Abstract
At birth, human infants and newborns of other primate species demonstrate the capacity to attend and to respond to facial stimuli provided by a caregiver. Newborn infants are also capable of exhibiting a range of facial expressions. Identification of the neural underpinnings of these capacities represents a formidable challenge in understanding social development. One possible neuronal substrate is the mirror-neuron system assumed to activate shared motor cortical representations for both observation and production of actions. We tested this hypothesis by recording scalp electroencephalogram (EEG) from 1–7 days old newborn rhesus macaques who were observing and producing facial gestures. We found that 5–6 Hz EEG activity was suppressed both when the infants produced facial gestures and while they were observing facial gestures of a human experimenter, but not when they were observing non-biological stimuli. These findings demonstrate the presence of neural reactivity for biological, communicatively-relevant stimuli which may be a likely signature of neuronal mirroring. The basic elements of the mirror-neuron system appear to operate from the very first days of life and contribute to the encoding of socially relevant stimuli.
Introduction
A fundamental issue in infant development is how the brain encodes facial gestures. The discovery of mirror neurons in the adult monkey (di Pellegrino, Fadiga, Fogassi, Gallese & Rizzolatti, 1992; Gallese, Fadiga, Fogassi & Rizzolatti,1996; Ferrari, Gallese, Rizzolatti & Fogassi, 2003) and studies suggesting a homolog system in humans (for a review, see Iacoboni, 2009) have prompted the idea that we encode others’ behaviour by mapping the observed actions/gestures onto our own neural motor representations. This mirror-neuron system (MNS) has been proposed to underlie important cognitive functions, such as understanding others’ behaviours and imitation. However, little is known about its early development.
Many of the neuroscience techniques used to study the neural correlates of the MNS in adult humans and monkeys (fMRI, MEG, PET, TMS, and single/multi-cell electrophysiology) are not feasible with infants. However, electroencephalography (EEG) can be used to tap neural responses associated with the MNS in human infants and children (Oberman et al., 2005; Pineda, 2005; Lepage & Theoret, 2006; Nyström, Ljunghammar, Rosander & von Hofsten, 2010; Marshall, Young, & Meltzoff, 2010; Southgate, Johnson, El Karoui & Csibra, 2010). When adults and school-aged children plan a motor action, the 8–13-Hz EEG activity recorded over the motor cortex is suppressed, a pattern similar to that seen when adults and children view others’ goal-directed actions (Pfurtscheller, Neuper, Andrew& Edlinger, 1997; Cochin, Barthelemy, Roux & Martineau, 1999; Muthukumaraswamy, Johnson & McNair, 2004; Lepage & Theoret, 2006; Hari & Salmelin, 2007). The assumption that mu-suppression during action observation reflects the activation of the MNS has been inferred based on data obtained during both observation and execution conditions. A recent study with human adult subjects examined mu suppression using both EEG and fMRI techniques (Arnstein, Cui, Keysers, Maurits & Gazzola, 2011). The results showed that during action observation there was a correlation between mu-suppression and activity in BA44 and the inferior parietal lobule, areas considered to be involved in the MNS, (Keysers & Gazzola 2009). Thus, there is at the very least some suggestion that mu suppression reflects the neural circuits that are activated during action observation.
Although EEG suppression has been reported during observation of action in human infants as young as 9–14 months old (Nyström, Ljunghammar, Rosander & von Hofsten, 2010; Marshall, Young, & Meltzoff, 2010; Southgate, Johnson, El Karoui & Csibra, 2010), no study has reported the presence of this phenomenon in the newborn.
In the present study we monitored EEG reactivity in newborn rhesus macaques (Macaca mulatta) during observation and production of facial gestures to test the hypothesis that a mirroring system may be present early in life. To do this, we developed an EEG cap that could be used with infant macaques. Using MRI and X-Ray scans of one-week-old infant macaque heads (Figure 1) we positioned electrodes on this cap to record from both anterior and posterior locations. EEG data with synchronized video recordings were then collected continuously while infants were shown live presentations (Figure 2) of (a) lip smacking (LS; a rapid opening and closing of the mouth), (b) tongue protrusion (TP; repetitive protrusion and retraction of the tongue), and (c) a non-biological control (CTRL; a white plastic disk with orthogonal red and black lines slowly rotated left and right). Previous behavioural work demonstrated that these stimuli elicit interest in macaque infants (Ferrari et al. 2006). Moreover, LS and TP elicited behavioral matched responses while the disk did not induce any significant motor activation of the mouth and the tongue. These behavioural matched responses have been interpreted as the consequence of the activation of a mirroring mechanism in which the observation of a gesture activates the same motor programs as those activated during specific motor action. We therefore considered these stimuli optimal to test our hypothesis.
Figure 1.
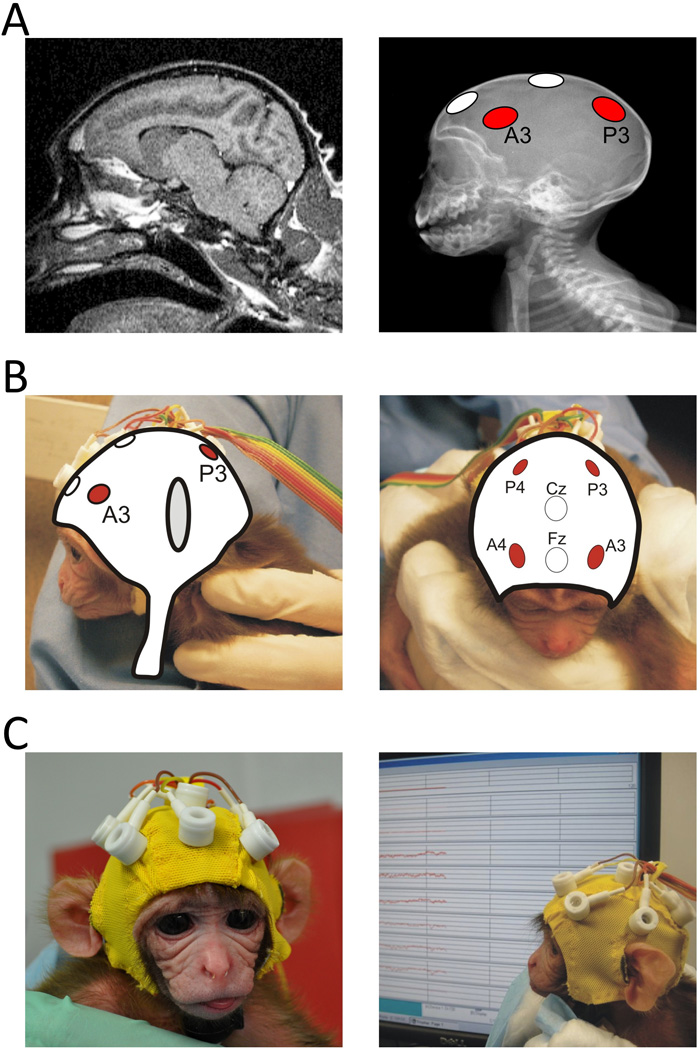
Custom lycra EEG cap fitted with 6 tin electrodes specifically designed for infant monkeys. A. Left a MRI scan image of a 1 week old infant. We used 2 MRI scan series of 1-week-old infant rhesus macaques to detect the approximate location of sulci and the lobes of the macroanatomical areas of the cerebral cortex. Right. An X-ray image taken from a 1-week-old infant that was used in conjunction with the MRI images to identify the location of the anterior and posterior electrode placement. The anterior electrodes were placed approximately above the premotor motor cortex while the posterior electrodes were placed above the posterior part of the parietal lobes. A plaster mold of a one-week-old infant skull was used to help with the construction of the infant cap with the coordinates assessed from the X-rays and MRI images. B. The two figures show, from two different views, an example of illustrated reconstructions that were used to design the cap. Cz: reference electrode; Fz: ground. C. A close-up view of the EEG cap fitted on a 1-day-old infant macaque.
Figure 2.

Examples of the settings and procedures used during EEG acquisition. A. Infants were held by one experimenter while a second experimenter acted as a live stimulus for facial gestures (top) or, in the case of control, presented the disk to the infant (bottom). B. Illustration of the experimental conditions: LS: lipsmacking condition; TP: tongue protrusion; CTRL: control condition in which a disk was presented in front of the infant during baseline period. During the stimulus period the disk was rotated clock and counter-clockwise.
Behavioral coding of the video records identified periods where the infant was quiet, still and observing or producing LS or TP. Epochs of EEG corresponding to these epochs were extracted and submitted to a fast Fourier transform. Event–related desynchronization (ERD) was computed from the EEG power to indicate either suppression or enhancement relative to a baseline level.
Here we demonstrate desynchronization of the 5–6 Hz frequency band during both the perception and production of facial gestures suggesting the presence of a MNS at birth.
Materials and Methods
Subjects
Subjects were 33 infant rhesus macaques (18 males and 15 females; average weight 511.9 ± 14.0 g) who were involved in ongoing experimental protocols that required separating the infants from their mother on day 1 post-partum. Infants were individually housed in plastic incubators (51 × 38 × 43 cm) that each contained a 25 cm-high inanimate “surrogate mother.” During the first week of life, the surrogate mother was composed of a 16.5 cm circumference polypropylene cylinder, wrapped in fleece fabric and attached by a flexible metal component to an 11.5-cm wide circular metal base. From the second week onwards, infants were provided with a hanging surrogate mother consisting of a plastic cylinder core (20 cm high and 19 cm circumference), with a wide soft cloth cover (20 × 25 cm; see Dettmer, Ruggiero, Novak, Jeyer & Suomi, 2008). The incubator was maintained at a temperature of ~27°C and at 50–55% humidity. Lights were on from 07:00 to 21:00. Infants could see and hear other infants, but not contact them physically. All animals were hand-fed with Similac Infant formula (Ross Laboratories, Columbus, Ohio, United States) until they were old enough to feed independently, usually by day 4. Formula was administered ad libitum until 4 months of age.
All testing was conducted in accordance with regulations governing the care and use of laboratory animals and had prior approval from the Institutional Animal Care and Use Committees of the National Institute of Child Health and Human Development (NICHD) and the University of Maryland.
Twelve out of the original 33 infants were excluded from further analyses: 4 because of insufficient artifact-free EEG epochs or non-compliance during testing, 3 because of equipment failure, 3 monkeys did not complete all tasks on a given testing day and 2 because their data were statistical outliers.
Due to our exclusion criteria and available testing days, we obtained usable recordings in a limited number of animals on all 4 testing days (n = 6 for LS and n = 5 for TP), while for most others we obtained reliable data on 3 testing days (n = 11 for LS and n = 12 for TP). The rest of the infants had reliable data, or have been tested only, on 1 or 2 testing days (n = 4 for LS and for TP).
Procedures
Infants were tested on days 1, 3, 5, and 7 post-partum. During each testing period, the infant was presented, in a random order, with all three conditions of the experimental procedure (see below), modified from Ferrari et al. (2006) for assessing imitation in infant macaques. Video and EEG data were recorded simultaneously while the infant observed the stimuli. A video camera (Sony Digital Video Camcorder ZR600) was positioned 0.5 m behind stimulus presentation so that the infants’ behavior and attention could be easily identified.
Experimental Setup
The setup is a modification of a neonatal imitation task developed for use with infant macaques (24). The task had three conditions in which the infants received live presentation of stimuli from a human experimenter: (a) tongue protrusion (TP), with repeated maximal extension and retraction, (b) lip smack (LS), a rapid opening and closing of the lips without sound production, and (c) a 15-cm diameter plastic disk (CTRL, control condition) with a red and black orthogonal bars painted on it and rotated 180° clockwise and counterclockwise (Figure 2).
At the beginning of a trial, a 40-s baseline period was conducted, in which the experimenter displayed a passive/neutral facial expression (or still disk in the CTRL condition). The experimenter then displayed the stimulus for 20 s (LS, TP, or rotating disk in CTRL) followed by 20 s of still face (or still disk). The sequence of 20-s moving stimuli and 20-s still periods was repeated three times to maximize artifact-free data obtained for each infant in each condition.
Video Recording
Testing sessions were recorded onto DVD for behavioral coding and synchronization to EEG. During acquisition, the video signal was time-stamped with a vertical interval time code (VITC) synchronized with the EEG acquisition time-bas, resulting in an accuracy of 33 milliseconds. The infant’s behavior was coded using the Video Coding System (VCS), James Long Company (Caroga Lake, NY). The start and end times of epochs where the infant was still and observing or imitating the stimuli, as well as epochs when the infant spontaneously produced lip smacking or tongue protrusion, were subsequently identified during behavioral analysis (frame-by-frame coding). These start and end times were then combined with the EEG analysis software and only epochs with both artifact-free EEG and identified behavior were included in the analyses.
Behavioral Analysis
All tapes were visually analyzed by two coders that were blind to the durations of the experimental conditions and the occurrence of behaviors relevant for the purpose of the study. The number of frames (30 frames per second) the infant spent looking at the experimenter were noted, as well as any motor responses in the body, arms and/or face.
The following behaviours were coded: (a) Visual attention to the model. The monkey orients and looks at the stimulus. (b) Lip smacking (opening and closing of the mouth; see also 24). (c) Tongue protrusion (forward movements of the tongue that cross the inner edge of the lower lip; see also 24). Lip smacking and tongue protrusion responses when coded during CTRL, LS and TP conditions. (d) Arm and/or hand movements. These could include a wide range of behaviors, from self-directed behaviors, such as scratching, to the uncontrolled movement of the arm in space with no apparent purpose. These movements could be often observed when newborns are trying to reach more stable posture. (e) Body movements, that is any movements, even minimal, of the trunk that could be or not associated to regain stability in the posture or to orient the body. Most of these movements are common reflexes that infant monkeys display early in life and could be due to general immaturity of the skeleton/muscle or to the search of a more stable position and posture during testing.
EEG Acquisition and Analysis
A custom lycra cap (Electro-Cap International, Eaton, OH) was made and fitted with 6 tin electrodes with their placement based on x-rays and MRI images of a 1-week-old typical infant rhesus macaque (Figure 1a). X-rays images were superimposed on the MRI scans to assess the locations of the main sulci of the brain to establish the placement of the electrodes on the cap (Figure 1b). A plaster cast of a month-old infant skull was used to help the construction of the cap. Two posterior electrodes (P3 and P4) were placed on scalp locations approximately over the parietal cortex and two anterior electrodes (A3 and A4) were placed approximately over the premotor cortex (Figure 1c). The vertex served as reference; the ground electrode was above the forehead. The infants’ heads were shaved and a mild abrading gel was applied to clean the scalp and improve impedances. Impedances were kept below 20 kΩ. EEG was band-pass filtered from 0.1 to 100 Hz, digitized with a 16-bit A/D converter (±5V input range) and sampled at 1000 Hz. Epochs contaminated with artifacts were removed from subsequent analyses. Epochs of clean EEG that coincided with epochs identified by the behavioral coding were analyzed with Fast Fourier Transform (FFT) using a 1-s Hanning window with 50% overlap, and spectral power (µV2) was computed for 1-Hz bins from 2 to 9 Hz. All data processing was performed using EEG Analysis System software, James Long Company. Infants providing less than 3 seconds of EEG uncontaminated by movement artifact (determined through both automatic artifact scoring and behavioral coding) were excluded from further analyses.
Event-related suppression of brain rhythms was computed for each testing day (i.e. 1, 3, 5, and 7) as ([S − B] / B) × 100, where S is the absolute power in a particular frequency band while the monkey observed the stimulus presentation (for observation analyses) or produced a facial gesture (for execution analyses) and B is the absolute power in a particular frequency band during periods of EEG in which the stimulus was still and the monkey’s gaze was directed towards the experimenter (see 10). The scores were then averaged across the total number of testing days for each monkey to quantify either suppression (i.e. decrease in band power relative to the baseline) or enhancement of the brain activity.
Statistical Analyses
We used within-subjects repeated measures ANOVA with Greenhouse-Geisser correction for violations of sphericity to examine the EEG data. Main effects and interactions were followed up using 2-tailed paired t-tests. The regions analyzed were as follows: anterior (A3 and A4) and posterior (P3 and P4).
Results
Thirty-three infant rhesus macaques were tested four times in their first week of life (i.e. at ages of 1–2 days, 3–4 days, 5–6 days, and 7–8 days); we report here on reliable EEG data of 21 infants during observe trials and 15 infants who produced either LS or TP during either task. We first considered EEG reactivity in the 2–4 Hz, 5–6 Hz, and 7–9 Hz frequency bands during LS and TP gesture production. The choice of these frequency bands was driven by a number of factors. First, the majority of EEG power in newborn infant macaque is located between 2 to 9 Hz. Second, studies in human infants as young as 9-month of age, show that frequencies between 5 and 9 Hz are responsive to biological visual stimulation (Nyström, Ljunghammar, Rosander & von Hofsten, 2010; Marshall, Young, & Meltzoff, 2010; Southgate, Johnson, El Karoui & Csibra, 2010; Marshall, Bar-Haim & Fox, 2002; Stroganova, Orekhova & Posikera, 1999) and, are suppressed during motor planning and observation of goal-directed actions (Nyström, Ljunghammar, Rosander & von Hofsten, 2010; Marshall, Young, & Meltzoff, 2010; Southgate, Johnson, El Karoui & Csibra, 2010).
To examine the EEG suppression during LS and TP execution we performed a 2 Condition (LS, TP) × 3 ERD (2–4 Hz, 5–6 Hz, 7–9 Hz) × 2 Region (Anterior, Posterior) × 2 Hemisphere (Left, Right) ANOVA on the 15 subjects that displayed both gestures (LS and TP) which revealed only a main effect for ERD (F(2, 28) = 4.24, p < 0.05, ε = 0.642). Follow-up comparisons revealed that the ERD in the 2–4 Hz band (mean ± SD = 1.8 ± 21.1) was significantly different from the ERD of the 5–6 Hz band (−16.0 ± 12.4) t(14) = 3.68, p < 0.005) but not from the ERD of the 7–9 Hz band (−9.5 ± 23.8; t(14) = 1.39, n.s.). The magnitude of the ERD of 5–6 Hz and 7–9 Hz bands did not differ from each other (t(14) = 1.30, n.s.). Because the effects of Condition, Region and Hemisphere were not significant, we averaged ERD across LS and TP execution conditions and across anterior and posterior electrodes for both hemispheres to quantify the suppression in the 2–4 Hz, 5–6 Hz and 7–9 Hz bands. The independent one-sample t-test revealed statistically significant desynchronization in the 5–6 Hz band (t(14) = 5.01, p < 0.001) but not in the 2–4 Hz or 7–9 Hz bands (t(14) = 0.34, n.s.; t(14) = 1.55, n.s. respectively). All of the fifteen infants show suppression in the 5–6 Hz band. Figure 3 summarizes these results. As the 5–6 Hz band displayed the greatest change from baseline during gesture execution, we used this frequency band to compare ERD while the infants observed biological versus non-biological movements.
Figure 3.
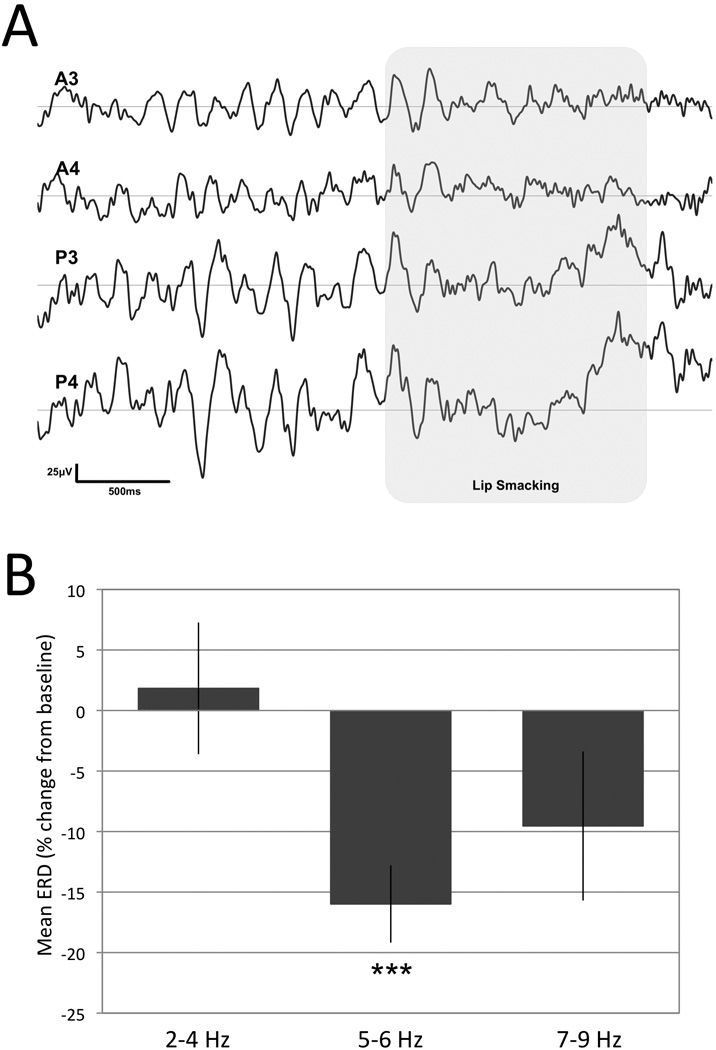
Summary of execution analyses identifying the frequency band of interest. A. Example of EEG signals at rest and during the infant’s own production of lip smacking (shaded area). A3 and A4 indicate the anterior and P3 and P4 the posterior electrodes. B. Mean ± SEM signal suppression during the production (N = 15) of facial gestures (both TP and LS) in three different frequency bands. The asteriscs indicate a statistically significant suppression (t(14) = 5.06, p < 0.005) in the 5–6 Hz band.
We compared the observation-related ERD suppression in the three experimental conditions. The 3 Condition (LS, TP, and CTRL) × 2 Region × 2 Hemisphere analysis on 21 infants revealed a main effect of Condition (F(2, 40) = 4.53, p < 0.05) qualified by a Condition by Region interaction (F(2, 40) = 3.48, p < 0.05). Paired comparisons (t-tests) revealed significant suppression in the LS and TP conditions in the anterior (A3/4; t(20) = 3.27, p < 0.005; t(20) = 2.26, p < 0.05 respectively) but not in the posterior (P3/4; t(20) = 1.22, n.s.; t (20) = 1.57, n.s. respectively) electrodes when compared with CTRL. LS and TP conditions did not differ at either region location. Sixteen out of twenty one subjects showed this pattern of suppression. Figure 4 illustrates a summary of these results.
Figure 4.
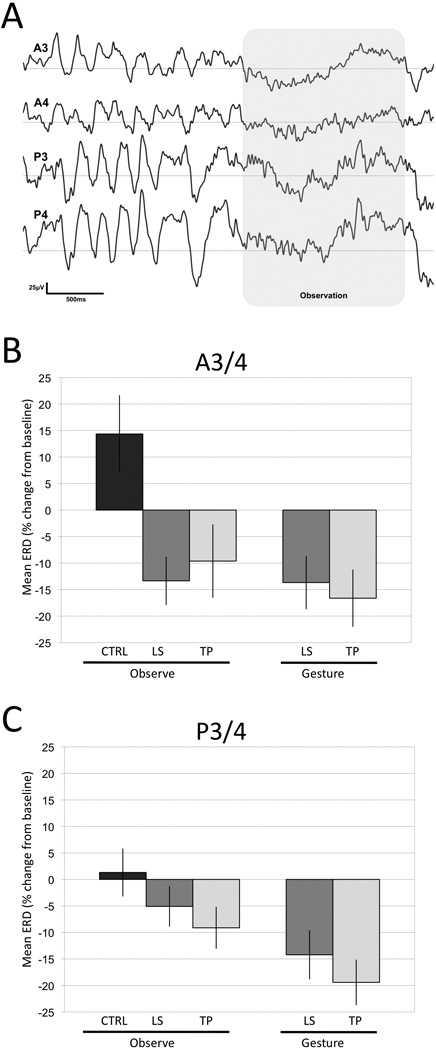
Reactivity of the 5–6-Hz frequency band during gesture observation. A. Example of EEG signals at rest and during the observation of lip smacking gesture (shaded area). A3 and A4 indicate the anterior and P3 and P4 the posterior electrodes. B. Mean ± SEM reactivity of the 5–6-Hz frequency band in anterior (A3/4) and (C) posterior (P3/4) electrodes during observation (N = 21) and production (N = 15) of facial gestures.
Discussion
To the best of our knowledge, this is the first study to examine scalp EEG in newborn rhesus macaque. Our results demonstrate the presence of a 5–6-Hz EEG rhythm that is suppressed both when the newborn macaque is observing facial gestures and when he/she is producing the same gesture. We did not find suppression in response to non–biological movements. This suppression cannot be attributed to skeletomotor activation because we excluded from the analysis, on the basis of behavioral video data, all epochs in which infants displayed even minimal movement. Although newborn monkeys often displayed poor motor control with involuntary muscle contraction, these involuntary contractions disappeared, or were attenuated, when the infants were attending to relevant stimuli; the movements were detectable in a detailed behavioral analysis of the video data. We are thus confident that the EEG epochs used for the observation analyses were not contaminated by overt movements.
The sensitivity of 5–6 Hz EEG activity to the production and observation of facial gestures but not of other relevant non-biological movements suggests that this frequency band acts similarly to the mu rhythm in humans (Hari et al. 1998; Oberman et al. 2005; Pineda, 2005; Lepage & Theoret, 2006; Muthukumaraswamy, Johnson, Gaetz & Cheyne, 2006; Caetano, Jousmäki & Hari, 2007; Cheng, Yang, Lin, Lee & Decety, 2008; Nyström, Ljunghammar, Rosander & von Hofsten, 2010; Marshall, Young, & Meltzoff, 2010; Southgate, Johnson, El Karoui & Csibra, 2010). In adults, EEG desynchronization is measured within the 9–13 Hz band (Marshall & Meltzoff, 2011). However, the mu frequency appears to be centered at lower frequencies (5 to 9 Hz) in human infants (Marshall & Meltzoff, 2011; Nyström, Ljunghammar, Rosander & von Hofsten, 2010; Southgate, Johnson, El Karoui & Csibra, 2010). A recent MEG study investigated how the mu rhythm changes from the first month of life in human infants through to preschool children by means of a motor task that required the subject to squeeze an object (Berchicci et al. 2011). The results showed that at around 18 weeks of age, infants exhibit a mu peak EEG frequency at 4.4 Hz. Thus, the EEG frequency band we identified as being sensitive during newborn monkeys executed/observed facial gestures appears compatible with the mu rhythm found in human infant studies.
In humans, the mu rhythm is suppressed during the execution and observation of hand actions (Pineda, 2005; Lepage & Theoret, 2006; Lepage & Theoret 2007; Muthukumaraswamy, Johnson, Gaetz & Cheyne, 2006; Marshall, Bouquet, Shipley & Young, 2009). However, this suppression is not limited to viewing hand or body actions. In fact, in a recent study, it has been found that in human adults the mu rhythm suppression is present while viewing facial gestures expressing different types of emotions (Moore, Gorodnitsky & Pineda, 2011), suggesting that desynchronization in EEG mu occurs in relation to biological meaningful actions as well as to facial gestures.
The correspondence in EEG activity between execution and observation has led to the proposal that the mu rhythm is a signature of MNS involvement in humans (Lepage & Theoret, 2006, 2007; Marshall et al., 2009, 2010; Marshall and Meltzoff, 2011; Arnstein, Cui, Keysers, Maurits & Gazzola, 2011). Our data are consistent with the human literature in demonstrating specific EEG suppression during observation and execution of facial gestures.
The current results have two major implications for our understanding of the ontogeny of the MNS. First, these data provide evidence (albeit indirect in that there were no direct recordings from specific neurons in monkey cortex) that a MNS is present very early in life. Previous studies with human infants as young as 9–14 months old have shown suppression of the mu rhythm during observation of hand actions (Nyström, Ljunghammar, Rosander & von Hofsten, 2010; Marshall, Young, & Meltzoff, 2010; Southgate, Johnson, El Karoui & Csibra, 2010), suggesting the presence of a MNS during the first year of life. However, until now no study has examined EEG suppression in the l period immediately following birth. We now demonstrate, as previously proposed (Ferrari et al., 2006; Ferrari, Bonini & Fogassi, 2009; Lepage & Theoret, 2007), that the basic elements of this system operate from the very first days of perinatal life.
Second, it has been proposed that the MNS underlies important behaviors, such as imitation (Iacoboni et al. 1999; Buccino et al. 2004; Iacoboni, 2009). In humans, the mu rhythm has been shown to suppress during the observation and later imitation of novel actions (Marshall et al., 2009, 2010). In human infants, neonatal imitation may be an early learning mechanism for the acquisition of more complex social behaviors (e.g. empathy and Theory of Mind; Meltzoff and Moore, 1977; Meltzoff, 2002). The current findings demonstrate that, during observation and the production of the same facial gestures, suppression of the 5–6 Hz EEG signal is a sensitive marker for biological movement. Furthermore, they suggest that the early elements of the MNS may contribute to the neonatal imitation phenomena (Ferrari et al., 2006, 2009) and may be foundational for learning complex social behaviors.
It should be noted that the EEG suppression during gesture production differed from that seen during the observation of action, as the former was associated with EEG suppression in both anterior and posterior electrodes. This may be a result of the additional contribution of cortical networks involved in motor control and associated somatosensory feedback likely involving parietal-premotor circuits. In monkey electrophysiological single cell recordings, mouth mirror neurons have been found in the ventral premotor but not consistently in the posterior parietal cortex, although both areas contain neurons firing for execution of mouth actions (Ferrari, Gallese, Rizzolatti & Fogassi, 2003; Rozzi, Ferrari, Bonini, Rizzolatti & Fogassi, 2008). Recent neurophysiological investigations in the monkey and human fMRI demonstrated activation of primary motor areas for execution and perception of others’ actions (Tkach, Reimer & Hatsopoulos, 2007; Evangeliou & Savaki, 2007; Arnstein, Cui, Keysers, Maurits & Gazzola, 2011). Thus, during the production of mouth gestures in addition to the ventral premotor areas, other motor and somatosensory cortical sectors are likely involved.
The presence of a functional mirroring system soon after birth provides infants important advantages, including the capacity to detect and respond to relevant social stimuli in their immediate environment. Such a mechanism might facilitate communicative exchanges with the caregiver and caregiving towards the infant. Our knowledge on the basic functioning of the MNS at birth will be of importance for understanding and detection of possible deficits emerging during development that may compromise infant’s social and cognitive faculties.
Acknowledgements
We thank Giacomo Rizzolatti, Leonardo Fogassi and Riitta Hari for useful comments on an earlier version of the manuscript.
This research was supported by P01HD064653-01 NIH grant to PFF and NAF, and division of Intramural Research, NICHD.
References
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. Journal of Neuroscience. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchicci M, Zhang T, Romero L, Peters A, Annett R, Teuscher U, Bertollo M, Okada Y, Stephen J, Comani S. Development of mu rhythm in infants and preschool children. Developmental Neuroscience. 2011 doi: 10.1159/000329095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Caetano G, Jousmäki V, Hari R. Actor’s and viewer’s primary motor cortices stabilize similarly after seen or heard motor actions. Proceedings of the National Academy of Sciences USA. 2007;104:9058–9062. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Yang C-Y, Lin C-P, Lee P-L, Decety J. The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. NeuroImage. 2008;40:1833–1840. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Roux S, Martineau J. Observation and execution of movement: Similarities demonstrated by quantified electroencephalography. European Journal of Neuroscience. 1999;11:1839–1842. doi: 10.1046/j.1460-9568.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Ruggiero AM, Novak MA, Jeyer MS, Suomi SJ. Surrogate mobility and orientation affect the early neurobehavioral development of infant rhesus macaques (Macaca mulatta) Developmental Psychobiology. 2008;50:418–422. doi: 10.1002/dev.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Bonini L, Fogassi L. From monkey mirror neurons to mirror-related behaviours: possible direct and indirect pathways. Philosophical Transactions of the Royal Society: B. 2009;364:2311–2323. doi: 10.1098/rstb.2009.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biology. 2006;4:e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti R, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. European Journal of Neuroscience. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a view through the skull. Trends in Neuroscience. 2007;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hari R, et al. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proceedings of the National Academy of Sciences USA. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annual Reviwes in Psychology. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Current Opinion in Neurobiology. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Theoret H. EEG evidence of an action-observation matching system in children. European Journal of Neuroscience. 2006;23:2505–2510. doi: 10.1111/j.1460-9568.2006.04769.x. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Theoret H. The mirror neuron system: grasping others' actions from birth? Developmental Science. 2007;10:513–523. doi: 10.1111/j.1467-7687.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bouquet CA, Shipley TF, Young T. Effects of brief imitative experience on EEG desynchronization during action observation. Neuropsychologia. 2009;47:2100–2106. doi: 10.1016/j.neuropsychologia.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems: exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1:110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Young T, Meltzoff AN. Neural correlates of action observation and execution in 14-month-old infants: an event-related EEG desynchronization study. Developmental Science. 2010 doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Imitation as a mechanism of social cognition: Origins of empathy, theory of mind, and the representation of action. In: Goswami U, editor. Blackwell Handbook of Childhood Cognitive Development. Oxford: Blackwell Publishers; 2002. pp. 6–25. [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of Facial and Manual Gestures by Human Neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Moore A, Gorodnitsky I, Pineda J. EEG mu component responses to viewing emotional faces. Behavioral Brain Research. 2011 doi: 10.1016/j.bbr.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, Gaetz WC, Cheyne DO. Neural processing of observed oro-facial movements reflects multiple action encoding strategies in the human brain. Brain Research. 2006;1071:105–112. doi: 10.1016/j.brainres.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Cognitive Brain Research. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Nyström P, Ljunghammar T, Rosander K, von Hofsten C. Using mu-rhythm perturbations to measure mirror neuron activity in infants. Developmental Science. 2010 doi: 10.1111/j.1467-7687.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythm. International Journal of Psychophysiology. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Mental simulation in the service of action perception. Journal of Neuroscience. 2007;27:12675–12683. doi: 10.1523/JNEUROSCI.2988-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of the inferior parietal lobule of the macaque monkey. European Journal of Neuroscience. 2008;28:1569–1588. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ online prediction of others’ goals. Psychological Science. 2010;21:355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Stroganova SA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action perception in motor cortex. Journal of Neuroscience. 2007;27:13241–13250. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


