Abstract
OBJECTIVE:
To examine the relationship between gestational age and mental and psychomotor development scores in healthy infants born between 37 and 41 weeks.
METHODS:
The cohort included 1562 participants enrolled during infancy in an iron deficiency anemia preventive trial in Santiago, Chile. All participants were healthy, full-term (37–41 weeks) infants who weighed 3 kg or more at birth. Development at 12 months was assessed using the Bayley Scales of Infant Development. Using generalized linear modeling, we analyzed the association between gestational age and 1-year-old developmental status, taking into account potential confounders including birth weight percentile, gender, socioeconomic status, the home environment, iron status, and iron supplementation.
RESULTS:
For each additional week of gestation, the Mental Development Index increased by 0.8 points (95% confidence interval = 0.2–1.4), and the Psychomotor Development Index increased by 1.4 points (95% confidence interval = 0.6–2.1) controlling for birth weight percentile, gender, socioeconomic status, and home environment.
CONCLUSIONS:
In a large sample of healthy full-term infants, developmental scores obtained using the Bayley Scales of Infant Development at 12 months increased with gestational age (37–41 weeks). There is increasing evidence that birth at 39 to 41 weeks provides developmental advantages compared with birth at 37 to 38 weeks. Because cesarean deliveries and early-term inductions have increased to 40% of all births, consideration of ongoing brain development during the full-term period is an important medical and policy issue.
Keywords: gestational age, term infants, development, cognition, Bayley Scales of Infant Development
What’s Known on This Subject:
Cognitive and motor developmental test scores of preterm and late preterm infants increase with gestational age. Developmental test scores in full-term infants have not previously been considered to relate to gestational age.
What This Study Adds:
In a cohort of healthy, full-term infants, 37 to 41 weeks, 12-month mental and psychomotor scores on the Bayley Scales of Infant Development increased with gestational age, suggesting that neurodevelopment is optimal in infants born at 39 to 41 weeks.
It is well established that prematurity (gestational age <37 weeks) is associated with poorer developmental outcomes compared with full-term birth (37–41 weeks), and developmental scores correlate with gestational age within the preterm range.1,2 Until recently, most published research has focused on infants born during the early preterm period (<34 weeks)1 when it is difficult to disentangle the role of developmental vulnerability of the immature brain from factors associated with illness or brain injury. On the basis of recent research, it is now clear that late preterm birth (34–36 weeks’ gestational age) is also associated with lower developmental scores compared with full-term birth.3–8 Furthermore, in the late preterm period, lower gestational age appears to have an effect on developmental outcomes independent of birth weight.4 Less attention has been paid to the relationship between gestational age and development within the full-term range, even though more than a decade ago, research showed that specific neurologic and physical characteristics of the infant change between 37 and 41 weeks’ gestation.9 New knowledge about brain development highlights the importance of the last 4 to 5 weeks of gestation.10,11 During this period, the fetus experiences impressive brain maturation. Brain weight increases by approximately one-third, and dramatic increases in gyri and sulci are noted.10 In addition, axons and dendrites experience rapid growth,10 and the subplate neurons begin to regress, and interconnections between the thalamus and the cerebral cortex develop. Therefore, a large portion of brain development and networking takes place in the last weeks of gestation.12
Evidence for an effect on developmental outcomes of gestational age during the full-term period comes from recent studies in school-age children and in young adult military recruits. A study of 13 824 healthy 6-year-old Belarusian children found higher IQ scores in those born at 39- to 40-weeks’ gestational age compared with those born at 37- to 38-weeks’ gestational age.13 US children (n = 128 050) born at 37 to 38 weeks had significantly lower third-grade, standardized math and reading scores, compared with those born at 39 to 41 weeks.14 Studies of military recruits in Norway (n = 317 761) and Sweden (n = 386 954) found associations between gestational age during the full-term period and IQ.5,15 In Great Britain, poorer school performance16 and need for special education services17 have also been correlated with decreasing gestational from 41 weeks. Furthermore, 9- to 15-week-old, full-term infants (n = 3224) had increased risk for nonoptimal development if born before the expected date of delivery and neuromotor development was associated with week of gestation (37–40 weeks).18
The issue of optimal timing of birth during the full-term period seems particularly relevant in an era when induction of labor is common and cesarian delivery rates are high.19,20 Others have suggested more research is needed to disentangle the role of obstetric and neonatal complications from that of gestational length.4 Although many factors are considered in deciding whether the benefits outweigh the risks related to the timing of delivery, infant development is currently considered to be homogenous during the full-term period and rarely taken into account. The purpose of this study was to examine the influence of gestational age, within the full-term range, on developmental outcomes at 12 months in healthy full-term singleton infants who had no apparent perinatal problems.
Methods
We conducted a secondary analysis of data collected in a study of 1657 infants who participated in a randomized controlled trial of iron supplementation, between 6 and 12 months, to prevent iron deficiency anemia (IDA).21 Infants were recruited from 4 contiguous urban, working-class communities in Santiago, Chile, from 1991 to 1996. Inclusion criteria for the trial have been described elsewhere.21 In brief, the study recruited healthy infants born between 37 and 42 completed weeks of gestation, who were born by spontaneous vaginal singleton delivery, weighing ≥3 kg, without any major infant or maternal health problems, including mental health. Infants were screened for IDA at 6 months, and those without IDA were randomly assigned to receive high- or low-iron supplementation or usual nutrition (no added iron). Iron status and developmental and behavioral outcomes were assessed at 12 months.
For this analysis, we included 1562 infants, with complete data on all variables, born between 37 and 41 weeks, excluding the 30 infants born at 42 weeks’ gestation. Gestational age (weeks) was assessed by the date of the last menstrual period. Prenatal ultrasound for assessing gestational age was not routinely used in Chile at the time.
The outcomes of interest included developmental scores at 12 months, measured by using the Mental and Psychomotor Bayley Scales of Infant Development (BSID).22 The test has a standardized mean of 100 and SD of 15, based on a representative US sample of 1409 infants.23 Standardized scores are corrected for exact age in postnatal days. The BSID was individually administered by trained psychologists, with the parent present, between 1991 and 1996. In 1993, an updated version of the BSID was published.24 Because data collection was well underway, we continued to use the BSID for comparability of scores.
Infant birth weight and gender were recorded from hospital records. Birth weight percentile (adjusted for gestational age) was computed based on the work of Oken et al.25 Socioeconomic status (SES) was measured by using a modified Graffar Index,26 which includes number of people in the home, father presence, head of household’s educational attainment, employment, home ownership, type and size of housing, running water supply, ownership of home appliances, and crowding. For the reverse coded Graffar variable, higher scores indicate higher SES with a range of 0 to 65. The mothers reported total years of education. We used the Infant Home Observation for Measurement of the Environment (HOME) inventory to assess the quality of emotional, social, and cognitive stimulation available to the child in the home. It consists of 45 items clustered into 5 subscales: parental responsivity, discipline, organization of the environment, learning materials, and variety of experience (Cronbach α = .80).27 Higher total HOME scores (range 0–45) indicate a home environment more supportive of child development. The assessments were performed by trained psychologists and study nurses. Interrater reliability was >80%.
Parents provided signed informed consent for enrollment in the randomized controlled trial. Ethics committees at the Institute of Nutrition and Food Technology, University of Chile, the University of Michigan, and the University of California, San Diego approved the study.
Statistical Analysis
Student t test and χ2 analyses were used for descriptive statistics. Using 1-way analysis of variance, we examined covariates by week of gestation. Multivariate linear regression was used to test our a priori hypothesis that gestational age was related to 12-month developmental scores, taking into account potential confounding variables, including gender, birth weight percentile, family SES, HOME, IDA, and random assignment to iron supplementation versus usual nutrition. We included birth weight percentile in the model because birth weight was correlated with gestational age. Maternal education was not included because it was highly correlated with SES. Significance level was set at P < .05. Analyses were performed by using SPSS version 19 (IBM SPSS Statistics, IBM Corporation, Armonk, NY).
Results
Mean birth weight was 3.55 kg, and 47% were female infants. The mean Graffar score, 27.7, indicated lower middle SES, and the HOME score of 30.3 was comparable to scores in similar US samples. Mothers’ mean education was 9.5 years, and 31% had ≥12 years. Approximately 10% of the infants had IDA at 12 months. The mean MDI was 104.2 (SD = 12.2), and the mean PDI was 97.1 (SD = 15.1). Background characteristics and outcomes by week of gestation are shown in Table 1. Birth weight was the only background characteristic related to week of gestation (Table 1).
TABLE 1.
Characteristics by Week of Gestationa
| 37 wk (n = 45) | 38 wk (n = 260) | 39 wk (n = 469) | 40 wk (n = 604) | 41 wk (n = 184) | |
|---|---|---|---|---|---|
| Age at BSID, d | 372 (8.2) | 373 (10.9) | 372 (10.6) | 372 (10.4) | 372 (10.5) |
| Birth wt, kgb | 3.3 (0.3) | 3.4 (0.3) | 3.5 (0.3) | 3.6 (0.4) | 3.6 (0.4) |
| Birth wt percentilec | 62.8 (15.8) | 56.9 (21.3) | 53.5 (24.1) | 55.4 (26.1) | 53.0 (25.7) |
| SESd | 26.0 (5.7) | 28.3 (6.4) | 27.5 (6.0) | 27.7 (6.6) | 27.3 (6.5) |
| HOME | 30.2 (4.5) | 30.2 (4.7) | 30.3 (4.7) | 30.2 (4.7) | 30.4 (4.9) |
| Maternal education, y | 10.0 (2.9) | 9.4 (2.6) | 9.5 (2.6) | 9.4 (2.8) | 9.6 (2.6) |
| Female gender | 53.3 | 42.7 | 46.3 | 49.7 | 48.9 |
| IDA at 6 or 12 mo | 13.3 | 17.7 | 10.9 | 11.4 | 10.3 |
| Iron assignmente | |||||
| High iron | 57.8 | 39.2 | 45.2 | 43.4 | 37.0 |
| Low iron | 15.6 | 26.5 | 22.6 | 24.3 | 25.5 |
| No added iron | 26.7 | 34.2 | 32.2 | 32.3 | 37.5 |
| MDI | 102.6 (11.4) | 103.4 (12.3) | 103.3 (12.9) | 105.1 (11.5) | 105.4 (12.2) |
| PDIb | 94.4 (14.9) | 94.4 (14.6) | 96.6 (15.4) | 98.5 (15.1) | 97.6 (14.8) |
Values are either mean (SD) or %.
P < .05.
Birth weight adjusted for gestational age.
SES was measured using the Graffar, a measure of SES that can differentiate poorer conditions even within lower- to lower-middle-class samples.
Iron assignment refers to assignment to high iron or low iron supplementation (drops) or fortification (formula) or to no-added iron, for the period from 6 mo to at least 12 mo.
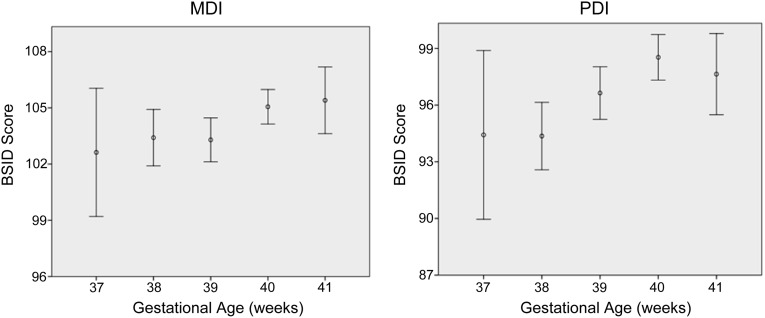
Figure 1 shows the unadjusted developmental scores by gestational week. In the multivariate linear regression model, gestational age was associated with MDI and PDI scores (P < .05; Table 2). With each additional week of gestation, the MDI increased by 0.8 (95% confidence interval = 0.2–1.4), and the PDI increased by 1.4 (95% confidence interval = 0.6–2.1) controlling for the covariates remaining in the model. For MDI only, birth weight percentile was modestly, but significantly, related to the outcome. Female gender was also positively associated with MDI score. For PDI only, SES was positively related to the outcome (P < .05). For both MDI and PDI, HOME score was significantly related to higher scores. IDA in infancy and iron supplementation group were not significantly related to either MDI or PDI and were subsequently removed from the final models.
FIGURE 1.
Mental Developmental Index and Psychomotor Developmental Index (BSID II) at 12 months according to week of gestation in a cohort of full-term healthy infants. MDI and PDI scores are expressed as means and 0.95 confidence intervals.
TABLE 2.
Multivariate Linear Regression of Factors Associated With Bayley MDI and PDI Scoresa
| MDI | PDI | |||
|---|---|---|---|---|
| β | 95% CI | β | 95% CI | |
| Gestational age, weeks | 0.81b | 0.21–1.41 | 1.35b | 0.60–2.10 |
| Birth wt percentilec | 0.03b | 0.01–0.05 | 0.03 | −0.01–0.06 |
| Female gender | 2.60b | 1.39–3.79 | −0.84 | −2.34–0.66 |
| SESd | 0.09 | −0.01–0.19 | 0.22b | 0.11–0.34 |
| HOME | 0.33b | 0.20–0.46 | 0.37b | 0.21–0.52 |
Mean MDI = 104.2 (SD = 12.2); mean PDI = 97.1 (SD = 15.1).
P < .05.
Birth wt adjusted for gestational age.
SES was measured using the Graffar, a measure of socioeconomic status which can differentiate poorer conditions even within lower- to lower-middle-class samples.
Discussion
Our findings extend the limited available literature on the relationship of gestational age to developmental scores. In these healthy, full-term infants weighing ≥3 kg at birth, 12-month mental and psychomotor BSID scores increased modestly, but significantly, between 37 and 41 weeks . This relationship was not attenuated after controlling for predictors of the MDI and PDI, including SES, HOME, birth weight, and gender.28–32
We consider several possible explanations for the effect of gestational age on developmental scores. It is possible this association might be explained, in part, by higher risk for mild brain injury in infants who are less mature at the time of labor, delivery, and the early postpartum period. Subclinical injury can occur during fetal life, such as with undetected placental insufficiency, even when intrauterine growth restriction is not present. Furthermore, postnatal signs of mild neurologic impairment such as self-limited apneic episodes or a single aspiration event are sometimes missed. It is clear that infants born during the late preterm period are at increased risk for adverse neurologic events compared with full-term infants. Adaptation to extrauterine life improves as neurologic development continues over this period.33 This suggests that optimality for birth is a developmental continuum improving with gestational age from preterm through late preterm and into the full-term period.
It is also possible brain maturation proceeds differently in the intrauterine environment compared with the extrauterine environment. A recent study showed differences in the development of gray matter density in school-age children, based on gestational age between 37 and 41 weeks.34 There was no history of perinatal risk (n = 64). Using MRI voxel-based morphometry, the investigators showed significant increases in gray matter density in the superior and middle temporal gyri by week of gestational age. In the temporal cortex, synaptogenesis followed by gyrification begins and progresses rapidly in the third trimester. The intrauterine and extrauterine environments differ dramatically related to maternal and placental hormones, which may play a role in brain development. For example, exposure to higher levels of maternal cortisol late in gestation has been related to more advanced infant cognitive development.35 Other possible environmental factors of interest include ambient temperature, light, sound, and other sensory inputs. In addition, important differences in cerebral arterial blood flow velocity and cerebral oxygen delivery occur before and after birth.36,37 The timing of these changes may influence brain development.
The results could also be explained by simple developmental maturation or as an artifact of no longer correcting for gestational age after 36 weeks. Although the BSID takes into account exact postbirth chronological age, gestational age is only corrected for infants born before 37 weeks gestational age. In this interpretation, a few weeks’ difference in postconceptual age comprises enough developmental maturation time to matter at 12 months. For example, a 12-month-old born at 41 weeks’ gestational age is 4% older, in postconceptual age, than a 12-month-old born at 37 weeks. We would not expect these differences to persist as gestational age becomes a smaller portion of overall age. However, previous studies have found relationships between gestational age, within the full-term range, and cognitive performance at 6 years in Belarus,13 8 years in the United States,14 and in young adult male military recruits in Norway5 and Sweden.15 Simple maturation would not explain such long-lasting differences in IQ.
Some studies have focused on the role of birth weight on developmental scores instead of, or in addition to, gestational age.38–41 Our research question related to gestational age and not birth weight. Nonetheless, we examined the role of birth weight percentile in this cohort. We found that gestational age remained independently associated with developmental scores after accounting for birth weight. As our study only included infants weighing ≥3 kg at birth, we were unable to examine the role of low birth weight or intrauterine growth retardation in the relationship between gestational age and development.
Our findings support the view that infants born at 37 weeks’ gestational age are more like late preterm infants than are those born at 40 weeks. Clinicians understand that 37-week neonates differ from their more mature counterparts in feeding, sleep patterns, motor development, and alertness. However, this is not routinely communicated to families. Furthermore, gestational age within the full-term period is not taken into account for developmental assessment and screening in the first year. Describing developmental differences by week of gestation in healthy full-term infants may help caregivers and clinicians to more accurately understand infant capabilities. Knowledge of developmental differences within the full-term period may prevent underestimation of abilities of infants born at 37 weeks’ gestation when compared with those born at 40 to 41 weeks. The modest yet significant developmental differences we observed could be meaningful on a population level. This is especially important given the recent trend toward earlier labor induction and the high rates of cesarean delivery. The rates of early-term induction increased from 2% to 8% of total births in the United States between 1991 and 2006.20 Similarly, the cesarean rate rose by 53% from 1996 to 2007, reaching 32% of all births, the highest ever reported in the United States.17 Such trends may shift the distribution of infant gestational age at birth downward by several weeks. Our findings add an additional reason to support current guidelines to avoid elective cesarean delivery and induction of labor before 39 weeks.42
This study has several limitations. The study population was composed of healthy, well-nourished, singleton Chilean infants. Thus, our findings may not generalize to multiples or other populations, especially those who are nutritionally at risk. We note for twins, perinatal mortality and morbidity increase after 37 weeks in parallel to the trend for singleton infants after 41 weeks, and the lowest risk for fetal death occurs at 37 to 38 weeks.43 However, we know of no studies in multiples that shed light on the optimal timing of birth related to infant development. We suspect that when there is placental sufficiency, optimal maternal health, and absence of pathology, neurodevelopment is likely to proceed at the same rate as that in singletons. Another limitation of our study is that gestational age was based on the date of the last menstrual period because dating by prenatal ultrasound was not available. Gestational age based on menstrual dates is correlated with ultrasound-based gestational age but tends to be longer by 1.3 to 2.8 days.44 This is a minor limitation because the within-group relationship between week of gestation and developmental scores should not be affected by the method of estimating gestational age. Another possible limitation is the small number of 37-week gestational age infants in our sample (n = 45). This reflects the lower overall prevalence of 37-week infants compared with those of 38, 39, 40, or 41 weeks and our selection criteria of birth weight of ≥3 kg. Although this smaller sample size may have decreased the precision of our estimate, it was adequate to show significant differences in developmental scores.
Although we examined a number of critical covariates, it is possible that residual confounding by unmeasured characteristics remains. For instance, birth order, nutrition, growth factors, and stress hormones were not investigated and may influence infant developmental scores.45–47 Although the BSID is a valid way to assess motor, language, and cognitive development of young infants, it has limitations as a measure of brain development. Furthermore, the BSID is best used to describe developmental functioning at the time of assessment,48,49 and caution related to prediction of later cognitive outcomes is appropriate. Although the BSID was not developed or standardized for use in our population, it has been widely and successfully used to discriminate between infants, within populations, exposed to different early-life conditions and interventions including supplementary feeding and educational stimulation in developing-country settings.50,51 For this Chilean sample, both the MDI and PDI were well within the normal range for US infants. Our study also has other strengths. Because detailed developmental evaluation is not typically performed in healthy full-term infants, the in-depth assessments of development, behavior, and family environmental characteristics in this cohort is valuable. Furthermore the fact that this sample was prospectively screened to exclude infants with any identifiable developmental risk makes it less likely that subclinical brain injury explains our findings. Additionally, the design of the original study allowed us to carefully control for SES and the home environment, decreasing the likelihood that the association found between gestational age and developmental scores was due to environmental factors.
Conclusions
We found higher gestational age within the full-term range was associated with higher MDI and PDI scores at 12 months, after controlling for infant and family characteristics in healthy infants. Our work contributes to a better understanding of development as a continuum from preterm through the full-term period and has implications for clinical care, research, and policy. For obstetricians, when singleton pregnancies are proceeding without identified risk to the mother or fetus, prioritizing timing of delivery for 40 to 41 weeks would allow more time for in utero brain development leading to more optimal developmental outcomes. In addition, we encourage the developmental pediatric academic community to grapple with the dilemma of whether to correct for gestational age within the full-term range for developmental assessment. Finally, more research is needed to improve our understanding of the developmental mechanisms that continue during the full-term range. This is an important area of research that should inform best practices related to timing of birth and care of infants after birth. Novel investigative methods to assess the in utero environment and new neuroimaging techniques could be paired with careful longitudinal assessment of infant development to better understand the role of environment on brain development between 37 and 41 weeks gestational age.
Acknowledgments
We are especially grateful for the careful review and substantive comments made by Claudine Amiel-Tison, MD, Association pour la Prise en Charge des Anomalies de Développement de l’Enfant, Paris, France. We express our gratitude to the participants and their families for their ongoing participation.
Glossary
- BSID
Bayley Scales of Infant Development
- HOME
Home Observation for Measurement of the Environment
- IDA
iron-deficiency anemia
- MDI
Mental Development Index
- PDI
Psychomotor Development Index
- SES
socioeconomic status
Footnotes
Dr Rose designed and conducted the data analysis and wrote the first draft of the manuscript; Ms Blanco participated in data analysis, interpretation of findings, and writing and editing of the manuscript; Dr Martinez and Mr Kang Sim participated in data analysis and interpretation; Dr Vaucher contributed to data interpretation; Dr Castillo participated in data collection and study design; Dr Lozoff contributed to study design and interpretation of findings; Dr Gahagan oversaw the research design, contributed to statistical design, and contributed substantially to writing the manuscript; and all authors read and approved the final version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants from the National Heart, Lung, and Blood Institute and the National Institute of Child Health and Human Development (grants R01HL088530, R01HD33487, principal investigator: Gahagan; grants R01HD14122 and R01HD33487, principal investigator: Lozoff). Funded by the National Institutes of Health (NIH).
References
- 1.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737 [DOI] [PubMed] [Google Scholar]
- 2.Hunt JV, Rhodes L. Mental development of preterm infants during the first year. Child Dev. 1977;48(1):204–210 [PubMed] [Google Scholar]
- 3.Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 2009;154(2):169–176 [DOI] [PubMed] [Google Scholar]
- 4.Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 2010;126(6):1124–1131 [DOI] [PubMed] [Google Scholar]
- 5.Eide MG, Oyen N, Skjaerven R, Bjerkedal T. Associations of birth size, gestational age, and adult size with intellectual performance: evidence from a cohort of Norwegian men. Pediatr Res. 2007;62(5):636–642 [DOI] [PubMed] [Google Scholar]
- 6.Morse SB, Zheng H, Tang Y, Roth J. Early school-age outcomes of late preterm infants. Pediatrics. 2009;123(4):e622–e629. Available at: www.pediatrics.org/cgi/content/full/123/4/e622. [DOI] [PubMed] [Google Scholar]
- 7.Kramer MS. Late preterm birth: appreciable risks, rising incidence. J Pediatr. 2009;154(2):159–160 [DOI] [PubMed] [Google Scholar]
- 8.Nomura Y, Halperin JM, Newcorn JH, et al. The risk for impaired learning-related abilities in childhood and educational attainment among adults born near-term. J Pediatr Psychol. 2009;34(4):406–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amiel-Tison C, Maillard F, Lebrun F, Bréart G, Papiernik E. Neurological and physical maturation in normal growth singletons from 37 to 41 weeks’ gestation. Early Hum Dev. 1999;54(2):145–156 [DOI] [PubMed] [Google Scholar]
- 10.Zacharia A, Zimine S, Lovblad KO, et al. Early assessment of brain maturation by MR imaging segmentation in neonates and premature infants. AJNR Am J Neuroradiol. 2006;27(5):972–977 [PMC free article] [PubMed] [Google Scholar]
- 11.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23(8):3308–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81–88 [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Platt RW, Kramer MS. Variation in child cognitive ability by week of gestation among healthy term births. Am J Epidemiol. 2010;171(4):399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble KG, Fifer WP, Rauh VA, Nomura Y, Andrews HF. Academic achievement varies with gestational age among children born at term. Pediatrics. 2012;130(2):e257–e264. Available at: www.pediatrics.org/cgi/content/full/130/2/e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Bergvall N, Cnattingius S, Kramer MS. Gestational age differences in health and development among young Swedish men born at term. Int J Epidemiol. 2010;39(5):1240–1249 [DOI] [PubMed] [Google Scholar]
- 16.Quigley MA, Poulsen G, Boyle E, et al. Early term and late preterm birth are associated with poorer school performance at age 5 years: a cohort study. Arch Dis Child Fetal Neonatal Ed. 2012;97(3):F167–F173 [DOI] [PubMed] [Google Scholar]
- 17.MacKay DF, Smith GC, Dobbie R, Pell JP. Gestational age at delivery and special educational need: retrospective cohort study of 407,503 schoolchildren. PLoS Med. 2010;7(6):e1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Batenburg-Eddes T, de Groot L, Arends L, et al. Does gestational duration within the normal range predict infant neuromotor development? Early Hum Dev. 2008;84(10):659–665 [DOI] [PubMed] [Google Scholar]
- 19.Menacker F, Hamilton BE. Recent trends in cesarean delivery in the United States. NCHS Data Brief. 2010;(35):1–8 [PubMed]
- 20.Murthy K, Grobman WA, Lee TA, Holl JL. Trends in induction of labor at early-term gestation. Am J Obstet Gynecol. 2011;204(5):e431–436 [DOI] [PubMed]
- 21.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112(4):846–854 [PubMed] [Google Scholar]
- 22.Bayley N. Bayley Scales of Infant Development. New York, NY: Psychological Corporation; 1969 [Google Scholar]
- 23.Bayley N. Comparisons of mental and motor test scores for ages 1–15 months by sex, birth order, race, geographical location, and education of parents. Child Dev. 1965;36:379–411 [PubMed] [Google Scholar]
- 24.Bayley N. Bayley Scales of Infant Development. San Antonio, TX: Psychological Corporation; 1993 [Google Scholar]
- 25.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez ML, Muzzo S, Ivanović D. Scale for measurement of socioeconomic level, in the health area [in Spanish]. Rev Med Chile. 1985;113(3):243–249 [PubMed] [Google Scholar]
- 27.Bradley RH, Caldwell BM. The relation of infants’ home environments to achievement test performance in first grade: a follow-up study. Child Dev. 1984;55(3):803–809 [DOI] [PubMed] [Google Scholar]
- 28.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399 [DOI] [PubMed] [Google Scholar]
- 29.Crosnoe R, Leventhal T, Wirth RJ, Pierce KM, Pianta RC, NICHD Early Child Care Research Network . Family socioeconomic status and consistent environmental stimulation in early childhood. Child Dev. 2010;81(3):972–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dearing E, McCartney K, Taylor BA. Does higher quality early child care promote low-income children’s math and reading achievement in middle childhood? Child Dev. 2009;80(5):1329–1349 [DOI] [PubMed] [Google Scholar]
- 31.Bornstein MH, Haynes OM. Vocabulary competence in early childhood: measurement, latent construct, and predictive validity. Child Dev. 1998;69(3):654–671 [PubMed] [Google Scholar]
- 32.Lung FW, Shu BC, Chiang TL, Chen PF, Lin LL. Predictive validity of Bayley scale in language development of children at 6–36 months. Pediatr Int. 2009;51(5):666–669 [DOI] [PubMed] [Google Scholar]
- 33.Stewart AL. Fetal and neonatal neurology and neurosurgery. Child Care Health Dev. 1989;15(5): 321–331 [Google Scholar]
- 34.Davis EP, Buss C, Muftuler LT, et al. Children’s brain development benefits from longer gestation. Front Psychol. 2011;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81(1):131–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baytur YB, Tarhan S, Uyar Y, et al. Assessment of fetal cerebral arterial and venous blood flow before and after vaginal delivery or cesarean section. Ultrasound Obstet Gynecol. 2004;24(5):522–528 [DOI] [PubMed] [Google Scholar]
- 37.Jones MD, Jr, Rosenberg AA, Simmons MA, Molteni RA, Koehler RC, Traystman RJ. Oxygen delivery to the brain before and after birth. Science. 1982;216(4543):324–325 [DOI] [PubMed] [Google Scholar]
- 38.Taylor HG, Minich N, Bangert B, Filipek PA, Hack M. Long-term neuropsychological outcomes of very low birth weight: associations with early risks for periventricular brain insults. J Int Neuropsychol Soc. 2004;10(7):987–1004 [DOI] [PubMed] [Google Scholar]
- 39.Doctor BA, O’Riordan MA, Kirchner HL, Shah D, Hack M. Perinatal correlates and neonatal outcomes of small for gestational age infants born at term gestation. Am J Obstet Gynecol. 2001;185(3):652–659 [DOI] [PubMed] [Google Scholar]
- 40.Vohr BR, Poindexter BB, Dusick AM, et al. NICHD Neonatal Research Network . Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1):e115–e123. Available at: www.pediatrics.org/cgi/content/full/118/1/e115. [DOI] [PubMed] [Google Scholar]
- 41.Hintz SR, Kendrick DE, Stoll BJ, et al. NICHD Neonatal Research Network . Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703 [DOI] [PubMed] [Google Scholar]
- 42.American Congress of Obstetricians and GynecologistsCommittee on Practice Bulletins—Obstetrics . ACOGPractice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386–397 [DOI] [PubMed] [Google Scholar]
- 43.Kahn B, Lumey LH, Zybert PA, et al. Prospective risk of fetal death in singleton, twin, and triplet gestations: implications for practice. Obstet Gynecol. 2003;102(4):685–692 [DOI] [PubMed] [Google Scholar]
- 44.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol. 2007;21(suppl 2):86–96 [DOI] [PubMed] [Google Scholar]
- 45.Kristensen P, Bjerkedal T. Explaining the relation between birth order and intelligence. Science. 2007;316(5832):1717. [DOI] [PubMed] [Google Scholar]
- 46.Richards M, Hardy R, Kuh D, Wadsworth ME. Birth weight and cognitive function in the British 1946 birth cohort: longitudinal population based study. BMJ. 2001;322(7280):199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu TC, Chen PH. Health consequences of nutrition in childhood and early infancy. Pediatr Neonatol. 2009;50(4):135–142 [DOI] [PubMed] [Google Scholar]
- 48.Harris SR, Megens AM, Backman CL, Hayes VE. Stability of the Bayley II Scales of Infant Development in a sample of low-risk and high-risk infants. Dev Med Child Neurol. 2005;47(12):820–823 [DOI] [PubMed] [Google Scholar]
- 49.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341 [DOI] [PubMed] [Google Scholar]
- 50.Black MM, Baqui AH, Zaman K, et al. Depressive symptoms among rural Bangladeshi mothers: implications for infant development. J Child Psychol Psychiatry. 2007;48(8):764–772 [DOI] [PubMed] [Google Scholar]
- 51.Torres-Sánchez L, Rothenberg SJ, Schnaas L, et al. In utero p,p’-DDE exposure and infant neurodevelopment: a perinatal cohort in Mexico. Environ Health Perspect. 2007;115(3):435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]