show that even when all participants are given an explanation consistent with GERD, the label nonetheless exerts a significant influence on judgments over and above that explanation. If participants who received no GERD label had also been given a different explanation of the symptoms, it is likely that we would have obtained even stronger effects than the ones reported here; however, the effects would not be attributable to the disease label, but rather to the combination of label and causal explanation.
Finally, this study presented parents with a hypothetical scenario, which allowed us to maximize internal validity and experimentally identify how specific components of that message affect judgment. Although there is always a question as to whether hypothetical studies predict real-world behavior, it is likely that parents who have infants with these symptoms are more anxious than parents in the current study. As a result, such parents might be even more susceptible to judgment biases that result from the disease label than the present research participants. Future research must address how disease labels influence medical decision-making during real-life interactions.
Conclusions
Although there are many reasons why GERD medications came into widespread use in irritable and regurgitating infants who are otherwise healthy, in the current study we tested the notion that this state of affairs could be perpetuated by the way that physicians label the infant’s symptoms. We found that a GERD disease label increases interest in using medication when the medications are known to be ineffective. Hence, doctors may inadvertently encourage the use of questionable medical interventions and foster medicalization of minor pediatric illnesses15 by using labels that increase patients’ perceived need for treatment.
Acknowledgments
The authors thank Adam Leavitt, Sussy Pan, Jacqueline Barkoski, Zachary Demertzis, and Emily Tran for their exceptional efforts in recruiting participants for this study.
Glossary
- GERD
gastroesophageal reflux disease
Appendix: Background
Imagine that you are the parent of a 1-month-old infant. At this point, your infant’s life mostly involves eating, pooping, and crying. You are tired all the time, because your infant is eating every 2 to 4 hours, even at night. The days and nights seem to blur together. At this point, your infant is developing normally and gaining an appropriate amount of weight.
Your infant also spits up a lot. Sometimes after feeding, your infant will spit-up a big mouthful onto your shirt or the floor. Often there is so much spit-up that you are amazed that there is anything left in your infant’s stomach. Sometimes the spit-up looks like breast milk or formula, but other times it looks curdled, like cottage cheese.
After spitting-up, your infant cries a lot. The crying and spitting seems especially bad after eating. But sometimes it seems like she is uncomfortable most of the time. The crying and fussing is beginning to take a toll on you. It seems like there is nothing that you can do to stop the crying or to soothe your infant.
The spit-up is so bothersome that you have been searching online for tips on how to control it. You have tried doing things like burping more frequently and keeping her upright for 30 minutes after feeding, but these haven’t seemed to help. Now you are starting to get anxious about the possibility that there is something seriously wrong. You are worried that an infant who is this uncomfortable, and that spits up this much, might not be healthy. So, you decided to take your infant to the doctor to be checked.
Footnotes
Dr Scherer carried out analyses and drafted the initial manuscript; Drs Zikmund-Fisher Fagerlin and Tarini reviewed and revised the manuscript; Dr Tarini coordinated communication between researchers and clinic site; and all authors conceptualized and designed the study and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Tarini is supported by a K23 Mentored Patient-Oriented Research Career Development Award from the National Institute for Child Health and Human Development (K23HD057994). Dr Zikmund-Fisher is supported by a Mentored Research Scholar Grant from the American Cancer Society (MRSG-06-130-01-CPPB). Funded by the National Institutes of Health (NIH).
COMPANION PAPER: A companion to this article can be found on page 991, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2013-0286.
References
- 1.Hassall E. Over-prescription of acid-suppressing medications in infants: how it came about, why it’s wrong, and what to do about it. J Pediatr. 2012;160(2):193–198 [DOI] [PubMed] [Google Scholar]
- 2.Nelson SP, Kothari S, Wu EQ, Beaulieu N, McHale JM, Dabbous OH. Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy. J Media Econ. 2009;12(4):348–355 [DOI] [PubMed] [Google Scholar]
- 3.Barron JJ, Tan H, Spalding J, Bakst AW, Singer J. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45(4):421–427 [DOI] [PubMed] [Google Scholar]
- 4.van der Pol RJ, Smits MJ, van Wijk MP, Omari TI, Tabbers MM, Benninga MA. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127(5):925–935 [DOI] [PubMed] [Google Scholar]
- 5.Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154(4):514–520.e4 [DOI] [PubMed] [Google Scholar]
- 6.Orenstein SR, Hassall E. Pantoprazole for symptoms of infant GERD: the emperor has no clothes! J Pediatr Gastroenterol Nutr. 2010;51(4):537–, author reply 537–539. [DOI] [PubMed] [Google Scholar]
- 7.Higginbotham TW. Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44(3):572–576 [DOI] [PubMed] [Google Scholar]
- 8.Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition . Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547 [DOI] [PubMed] [Google Scholar]
- 9.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–1802 [DOI] [PubMed] [Google Scholar]
- 10.Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. [DOI] [PubMed]
- 11.Welch HG, Schwartz L, Schwartz LM, Woloshin S. Overdiagnosed: Making People Sick in the Pursuit of Health. Beacon Press: Boston; 2011 [Google Scholar]
- 12.Hadler NM. The Last Well Person: How to Stay Well Despite the Health-Care System. McGill-Queen’s University Press Montreal; 2004.
- 13.Haynes RB, Sackett DL, Taylor DW, Gibson ES, Johnson AL. Increased absenteeism from work after detection and labeling of hypertensive patients. N Engl J Med. 1978;299(14):741–744 [DOI] [PubMed] [Google Scholar]
- 14.van Weel C. Does labelling and treatment for hypertension increase illness behaviour? Fam Pract. 1985;2(3):147–150 [DOI] [PubMed] [Google Scholar]
- 15.Carey WB, Sibinga MS. Avoiding pediatric pathogenesis in the management of acute minor illness. Pediatrics. 1972;49(4):553–562 [PubMed] [Google Scholar]
- 16.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465 [DOI] [PubMed] [Google Scholar]