Abstract
CoQ10 deficiency has been associated with five major clinical phenotypes: encephalomyopathy, severe infantile multisystemic disease, nephropathy, cerebellar ataxia, and isolated myopathy. Primary CoQ10 deficiency is due to defects in CoQ10 biosynthesis while secondary forms are due to other causes. Review of 149 cases, including our cohort of 76 patients, confirms that CoQ10 deficiency is a clinically and genetically heterogeneous syndrome that predominantly begins in childhood and predominantly manifests as cerebellar ataxia. CoQ10 measurement in muscle is the gold standard for diagnosis. Identification of CoQ10 deficiency is important because it frequently responds to treatment. Causative mutations have been identified in a small proportion of patients.
INTRODUCTION
Coenzyme Q10 (CoQ10 or ubiquinone) deficiency in human is associated with clinically heterogeneous diseases.1 Five major phenotypes have been described: 1) encephalomyopathy; 2) cerebellar ataxia; 3) infantile multisystemic form; 4) nephropathy; and 5) isolated myopathy (Table 1).
Table 1.
Clinical features of major forms of CoQ10 deficiency
| Syndrome | Number of cases (new cases) |
Clinical features | Natural history | Responsible genes (numbers of patients/families) |
References |
|---|---|---|---|---|---|
| Encephalomyopathy | 4 | Juvenile-onset mitochondrial myopathy, recurrent myoglobinuria, and encephalopathy |
Slow progression of weakness | None known | 4-6 |
| Isolated myopathy | 14 (4) | Juvenile or adult-onset muscle weakness, myoglobinuria, exercise intolerance, cramps, myalgias, elevated CK |
Slow progression of weakness | ETFDH (7/5) | 6-9 |
| Nephropathy | 11 | Infantile or early childhood-onset steroid-resistant nephrotic syndrome. |
May progress to renal failure. Encephalopathy may develop. |
COQ2 (2/2), | 2,10-19 |
| Infantile or juvenile-onset steroid- resistant nephrotic syndrome typically with congenital or juvenile- onset sensorineural deafness |
Nephropathy typically progresses to renal failure. Seizures and ataxia may develop. May be fatal (2/8=25%) |
COQ6 (8/4) | |||
| Infantile multisystemic disease |
17 (2) | Infantile-onset psychomotor regression, encephalopathy, optic atrophy, retinopathy, hearing loss, renal dysfunction (mainly nephrotic syndrome). Less common: liver, cardiac, and pancreatic dysfunction and obesity. |
Typically progresses rapidly with high mortality (11/17=65%) |
COQ2 (4/3?), PDSS2 (1/1), COQ9 (1/1) PDSS1 (1/1), COQ6 (3/?) |
14-18 |
| Cerebellar ataxia | 94 (23) | Typically juvenile-onset cerebellar ataxia. Multiple additional neurological and non-neurological manifestations may occur. |
Slow or minimal progression of ataxia. |
ADCK3 (22/13?), APTX (12/8?) |
3,20-34 |
| Atypical presentations | 9 (5) | Adults with childhood-onset Leigh syndrome |
Subacute progression. | None known | 35-37 |
| Cardiofaciocutaneous syndrome | Uncertain. | BRAF (1/1) | |||
| Neonatal hypotonia, infantile spasms |
? | None known | |||
| Adult-onset cerebellar ataxia and nephrotic syndrome |
? | None known | |||
| Childhood-onset encephalomyopathy, short stature, hearing loss and retinopathy |
? | None known |
METHODS
Seventy-six patients with CoQ10 deficiency (36 previously unreported) were studied at the H. Houston Merritt Clinical Research Center, Columbia University Medical Center (CUMC), New York, NY, USA under CUMC Institutional Review Board protocols. CoQ10 levels were measured in muscle, cultured fibroblasts, and/or lymphoblasts.2,3 CoQ10 levels reduced more than 2 SD below mean control values were considered deficient. Patients with CoQ10 level decreased in one member of the family with similar phenotype and/or genetic mutation were considered to have CoQ10 deficiency.
Dideoxy-sequencing was performed on all exons and flanking intronic regions of genes involved in CoQ10 biosynthesis, associated with secondary CoQ10 deficiencies, or encoding proteins with similar function, structure or both as proteins associated with CoQ10 deficiency. In addition, PSAP encoding saposin B, a cytosolic protein that binds and transfers CoQ10, and POLG were sequenced (eTable 1).
CLINICAL FEATURES
Since the first description of human ubiquinone deficiency, 113 patients have been reported (Tables 1-3). Of 455 patients’ samples referred to our center for possible CoQ10 deficiency from 1997 to 2010, 76 patients (64 families) had CoQ10 deficiency; 40 were previously described. The reported patients and our 36 new patients comprised 149 cases: 4 encephalomyopathy, 14 isolated myopathy, 17 infantile-onset multisystemic disease, 11 nephropathy (with our without sensorineural hearing loss), 94 cerebellar ataxia, and 9 patients with atypical presentations.
Table 3.
Clinical response to CoQ10 supplementation in major forms of CoQ10 deficiency
| Syndrome | CoQ10 doses and duration | Response to therapy |
|---|---|---|
| Encephalomyopathy | ||
| Isolated myopathy | ||
| Isolated nephropathy | ||
| Infantile multisystemic disease |
||
| Cerebellar ataxia | ||
| Atypical presentations |
Onset was predominantly in childhood (<13 years old: 82%) including 23% in infancy (<12 months old). Onset during adolescence (13-18 years-old: 7%) and adulthood (>18 years-old: 11%) were uncommon. The mortality rate was low (8%) and mainly seen in the infantile multisystemic and renal forms.
The encephalomyopathic form of CoQ10 deficiency manifesting as a triad of mitochondrial myopathy, recurrent myoglobinuria, and encephalopathy, has been reported in 4 patients (eTable 2).4-6 Neurological features included cerebellar ataxia, seizures, mental retardation, delayed motor development; progressive external ophthalmoplegia and ptosis.
The myopathic form of CoQ10 deficiency presents with muscle weakness, myoglobinuria, exercise intolerance, cramps, myalgia, and elevated CK. This condition has been described in 10 patients.6-9 We have identified 4 additional patients (eTable 3).
Among the 17 patients (including 2 new patients) with the infantile multisystemic form, the combination of encephalopathy and nephropathy has been the most common presentation (eTable 4).2,10-16 Neurological manifestations are: psychomotor regression, ataxia, hypotonia, seizures, pyramidal syndrome, optic atrophy and retinopathy, deafness and Leigh syndrome. The renal involvement is mainly nephrotic syndrome but occasionally tubulopathy. Three patients required kidney transplantation.15,17 In addition, liver, cardiac and pancreatic involvement, and obesity have been described in literature and in our cohort.10,12,18 Eleven patients (65%) died in early infancy, and causes of death were opportunistic infections, kidney failure, encephalopathy, or multi-organ failure. Early onset isolated steroid resistant nephrotic syndrome (SRNS), due to collapsing glomerulopathy and focal segmental glomerulosclerosis (FSGS), has been reported in 2 patients.14 Moreover, the association between SRNS with sensorineural hearing loss has been described in patients with mutations in the CoQ10 biosynthetic gene, COQ6; however, CoQ10 level was not measured in these patients (eTable 5).16
Cerebellar ataxia is the most common phenotype, with 94 patients (including 23 new patients) (eTable 6).3,19-33 Other manifestations include neuropathy, seizures, mental retardation, migraine, psychiatric disorders, muscle weakness and exercise intolerance, congenital hypotonia, upper motor neuron signs, dystonia and chorea, ptosis and ophthalmoplegia, retinitis pigmentosa, optic atrophy, oculomotor apraxia, deafness, lipomas, Dandy-Walker syndrome, agenesis of corpus callosum, hypogonadism and other endocrinological problems, hypoalbuminemia, and hypercholesterolemia.
Among the patients classified as “atypical cases”, there are two adult sisters with childhood onset Leigh-syndrome, growth retardation, infantilism, ataxia, deafness, and lactic acidosis, 34 a 4-year-old Moroccan girl with cardiofaciocutaneous syndrome (CFC),35 two unrelated patients with neonatal hypotonia and infantile spasm (one reported)36, two Portuguese sisters with adult-onset cerebellar ataxia and nephrotic syndrome, and a 16-year-old girl with onset at age 4 years of exercise intolerance, fatigue, recurrent headaches, short stature, deafness, retinopathy and mental retardation. One new patient is the father of a child with cerebellar ataxia (P119 in eTable 6), with mild CK elevation, but normal examination and brain MRI.
DIAGNOSIS
Initial biochemical evaluation of patients with suspected CoQ10 deficiency should include blood lactate measurement, although normal values do not exclude ubiquinone deficiency. Muscle biopsies occasionally show mitochondrial proliferation or lipid droplets, but can be normal or show only non-specific changes.
Reduced biochemical activities of respiratory chain complexes, in particular, complexes I+III (NADH:cytochrome c oxidoreductase) and II+III (succinate:cytochrome c oxidoreductase) in muscle suggest CoQ10 deficiency, although activities of these enzymes may be normal particularly when the deficiency is mild. Reduction of these enzyme activities and deficiency of CoQ10 in skin fibroblasts can be an important confirmation of ubiquinone deficiency; however normal levels do not exclude deficiency in muscle. Direct measurement of CoQ10 in skeletal muscle by high performance liquid chromatography is the most reliable test for the diagnosis. In contrast, plasma concentrations of ubiquinone are significantly influenced by dietary uptake, therefore not reliable. Measurements of CoQ10 in peripheral blood mononuclear cells (MNC) has detected deficiency in a small number of patients; however, correlations with muscle CoQ10 measurements in a larger cohort of patients will be necessary to assess clinical utility of MNC ubiquinone levels.
Morphological and biochemical findings differ in the various clinical forms. In patients with the encephalomyopathic, myopathic, or infantile multisystemic forms, muscle biopsies have typically revealed abnormal mitochondrial proliferation (RRF or excessive SDH histochemical activity) and lipid accumulation as well as reduced biochemical activities of respiratory chain enzyme complexes I+III and II+III while all muscle samples showed decreased CoQ10 levels. In contrast, fibroblast CoQ10 levels have varied and was normal in one patient with encephalomyopathy, but low in 1/2 with the myopathic form and 7/8 with the infantile multisystemic form.
In two patients with isolated nephropathy, CoQ10 levels and respiratory chain enzyme activities were reduced in either fibroblasts or muscle. RRF-like were observed in the only patient who underwent muscle biopsy.14
In patients with the ataxic form, muscle biopsies revealed mitochondrial proliferation, COX-negative fibers, or lipid accumulation in 15/49, and reduced respiratory chain enzyme activities in 27/51. Levels of CoQ10 were low in muscle, but reduced in 18/30 fibroblasts.
GENETICS
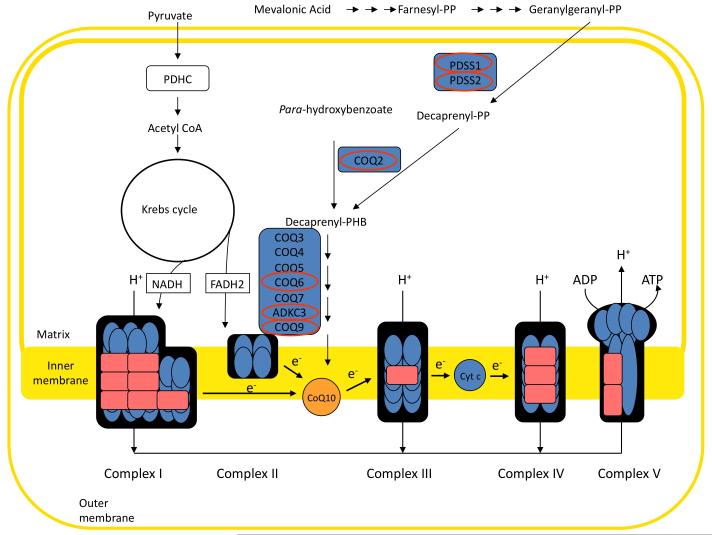
Primary CoQ10 deficiency is due to mutations in genes involved in CoQ10 biosynthesis (Figure 1). Secondary deficiencies include diseases caused by mutations in genes unrelated to ubiquinone biosynthesis, for example aprataxin (APTX) gene, causing ataxia and oculomotor apraxia,21,27,28,30 electron-transferring-flavoprotein dehydrogenase gene (ETFDH), causing isolated myopathy,9 and BRAF gene, causing CFC syndrome.35 Moreover, CoQ10 deficiency has been reported in association with mitochondrial DNA mutations.1
Figure 1.
Biosynthesis of coenzyme Q10 (CoQ10). Mutations in CoQ10 biosynthetic genes (indicated by red ovals) cause primary CoQ10 deficiency. CoQ10 transport electrons from mitochondrial respiratory chain complexes I and II to complex III.
In most cases, family history suggests autosomal recessive inheritance. Pathogenic mutations have been reported in 63 patients (Table 1).
No mutations have been described among the encephalomyopathic patients. Except for the patients reported by Gempel in 2007,9 we did not find other mutations in ETFDH in our cohort of patients with isolated myopathy, indicating that other genes can be responsible for this phenotype.
Most patients with the infantile-onset multisystemic variant have genetically confirmed primary CoQ10 deficiency. Mutations have been described in COQ2 (3 patients)13,14,18, PDSS2 (1 patient)2, COQ9 (1 patient)11, PDSS1 (2 patients)18, and COQ6 (3 patients)16. Interestingly, two patients with isolated nephrotic syndrome have COQ2 mutations.13,14 Thus, patients with COQ2 mutations have presented with either infantile multisystemic syndrome or isolated nephropathy. There are three potential explanations for the divergent phenotypes. First, variations in the phenotypes may be due to genetic or environmental modifiers. Second, patients with isolated nephropathies may develop multisystemic disease later in life. Third, CoQ10 treatment may have altered the clinical course of the disease by preventing neurological complications in both patients with only renal disease. In contrast, in 9 patients, mutations in COQ6 gene have been associated with kidney involvement (nephrotic syndrome, and nephrolithiasis) and sensorineural hearing loss.16 A subgroup of patients with juvenile-onset cerebellar ataxia have primary CoQ10 deficiency due to mutations in the ADCK3 gene (22 patients).3,25,29,31-33 Secondary forms have been described in association with mutations in the APTX gene (12 patients) including four affected members of one family that we studied.21,27,28,30 The majority of patients with cerebellar ataxia and CoQ10 deficiency still lack molecular diagnosis.
THERAPY
Patients with CoQ10 deficiency showed variable responses to CoQ10 treatment (Table 3). We recommend oral supplementation doses up to 2,400 mg daily in adult patients and up to 30mg/kg daily in pediatric patients, divided into three doses per day.
In patients with encephalomyopathy, muscle symptoms improved after therapy.4-6 In one of our patients, muscle symptoms and seizures resolved, CK and lactic acid normalized, and a muscle biopsy showed CoQ10 level normalization.6 In contrast, another patient, developed cerebellar ataxia.5
Six patients with pure myopathy6-9 (including one unreported) improved after CoQ10 supplementation, while 2 patients with ETFDH mutations improved only after addition of riboflavin (100 mg/day).9
In some patients with the infantile multisystemic form, CoQ10 supplementation has halted progression of the encephalopathy and improved the myopathy.17 One patient with a homozygous COQ2 mutation, on therapy showed neurological but not renal improvement, and underwent kidney transplant, but his sister, with isolated nephropathy, received coenzyme Q10 and has had progressive recovery of renal function, reduced proteinuria and no neurological manifestations.13-15 In contrast, a patient with a homozygous COQ9 mutation, on therapy, had reduction of blood lactate, but neurological and cardiac worsening, and died at age 2 years.11 Similarly, despite treatment, a patient with PDSS2 mutations developed intractable seizures and died at age 8 months2 and a patient with infantile-onset Leigh syndrome, hepatopathy, and hypertrophic cardiomyopathy after initial clinical improvement, died at age 3 years. Published data for 2 patients with mutations in COQ6 gene noted decreased proteinuria in both patients after CoQ10 treatment, but hearing improved only in one patient.16
Response to CoQ10 supplementation in patients with cerebellar ataxia is also variable. Three patients with mutations in ADCK3 gene showed mild clinical improvement after treatment,3,31,32 but 7 patients carrying mutations in the same gene did not improve,25,33 and another patient, despite dramatic muscle improvement, developed tremor, myoclonic jerks, and cerebellar atrophy.24,25 In 3 siblings with mutations in APTX gene, CoQ10 supplementation was associated with clear improved ambulation, and resolution of seizures in one patient.21 Nevertheless, another patient with APTX mutations did not improve after therapy.28 Improvement in muscle but not neurological signs and symptoms has been noted in one new and 2 reported patients.19,20 A reduction in ICARS score in 9 patients with unknown genetic defect and in one patient with ADCK3 gene mutations has been documented.23,26,31 Eleven patients with undefined molecular defect did not respond to therapy.22,32
Among the clinically atypical cases of CoQ10 deficiency, two sisters with Leigh syndrome and one patient with CFC syndrome improved after CoQ10 supplementation.34,35 One patient with neonatal hypotonia and infantile spasm showed no improvement.36
CONCLUSIONS
It is important to identify CoQ10 deficiency as this condition often responds to supplementation. The diagnosis can be made by direct measurement of CoQ10 in muscle, and reinforced by the presence of reduced biochemical activities of respiratory chain complexes, in particular, complexes I+III and II+III. Molecular genetic testing has revealed causative mutations in a small proportion of patients indicating that screening for DNA mutations is not yet effective for diagnosing CoQ10 deficiency. Our observations not only highlight the clinical heterogeneity of CoQ10 deficiency, but also the genetic heterogeneity that is likely related to the large number of proteins involved in ubiquinone biosynthesis and regulation and of secondary CoQ10 deficiencies. Clinical improvement after CoQ10 supplementation was documented in many patients, but treatment protocols have not been standardized, and results have not been uniform. Progress in our knowledge of the genetic bases of CoQ10 deficiencies may help us develop a more accurate molecular classification of this syndrome, while additional studies of the pathogenesis of CoQ10 deficiency may lead to more effective therapies.
Supplementary Material
Table 2.
Laboratory features of major forms of CoQ10 deficiency
| Syndrome | Blood lactate | Serum CK | Muscle Histology | Mitochondrial respiratory chain enzyme activities |
CoQ10 levels |
|---|---|---|---|---|---|
| Encephalomyopathy | |||||
| Isolated myopathy | |||||
| Isolated nephropathy | |||||
| Infantile multisystemic disease |
|||||
| Cerebellar ataxia | |||||
| Atypical presentations |
ACKNOWLEDGEMENTS
We are grateful to all of the patients and relatives for their collaboration. We thank all of the clinicians who referred us the patients and samples The project described was supported by K23HD065871 (CMQ) and 1R01HD057543 (MH) from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. MH is supported by NIH grants R01HD056103 and 1RC1NS070232, a Muscular Dystrophy Association grant, and by the Marriott Mitochondrial Disorder Clinical Research Fund (MMDCRF).
Footnotes
Author Contributions: All authors contributed to this manuscript. Study concept and design: Emmanuele, DiMauro, Quinzii, and Hirano. Acquisition of data: Emmanuele, López, Berardo, Naini, Tadesse, Wen, D’Agostino, Solomon, Quinzii, and Hirano. Analysis and interpretation of data: Emmanuele, López, Berardo, DiMauro, Quinzii, and Hirano. Drafting of the manuscript: Emmanuele, López, Solomon, Quinzii, and Hirano. Critical revision of the manuscript for important intellectual content: Emmanuele, Berardo, Naini, Tadesse, Wen, D’Agostino, DiMauro, Quinzii, and Hirano. Statistical analysis: Emmanuele and Solomon. Obtained funding: Quinzii and Hirano. Administrative, technical, and material support: Emmanuele, López, Naini, Tadesse, Wen, and Hirano. Study supervision: Emmanuele, DiMauro, and Quinzii.
Financial Disclosure: None reported.
References
- 1.Quinzii CM, Hirano M. Primary and secondary CoQ(10) deficiencies in humans. Biofactors. 2011;37(5):361–365. doi: 10.1002/biof.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez LC, Schuelke M, Quinzii CM, et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79(6):1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagier-Tourenne C, Tazir M, Lopez LC, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82(3):661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogasahara S, Engel AG, Frens D, Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc Natl Acad Sci U S A. 1989;86(7):2379–2382. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobreira C, Hirano M, Shanske S, et al. Mitochondrial encephalomyopathy with coenzyme Q10 deficiency. Neurology. 1997;48(5):1238–1243. doi: 10.1212/wnl.48.5.1238. [DOI] [PubMed] [Google Scholar]
- 6.Di Giovanni S, Mirabella M, Spinazzola A, et al. Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology. 2001;57(3):515–518. doi: 10.1212/wnl.57.3.515. [DOI] [PubMed] [Google Scholar]
- 7.Lalani SR, Vladutiu GD, Plunkett K, et al. Isolated mitochondrial myopathy associated with muscle coenzyme Q10 deficiency. Arch Neurol. 2005;62(2):317–320. doi: 10.1001/archneur.62.2.317. [DOI] [PubMed] [Google Scholar]
- 8.Horvath R, Schneiderat P, Schoser BG, et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology. 2006;66(2):253–255. doi: 10.1212/01.wnl.0000194241.35115.7c. [DOI] [PubMed] [Google Scholar]
- 9.Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130(Pt 8):2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman S, Hargreaves I, Clayton P, Heales S. Neonatal presentation of coenzyme Q10 deficiency. J Pediatr. 2001;139(3):456–458. doi: 10.1067/mpd.2001.117575. [DOI] [PubMed] [Google Scholar]
- 11.Duncan AJ, Bitner-Glindzicz M, Meunier B, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84(5):558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leshinsky-Silver E, Levine A, Nissenkorn A, et al. Neonatal liver failure and Leigh syndrome possibly due to CoQ-responsive OXPHOS deficiency. Mol Genet Metab. 2003;79(4):288–293. doi: 10.1016/s1096-7192(03)00097-0. [DOI] [PubMed] [Google Scholar]
- 13.Quinzii C, Naini A, Salviati L, et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet. 2006;78(2):345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18(10):2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 15.Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. New Engl J Med. 2008;358(26):2849–2850. doi: 10.1056/NEJMc0800582. [DOI] [PubMed] [Google Scholar]
- 16.Heeringa SF, Chernin G, Chaki M, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121(5):2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotig A, Appelkvist EL, Geromel V, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356(9227):391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- 18.Mollet J, Giurgea I, Schlemmer D, et al. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;117(3):765–772. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boitier E, Degoul F, Desguerre I, et al. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J Neurol Sci. 1998;156(1):41–46. doi: 10.1016/s0022-510x(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 20.Musumeci O, Naini A, Slonim AE, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56(7):849–855. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- 21.Quinzii CM, Kattah AG, Naini A, et al. Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology. 2005;64(3):539–541. doi: 10.1212/01.WNL.0000150588.75281.58. [DOI] [PubMed] [Google Scholar]
- 22.Lamperti C, Naini A, Hirano M, et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology. 2003;60(7):1206–1208. doi: 10.1212/01.wnl.0000055089.39373.fc. [DOI] [PubMed] [Google Scholar]
- 23.Gironi M, Lamperti C, Nemni R, et al. Late-onset cerebellar ataxia with hypogonadism and muscle coenzyme Q10 deficiency. Neurology. 2004;62(5):818–820. doi: 10.1212/01.wnl.0000113719.67643.b7. [DOI] [PubMed] [Google Scholar]
- 24.Aure K, Benoist JF, Ogier de Baulny H, et al. Progression despite replacement of a myopathic form of coenzyme Q10 defect. Neurology. 2004;63(4):727–729. doi: 10.1212/01.wnl.0000134607.76780.b2. [DOI] [PubMed] [Google Scholar]
- 25.Mollet J, Delahodde A, Serre V, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82(3):623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artuch R, Brea-Calvo G, Briones P, et al. Cerebellar ataxia with coenzyme Q10 deficiency: diagnosis and follow-up after coenzyme Q10 supplementation. J Neurol Sci. 2006;246(1-2):153–158. doi: 10.1016/j.jns.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Le Ber I, Dubourg O, Benoist JF, et al. Muscle coenzyme Q10 deficiencies in ataxia with oculomotor apraxia 1. Neurology. 2007;68(4):295–297. doi: 10.1212/01.wnl.0000252366.10731.43. [DOI] [PubMed] [Google Scholar]
- 28.D’Arrigo S, Riva D, Bulgheroni S, et al. Ataxia with oculomotor apraxia type 1 (AOA1): clinical and neuropsychological features in 2 new patients and differential diagnosis. J Child Neurol. 2008;23(8):895–900. doi: 10.1177/0883073808314959. [DOI] [PubMed] [Google Scholar]
- 29.Gerards M, van den Bosch B, Calis C, et al. Nonsense mutations in CABC1/ADCK3 cause progressive cerebellar ataxia and atrophy. Mitochondrion. 2010;10(5):510–515. doi: 10.1016/j.mito.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Castellotti B, Mariotti C, Rimoldi M, et al. Ataxia with oculomotor apraxia type1 (AOA1): novel and recurrent aprataxin mutations, coenzyme Q10 analyses, and clinical findings in Italian patients. Neurogenetics. 2011;12:193–201. doi: 10.1007/s10048-011-0281-x. [DOI] [PubMed] [Google Scholar]
- 31.Pineda M, Montero R, Aracil A, et al. Coenzyme Q(10)-responsive ataxia: 2-year-treatment follow-up. Mov Disord. 2010;25(9):1262–1268. doi: 10.1002/mds.23129. [DOI] [PubMed] [Google Scholar]
- 32.Terracciano A, Renaldo F, Zanni G, et al. The use of muscle biopsy in the diagnosis of undefined ataxia with cerebellar atrophy in children. Eur J Paediatr Neurol. 2011 doi: 10.1016/j.ejpn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvath R, Czermin B, Gulati S, et al. Adult-onset cerebellar ataxia due to mutations in CABC1/ADCK3. J Neurol Neurosurg Psychiatry. 2011 doi: 10.1136/jnnp-2011-301258. [DOI] [PubMed] [Google Scholar]
- 34.Van Maldergem L, Trijbels F, DiMauro S, et al. Coenzyme Q-responsive Leigh’s encephalopathy in two sisters. Ann Neurol. 2002;52(6):750–754. doi: 10.1002/ana.10371. [DOI] [PubMed] [Google Scholar]
- 35.Aeby A, Sznajer Y, Cave H, et al. Cardiofaciocutaneous (CFC) syndrome associated with muscular coenzyme Q10 deficiency. J Inherit Metab Dis. 2007;30(5):827. doi: 10.1007/s10545-007-0612-0. [DOI] [PubMed] [Google Scholar]
- 36.Huntsman RJ, Lemire EG, Dunham CP. Hypotonia and infantile spasms: a new phenotype of coenzyme Q10 deficiency? Can J Neurol Sci. 2009;36(1):105–108. doi: 10.1017/s0317167100006429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.