Summary
In comparison to murine dendritic cells (DCs), less is known about the function of human DCs in tissues. Here, we analyzed, using lung tissues from humans and humanized mice, the role of human CD1c+ and CD141+ DCs in determining the type of CD8+ T cell immunity generated to live-attenuated influenza virus (LAIV) vaccine. We found that both lung DC subsets acquired influenza antigens in vivo and expanded specific cytotoxic CD8+ T cells in vitro. However, lung-tissue-resident CD1c+ DCs but not CD141+ DCs were able to drive CD103 expression on CD8+ T cells and promoted CD8+ T cell accumulation in lung epithelia in vitro and in vivo. CD1c+ DCs induction of CD103 expression was dependent on membrane-bound cytokine TGF-β1. Thus, CD1c+ and CD141+ DCs generate CD8+ T cells with different properties, and CD1c+ DCs specialize in the regulation of mucosal CD8+ T cells.
Introduction
Dendritic cells (DCs) are key regulators of innate and adaptive immune responses (Banchereau and Steinman, 1998). DCs are very efficient and can elicit T cell responses at much lower DC/T cell ratios than other antigen presenting cells (APC) such as macrophages (Steinman, 2011). Tissue-resident DCs refer to those DCs that are present in normal non-inflamed tissues. Recent studies in the mouse have established that tissue-resident DCs arise from two distinct lineages, the Batf3, IRF8, Id2-dependent and Batf3, IRF8, Id2-independent lineage (Edelson et al., 2010; Ginhoux et al., 2009; Hashimoto et al., 2011; Hildner et al., 2008). These studies also established that Batf3, IRF8, Id2-dependent DCs, which include both lymphoid-tissue-resident CD8+ DCs and non-lymphoid-tissue-resident CD103+ DCs, have a superior ability to drive CD8+ T cell immune responses compared to CD8− and CD103− DCs (Heath and Carbone, 2009). Considerably less is known about the origin of human DCs, their differentiation program, and their functional differentiation in situ due to their rarity in the blood and poor accessibility of human tissues. Most of the studies that probed the specialization of human DC subsets have focused on blood-circulating and skin DCs (reviewed in (Ueno et al., 2010)). These studies have distinguished human-blood-circulating DC subsets based on three main cell surface markers: CD303 (BDCA-2) on plasmacytoid DCs (pDCs), CD1c (or BDCA-1) expressed on the majority of circulating DCs, and CD141 (or BDCA-3) expressed on a minute population (Dzionek et al., 2000; MacDonald et al., 2002). These markers were also utilized to establish the presence of DC subsets in the human lung (Demedts et al., 2005). Human CD141+CD1c− DCs were found to uniquely express Toll-like receptor 3 (TLR3); they excel in the production of IL-12 and the cross-presentation to CD8+ T effector cells when activated with poly I:C (Bachem et al., 2010; Crozat et al., 2010; Haniffa et al., 2012; Jongbloed et al., 2010; Lauterbach et al., 2010; Mittag et al., 2011; Poulin et al., 2010). However, other human DCs such as epidermal Langerhans cells (LCs) (Klechevsky et al., 2010; Klechevsky et al., 2008) and CD1c+ DCs were also found to cross-present antigens to CD8+ T cells (Jongbloed et al., 2010; Mittag et al., 2011; Poulin et al., 2010). Skin LCs’ efficiency in priming naive CD8+ T cells can be at least partially explained by their surface expression of IL-15 (Banchereau et al., 2012; Romano et al., 2012) and/or upregulation of CD70 upon viral exposure (van der Aar et al., 2011). Yet, upon exposure to some viruses, LCs are unable to generate CD8+ T cell immunity (van der Vlist et al., 2011). Thus, it remains to be determined how and via which mechanisms all of these DC subsets cooperate in shaping adaptive immunity.
To assess the role of human respiratory mucosal DCs in vaccine immunity in vivo, we reconstituted immunodeficient mice with human CD34+ hematopoietic progenitor cells (HPCs). A few weeks after transplant, mice generate human B cells and all human DC subsets including pDCs and classical DCs (cDCs) in the bone marrow and spleen as well as cDCs in peripheral tissues (Palucka et al., 2003; Yu et al., 2008). In one version of the model, human T cells were adoptively transferred, thereby permitting the analysis of T cell subsets and memory T cell responses. These humanized mice, when vaccinated with live attenuated influenza vaccine (LAIV), generated CD8+ T cells specific to influenza matrix protein 1 (FluM1) and nonstructural protein 1 (NS1) in blood, spleen, and lungs. The expansion of antigen-specific CD8+ T cells is dependent on the reconstitution of the human myeloid compartment (Yu et al., 2008). Therefore, we used these mice and human lung tissues herein to analyze the role of human lung CD1c+ and CD141+ DC subsets in the induction of anti-viral CD8+ T cell responses. Here we show that whereas both DC subsets could acquire viral antigens and drive antiviral effector CD8+ T cell responses, CD1c+ DCs were uniquely able to drive the differentiation of CD103+CD8+ mucosal T cells.
Results
Characterization of CD1c+ and CD141+ DCs in lungs
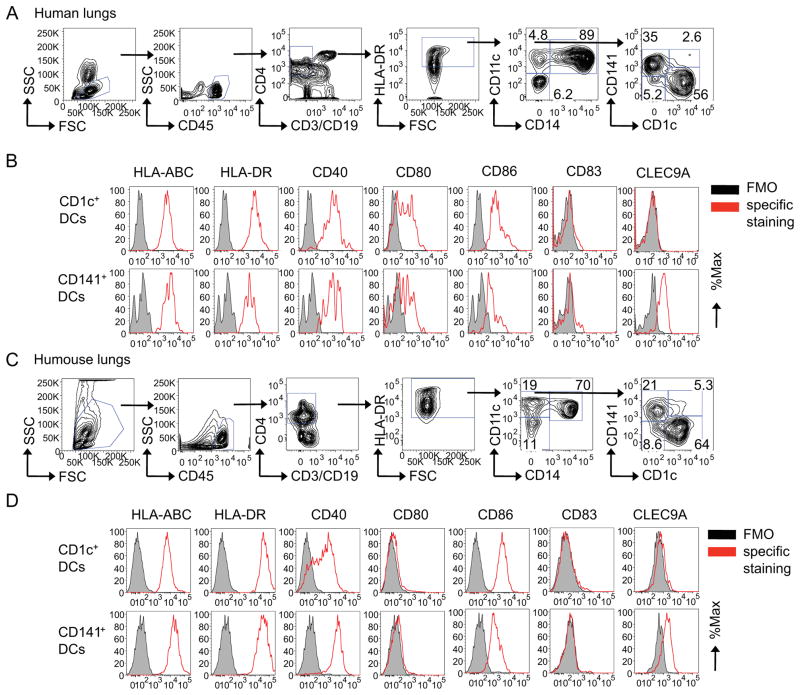
We first characterized the distribution of CD1c+ and CD141+ DCs in human lungs and in the lungs of humanized mice in the steady state. Human lung samples were obtained during surgical resection in patients with lung cancer as macroscopically uninvolved normal tissue. DCs were analyzed using flow cytometry by gating on CD45+CD4+HLA-DR+CD11c+CD14− cells (as shown in Figure 1 and S1 and in ref. (O’Doherty et al., 1993)) with differential expression of CD1c and CD141 (Figure 1A). Single-cell suspensions of human lungs contained CD1c+ DCs (n=8: mean±SD=62±8.7%, range=48–72%) and CD141+ DCs (mean±SD=20±10%, range=11–41%). Both DC subsets displayed expression of MHC class I and HLA-DR as well as co-stimulatory molecules CD40, CD80 and CD86. CD1c+ DCs displayed a higher expression of HLA-DR and CD86 (Figure 1B). Immunofluorescence on frozen sections showed CD1c+ DCs in close proximity to cytokeratin-expressing epithelial cells whereas CLEC9A-expressing CD141+ DCs (Jongbloed et al., 2010) were found predominantly in the interstitium (Figure S1F). To facilitate functional studies of lung DC subsets, we next analyzed their reconstitution in vivo in the lungs of humanized mice. Sublethally irradiated NOD-SCID B2m−/− mice were transplanted with 3×106 CD34+ HPCs isolated from G-CSF-mobilized blood of HLA-A*0201+ healthy donors (Yu et al., 2008). Flow cytometry analysis on single-cell suspensions from lungs 4–6 weeks later showed the presence of CD1c+ DCs (n=7: mean±SD=63±6.4%, range=55–69%) and CD141+ DCs (mean±SD=25±6.7%, range=20–36%) at ratios comparable to those in human lungs (Figure 1C). CD141+ DCs expressed higher amounts of MHC class I and CD40 and lower amounts of CD86 (Figure 1D). Upregulation of CD80 expression on DCs from human lungs might be ascribed to lengthy ex vivo isolation procedures. Thus, the distribution and phenotype of CD1c+ and CD141+ DC subsets in the lungs of humanized mice are comparable to that of human lungs.
Figure 1. Human CD1c+ and CD141+ DC subsets in the lung.
Single-cell suspensions from lung tissues obtained from (A–B) human subjects or humanized (C–D) mice were stained with specific Abs and analyzed by flow cytometry. (A, C) DCs were gated as CD45+, CD3−, CD19−, CD4+, HLA-DR+, CD14− and CD11c+ cells with differential expression of CD1c and CD141. Flow cytomtery characterization of surface marker expression relative to a fluorescence-minus-one (FMO) control on CD1c+ and CD141+ DCs obtained from (B) human lungs or from the lungs of (D) humanized mice. One of three experiments shown. See also Figure S1.
CD1c+ and CD141+ DCs can expand influenza-specific memory CD8+ T cells
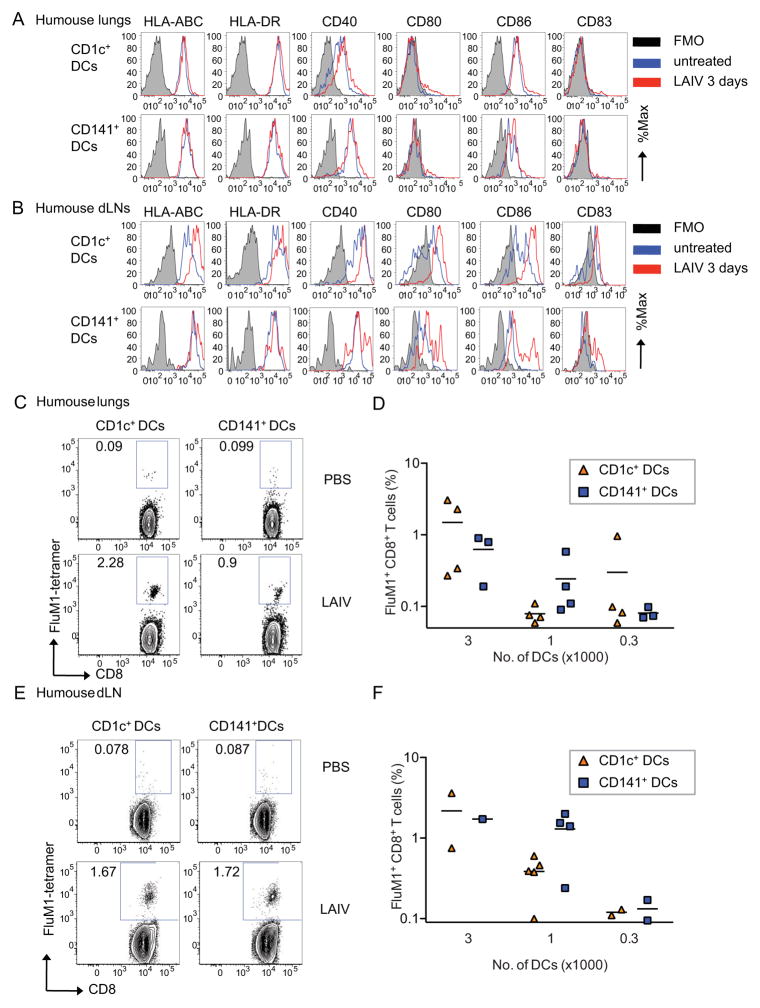
To define the role of lung CD1c+ and CD141+ DCs in the differentiation of CD8+ T cells, humanized mice were inoculated intranasally with LAIV as this route allows expansion of FluM1-specific CD8+ T cell numbers both in blood and lungs (Yu et al., 2008). Both DC subsets could be detected in lungs and draining lymph nodes (LNs) at day 3 post inoculation (Figure 2A–B and S2A–D). Then, DCs were sorted from the lungs, spleen and draining LNs and co-cultured with CFSE-labeled autologous total T cells for 8 days without exogenous cytokines. Expansion of antigen-specific CD8+ T cells ex-vivo was defined as the percentage of CFSE-negative CD3+CD8+ T cells that bound FluM1-peptide-loaded HLA-A*0201 tetramer by flow cytometry (Figure 2C–F). T cells cultured with either purified lung CD1c+ or CD141+ DCs showed comparable expansion of FluM1-tetramer+ CD8+ T cells at different DC:T cell ratios (Figure 2C–D) and CFSE dilutions (Figure S2E). Similarly, the two DC subsets sorted from draining LNs induced comparable expansion of FluM1-tetramer-binding CD8+ T cells in vitro (Figure 2E–F). The ability of both CD1c+ and CD141+ DC subsets to capture and present influenza antigens to memory T cells was further confirmed using DC subsets sorted from the lungs of humanized mice and loaded with LAIV in vitro (Figure S2F). These results show that CD1c+ and CD141+ DCs can capture vaccine antigens both in vivo and in vitro. They can also present these antigens to CD8+ T cells, resulting in the expansion of FluM1-specific recall T cell responses. Thus, both CD1c+ and CD141+ DCs can contribute to the generation of CD8+ T cell immunity against influenza virus.
Figure 2. Generation of influenza-specific recall T cell responses by CD1c+ and CD141+ lung DC subsets.
(A–B) Flow cytometry analysis of CD1c+ and CD141+ DCs isolated from the (A) lungs and(B) draining LNs (dLNs) of humanized mice before and 3 days after LAIV inoculation. One of three experiments shown. (C–F) Humanized mice were inoculated intranasally with PBS or LAIV. Three days later, CD1c+CD141− and CD1c−CD141+DC subsets were purified from the lungs and draining LNs (dLNs), cocultured with autologous T cells for 8 days, and the expansion of specific CD8+ T cells was measured using FluM1-tetramer. Dot plots show the percentage of FluM1 specific CD8+ T cells upon coculture with DC subsets sorted from the lungs (C) or dLNs (E) of PBS- (upper plots) and LAIV- (lower plots) inoculated humanized mice. (D, F) The percentages of FluM1 tetramer+ CD8+ T cells (ordinate) obtained upon coculture with titrated doses of CD1c+ (red) and CD141+ (blue) DCs isolated from the lungs (D) or dLNs (F). N = 2 experiments from 8 different mice cohorts. See also Figure S2.
CD1c+ DCs induce the differentiation of CD103+CD8+ T cells
To further probe the functions of these two DC subsets, we assessed their capacity to induce allogeneic naïve CD8+ T cell responses. To this end, titrated numbers of CD1c+ DCs and CD141+ DCs sorted from lungs, draining LNs and spleens of humanized mice inoculated with LAIV and from the lungs and blood of human subjects, were co-cultured with CFSE-labeled, allogeneic, naïve T cells for 6 days. CD8+ T cells cultured with CD141+ DCs from the lungs of humanized mice proliferated more than those cultured with CD1c+ DCs at all DC doses (mean: 21% vs. 42% for 1K DCs, p<0.0001; 6.5% vs. 33% for 500 DCs, p<0.0001; 6.1% vs. 20% for 250 DCs, p<0.01, of CFSE-negative CD8+ T cells, respectively; p<0.0001; Figure S3A–B). Similar results were observed with DCs sorted from human lungs (mean: 22% vs. 10% for 1K DCs, p<0.01; 15% vs. 5.7% for 500 DCs, p<0.01, of CFSE-negative CD8+ T cells, respectively; Figure S3C). These results suggest that CD141+ DCs are more efficient in initiating naïve CD8+ T cell proliferation, both in the steady state (human lungs) and after viral challenge (humanized mice).
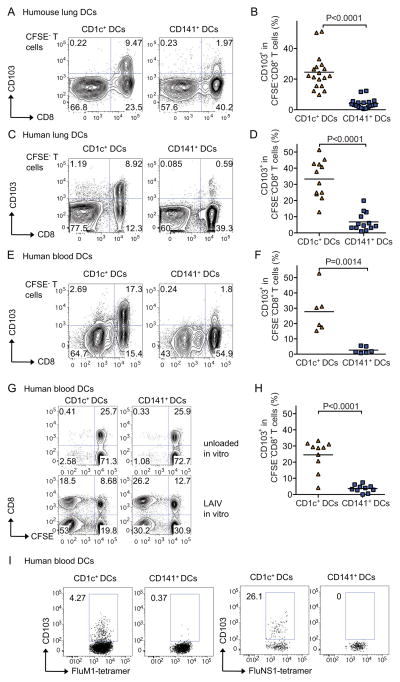
These results prompted us to further examine whether these two distinct DC subsets would allow the generation of CD8+ T cells of different phenotype and function. Thus, we used a large panel of cell surface antibodies to identify mucosal markers differentially expressed by CD8+ T cells educated by CD1c+ or CD141+ DCs. Naïve allogeneic CD8+ T cells cocultured with CD1c+ DCs purified from humanized mice lungs showed significantly higher expression of the αE integrin (CD103) (mean±SD=25±11%, range=9.8–51%, n=18 for CD1c+ DCs vs. mean±SD=4.2±3.3%, range=0.5–12%, n=18 for CD141+ DCs, p<0.0001; Figure 3A–B). CD103 is known to promote T cell retention in mucosa (Cepek et al., 1994). Similar to humanized mice, CD1c+ DCs purified from human lungs were also superior to CD141+ DCs at eliciting CD103 expression on allogeneic CD8+ T cells (mean±SD=33±12%, range=13–51%, n=12 vs. mean±SD=6.8±5.6%, range=1.0–20%, n=13, respectively; p<0.0001; Figure 3C–D). The differential induction of CD103 on CD8+ T cells was also observed with DC subsets purified from the spleen of humanized mice (Figure S3D–E) and from human blood (mean±SD=28±14%, range=15–53%, n=6 for CD1c+ DCs vs. mean±SD=2.6±2.1%, range=0.7–5.5%, n=6 for CD141+ DCs, p=0.0014; Figure 3E–F). Kinetic studies showed that the CD103 induction on allogeneic CD8+ T cells was linked with T cell proliferation appearing at day 4 of co-culture (Figure S3F). This suggests that the induction of CD103 expression is dependent on cognate interactions as previously proposed (Glader et al., 2005).
Figure 3. CD1c+ DCs elicit the differentiation of CD103+ CD8+ T cells.
CD1c+ and CD141+ DCs were sorted from lungs of humanized mice after LAIV inoculation (A–B), human lungs (C–D), and human blood (E–I). (A–F) Sorted DCs were cocultured with 105 CFSE-labeled allogeneic naïve T cells for 6 days. T cell proliferation was measured by CFSE dilution and CD103 expression in CSFE-negative CD8+ T cells. A, C and E panels show representative dot plots illustrating the phenotype of proliferating-gated CFSE-negative CD8+ T cells in cocultures with CD1c+ and CD141+ DC subsets isolated from different tissues. B, D and F panels show summaries of data from more than 5 experiments per each condition. (G–I) A similar experiment was carried out with autologous T cells using CD1c+ and CD141+ DC subsets sorted from the blood and loaded with LAIV ex vivo. T cell proliferation and CD103 expression were assessed on proliferating CFSE−CD8+ T cells and tetramer positive cells to FluM1 or NS1 epitopes at day 8. One of five experiments shown. See also Figure S3.
Experiments performed with autologous influenza-antigen-specific T cells yielded similar results. Whereas both CD1c+ and CD141+ DCs exposed to LAIV expanded autologous CD8+ T cells (Figure 3G), the frequency of CD103-expressing CD8+ T cells was significantly higher in cocultures with CD1c+ DCs (mean±SD=25±9.4%, range=4.2–33%, n=10 vs. mean±SD=3.7±2.3%, range=0–7.4%, n=10, p<0.0001; Figure 3H). Moreover, CD103+CD8+ T cells educated by blood CD1c+ DCs expressed VLA-1, NKG2D, but lacked NKG2A (Figure S3G). Kinetic experiments indicated that the expression of CD103 on autologous antigen-specific CD8+ T cells was induced around day 6 of co-culture (Figure S3H). To determine if CD1c+ DCs’ superior ability to expand CD103+CD8+ T cells reflects their ability to induce the differentiation of CD103− T cells into CD103+ T cells, or rather drive the proliferation of existing CD103+CD8+ T cells, purified CD103− T cells were exposed to autologous human blood DCs. As shown in Figure S3I, CD1c+ DCs were uniquely able to induce CD103 expression on CD103− CD8+ T cells. Furthermore, CD1c+ DCs were also uniquely able to drive the differentiation of autologous CD103+ FluM1 and NS1-specific CD8+ T cells from HLA-A*0201 donors (Figure 3I). Thus, CD1c+ DCs have a unique capacity to induce CD103 expression in both naïve and memory CD8+ T cells.
CD103+CD8+ T cells elicited by CD1c+ DCs bind E-Cadherin and express an effector phenotype
Both CD1c+ and CD141+ DCs isolated from the lungs of LAIV-inoculated humanized mice and co-cultured with allogeneic naive T cells were able to expand CD8+ T cells expressing granzyme B (Figure S4A). Similarly, proliferating CFSE−CD8+ T cells expanded by allogeneic LAIV-loaded human blood DC subsets also displayed a cytotoxic phenotype expressing both granzyme A, granzyme B and perforin (Figure S4B). Furthermore, as illustrated in Figure S4C, CD8+ T cells expanded ex vivo by LAIV-loaded blood DC subsets recognized and killed T2 targets pulsed with FluM1 peptide even at very low E:T ratios. As T2 cells do not express E-cadherin, adherence of CD8+ T cells to target cells via CD103 cannot modulate their cytotoxic activity thereby allowing us to compare the TCR-dependent lytic activity of CD8+ T cells elicited by the two DC subsets. These results show that CD8+ T cells elicited by both CD1c+ and CD141+ DCs display comparable lytic activity in vitro, which is consistent with their comparable intensity of FluM1 tetramer staining (Figure 2) and their comparable levels of effector molecule expression.
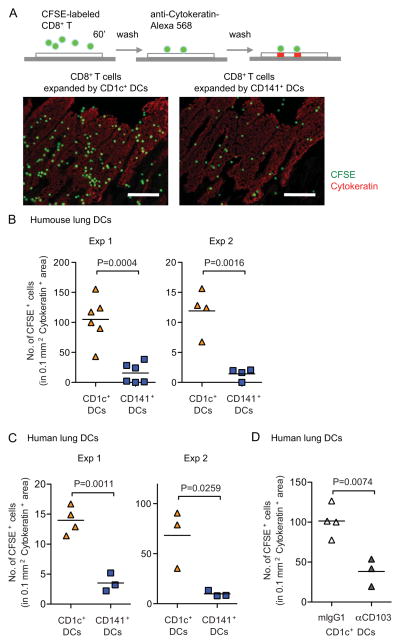
Through binding to E-cadherin, CD103 integrins control effector and memory T cell retention in epithelial compartments (Cepek et al., 1994). To assess whether T cells educated by the CD1c+ DC subset have a superior ability to adhere to epithelial cells, we used a modified Stamper-Woodruff tissue-binding assay (Bell et al., 1999). Proliferating CFSE-negative allogeneic CD8+ T cells were sorted from cocultures with DC subsets, re-labeled with CFSE and overlaid on frozen lung tissue sections to allow adherence. After one-hour incubation, tissue sections were washed and counter-stained with anti-cytokeratin mAbs to visualize the epithelial sections (Figure 4A). The numbers of T cells bound to the lung epithelium were quantified in 0.1 mm2 cytokeratin+ areas using a series of consecutive tissue sections. T cells isolated from cocultures with CD1c+ DCs from the lungs of humanized mice (Figure 4B) or from human lungs (Figure 4C) bound at much higher numbers to the frozen lung tissue sections. Blockade of CD103 on T cells resulted in a significant decrease in the number of CD8+ T cells bound to lung tissue sections (Figure 4D). These results show that CD1c+ DCs expand CD8+ T cells with increased adherence to lung tissue in a CD103-dependent manner.
Figure 4. CD103+CD8+ T cells expanded by CD1c+ DCs show features of mucosal lymphocytes.
CD1c+ and CD141+ DCs were purified from the lungs of humanized mice after LAIV inoculation (A–B) and from human lungs (C–D) and co-cultured with allogeneic naive T cells. At day 6 of coculture, proliferating CFSE− CD8+ T cells were sorted and further labeled with CFSE for Stamper-Woodruff assays on human lung sections. (A) T cells from cocultures with different DC subsets were overlaid on human lung tissue sections; and after one hour of incubation, the sections were washed and counterstained with cytokeratin. T cells were identified by CFSE (green) and airway epithelium was identified as cytokeratin+ (red, scale bar = 100 μm). (B–C) The numbers of T cells bound to lung airway epithelium locations were quantified in 0.1 mm2 cytokeratin+ areas. Two experiments are shown using T cells elicited by CD1c+ and CD141+ DCs from the lungs of humanized mice (B) and from human lungs of two different donors (C). (D) Proliferating CFSE− CD8+ T cells sorted from cocultures with human lung CD1c+ DCs were pre-treated with anti-CD103 Abs or isotype control prior to performing the Stamper-Woodruff assay as described above. Two-tailed t-test was used for statistical analysis. See also Figure S4.
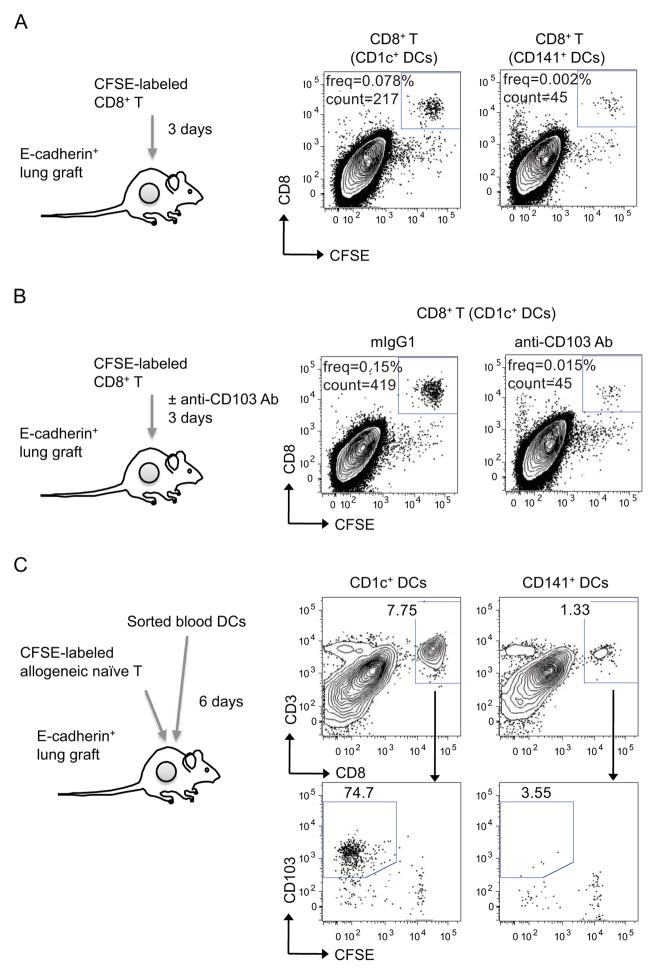
CD1c+ DCs elicit the accumulation of CD103+CD8+ T cells in vivo
To assess whether T cells educated by CD1c+ DCs have a superior ability to accumulate in human epithelial compartments in vivo, we established a human epithelial environment by engrafting immunodeficient animals with human E-cadherin+ HLA-A*0201+ lung-epithelia-derived cell lines and assessed T cell accumulation in these epithelial compartments upon adoptive transfer in vivo. Proliferating CFSE-negative HLA-A*0201+CD8+ T cells purified from co-cultures with allogeneic CD1c+ or CD141+ DCs loaded with LAIV were relabeled with CFSE and injected directly into human epithelial grafts in mice. Three days later, only T cells that were expanded by CD1c+ DCs remained in the human epithelial grafts (Figure 5A). The retention of CD1c+ DC-educated T cells was dependent on CD103 as daily treatment with anti-CD103 but not with isotype control antibody abolished T cell retention in human epithelial grafts in vivo (Figure 5B). Furthermore, CD1c+ DC-educated T cells retained CFSE labeling, indicating their lack of proliferation (Figure 5B). These results suggest that the superior ability of T cells educated by the CD1c+ DC subset to accumulate in epithelial sites is not due to a higher proliferation potential but rather to an increased adherence to the epithelial sites.
Figure 5. CD1c+ DCs elicit the differentiation of CD103+CD8+ T cells in vivo.
(A–B) CD1c+ and CD141+ DCs were purified from human blood and cocultured with allogeneic naïve CFSE labeled CD8+ T cells. Proliferating CFSE− CD8+ T cells cultured with either CD1c+ or CD141+ DCs were sorted and further labeled with CFSE. CFSE-relabeled T cells (1×105 cells) were injected into the human epithelial grafts and single-cell suspensions were analyzed by flow cytometry at day 3. Dot plots show the presence of CD8+CFSE+ T cells. (B) Anti-CD103 or isotype control Abs (50 μg) were given daily to block T cell binding. (C) CD1c+ and CD141+ DCs subsets sorted from human blood were co-injected with allogeneic naïve CFSE-labeled T cells (3×105 DCs and 3×106 T cells) into human epithelial environments. Human epithelia were harvested 6 days later and analyzed by flow cytometry. Dot plots show the presence of CD3+CD8+ human T cells (upper plots) and the percentages of CFSE-negative CD103+ cells in gated CD3+CD8+ T cells (lower plots). One of two experiments shown. See also Figure S5.
Next, we determine whether CD1c+ DCs can elicit the differentiation of CD103+CD8+ T cells directly at the epithelial site in vivo. To this end, immunodeficient mice engrafted with human E-cadherin+ epithelial compartments were injected with human blood CD1c+ or CD141+ DCs and CFSE-labeled allogeneic naive T cells. Substantially higher percentages of T cells were present in human lung epithelial grafts reconstituted with CD1c+ DCs compared to lung epithelial grafts reconstituted with CD141+ DCs (Figure 5C). A majority of T cells adoptively transferred into epithelial compartments reconstituted with CD1c+ DCs had lost CFSE labeling, indicating that they had proliferated in vivo and acquired CD103 expression (Figure 5C). The differential accumulation of T cells in human epithelia reconstituted with CD141+ or CD1c+ DCs was not due to differential survival since the same numbers of T cells were present in the spleen of these mice (Figure S5). Thus, these results establish that CD1c+ DCs play a critical role in the generation and maintenance of epithelial CD103+CD8+ T cells in vivo.
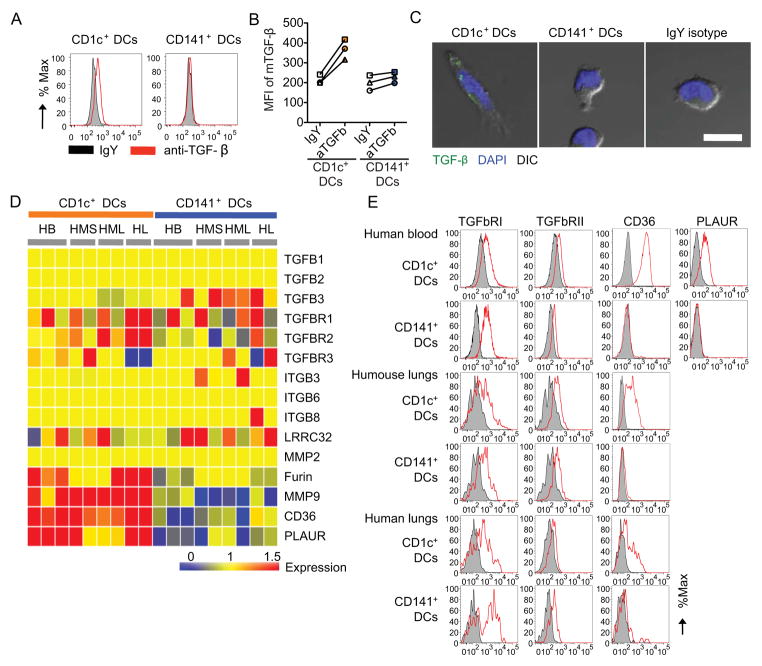
CD1c+ DCs elicit CD103+CD8+ T cells via TGF-β
Granulocyte macrophage colony stimulating factor (GM-CSF) has been shown to control CD103 expression and enhance cross-presentation function in murine DCs (Edelson et al., 2011; Zhan et al., 2011). However, GM-CSF blockade did not affect the ability of CD1c+ DCs to induce CD103 expression on CD8+ T cells in vitro (Figure S6). TGF-β1 controls CD103 expression on T cells in mice and humans (Parker et al., 1992; Rihs et al., 1996). Accordingly, flow cytometry analysis of blood DC subsets reproducibly showed that CD1c+ but not CD141+ DCs displayed membrane TGF-β1 expression (Figure 6A–B). This was further confirmed by confocal microscopy analysis (Figure 6C). To further analyze the contribution of TGF-β1 to CD1c+ DC-mediated regulation of CD103 in CD8+ T cells, we analyzed transcriptional profiles of CD1c+ and CD141+ DCs sorted from human blood and lungs, and from humanized mouse spleens and lungs at the steady state. As illustrated in Figure 6D, both DC subsets in all analyzed tissues displayed similar abundance of TGF-β and TGF-β receptor transcripts. Thus, both DC subsets in all analyzed tissues expressed TGF-β receptors by flow cytometry analysis (Figure 6E). These results suggested that CD1c+ DCs might be equipped with molecular machinery allowing the binding and activation of latent TGF-β1. Indeed, CD1c+ DCs in all tissues showed enhanced transcription of genes coding molecules involved in TGF-β1 activation including: furin (Dubois et al., 1995) and matrix metalloproteinase 9 (MMP-9) (Yu and Stamenkovic, 2000), involved in proteolytic cleavage of TGF-β1; CD36, a co-receptor involved in the interaction with thrombospondin-1 (Crawford et al., 1998; Silverstein et al., 1992; Wang et al., 2009, 2010); and PLAUR, urokinase plasminogen activator (CD87) that contributes to TGF-β1 activation in myeloid cells (Godar et al., 1999) (Figure 6D). The unique expression of CD36 and PLAUR by CD1c+ DCs was further confirmed by flow cytometry (Figure 6E).
Figure 6. TGF-β expression in CD1c+ DCs.
(A–B) Histogram represents membrane-bound TGF-β1 expression (red) and isotype control (black) in DC subsets isolated from human blood (A). Mean fluorescence intensity of TGF-β1 expression in gated human blood CD1c+ and CD141+ DCs from three different donors (B). (C) Immunofluorescence staining of TGF-β (green) on sorted human blood CD1c+ DCs (left panel) or CD141+ DCs (middle panel) and isotype control (green, right panel). Cells are defined by DAPI staining (blue) and phase contrast (grey). Scale bar=10 μm. (D) TGF-β related gene expression. Transcript intensity values are normalized to the median of all samples including DC subsets from human blood (HB), spleen (HMS) and lungs (HML) of humanized mouse, and human lungs (HL). Red transcripts are relatively over-abundant and blue transcripts under-abundant. (E) Histograms represent surface expression of TGF-β RI, TGF-β RII, CD36, and PLAUR on CD1c+ DCs and CD141+ DCs present in human blood, the lungs of humanized mice, and human lungs. One of two experiments shown. See also Figure S6.
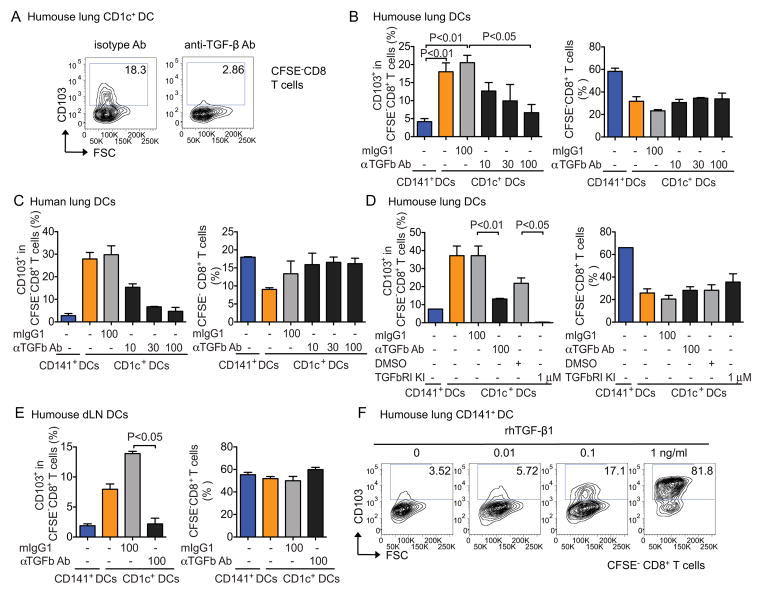
Consistent with the above finding, TGF-β1 neutralizing antibodies substantially reduced the ability of CD1c+ DCs to induce CD103 expression on CD8+ T cells in vitro (Figure 7A). The generation of CD103+CD8+ T cells by CD1c+ DCs purified from the lungs of humanized mice (Figure 7B) and humans (Figure 7C) was reproducibly blocked in a dose-dependent manner by TGF-β neutralizing antibodies, and nearly complete inhibition was observed at a dose of 100 μg/mL (Figure 7D). TGF-β-dependent expression of CD103 was observed in the experiments using DCs sorted from the lungs and lung-draining LNs of humanized mice as well as human lungs (Figure 7B-E). The involvement of TGF-β was further confirmed by the pharmacological blockade of TGF-β using TGF-β RI kinase inhibitor II (Ito et al., 2008) in DC-T cocultures (Figure 7D). Inhibition of CD103 expression was not associated with the global inhibition of T cell proliferation as shown in Figure 7B–E. Conversely, adding human recombinant TGF-β1 in co-cultures of allogeneic naïve T cells with CD141+ DCs led to dose-dependent expansion of CD103-expressing CD8+ T cells (Figure 7F). This, together with transcriptional profile analysis, suggests that the inability of CD141+ DCs to drive CD103 expression on CD8+ T cells might simply reflect their inability to present and activate TGF-β1. Altogether, these results establish that the unique ability of CD1c+ DCs to present membrane-bound TGF-β1 to CD8+ T cells contributes to their superior ability to promote the differentiation of CD103+CD8+ T cells and contribute to mucosal immunity.
Figure 7. Human lung CD1c+ DCs expand CD103+CD8+ T cells via TGF-β.
Sorted DCs were cocultured with CFSE-labeled allogeneic naïve T cells at a 1:100 ratio in the presence or absence of TGF-β1 Ab blockade as indicated. At day 6, CFSE−CD8+ T cells were analyzed for CD103 expression by flow cytometry. (A) Representative Scatter plots showing CD103 expression on CFSE−CD8+ T cells cocultured with CD1c+ DCs in the presence of 100 μg/ml of TGF-β neutralizing Abs or isotype control. (B–E) Percentages of CD103-expressing CFSE−CD8+ T cells and percentages of CFSE−CD8+ total T cells in cocultures with DC subsets isolated from the lungs of humanized mice (B) human lungs (C) in the presence of titrated doses of TGF-β neutralizing Abs or TGF-βRI kinase inhibitor (D). One representative experiment of more than three is shown (one-way ANOVA with Bonferroni post-test). Data are shown as mean ± SEM. (E) The same type of experiment as in (B) but DCs are sorted from the draining LNs of humanized mice. (F) Scatter plots illustrated CD103 expression on CFSE−CD8+ T cells cocultured with CD141+ DCs in the presence of recombinant human TGF-β1.
Discussion
DCs are composed of subsets that differ in their localization, phenotype, and functions. This appears to provide a critical path for the generation and maintenance of a highly diverse functional T cell repertoire including for example Th1, Th2, Th17 and Treg cells. However, the contribution of the distinct human DC subsets to this extensive functional T cell repertoire in vivo is not fully understood particularly as it relates to the generation of cytotoxic effector CD8+ T cells. In our humanized mouse model, intranasal vaccination with LAIV demonstrated that CD11c+CD14−CD303− DCs are able to present viral antigens to CD8+ T cells. As CD11c+CD14−CD303− DCs consist of two subsets that include CD1c+ and CD141+ DCs (Haniffa et al., 2012), the goal of the current study was to determine the contribution of CD1c+ and CD141+ DC subsets to the quality of the antiviral CD8+ T cell response.
The results show that human CD1c+ DCs, but not CD141+ DCs isolated from humanized mouse lungs and human lung explants, drive the differentiation of both naïve and memory CD8+T cells into CD8+ T cells expressing CD103. CD103 forms a heterodimer with the integrin β7 allowing peripheral CD8+ T cells to access the epithelial compartments (Shaw and Brenner, 1995; Sheridan and Lefrancois, 2011). CD103 specifically binds E-cadherin that is expressed on murine and human epithelial cells (Agace et al., 2000; Schon et al., 1999). The expression of CD103 by CD8+ T cells allows the retention of CD8+ T cell in the lungs in mice (Lee et al., 2011) and humans (Piet et al., 2011). Although lung-tissue-resident memory CD103+CD8+ T cells do not constitutively express perforin or granzyme B, they can rapidly upregulate these cytotoxic molecules upon TCR engagement (Smyth et al., 2007). The current study further established that CD1c+ DCs were uniquely able to induce the expression of CD103 on activated CD8+ T cells and enable their adherence to lung tissue sections ex vivo as well as their accumulation in vivo in a cell bed composed of human lung epithelial cell lines that are engrafted into immunodeficient mice. Following acute infection microbe-specific memory CD8+ T cells persist in the blood while accumulating in non-lymphoid tissues, where their numbers may actually exceed those in circulation (Sheridan and Lefrancois, 2011). These tissue-resident memory T cells can be superior to circulating central memory T cells at providing rapid protection against re-infection in mice (Gebhardt et al., 2009; Liu et al., 2010) and humans (Clark et al., 2012). Therefore, an active mechanism of T cell retention in the periphery likely exists not only to facilitate the clearance of infected cells but also to promote the accumulation of CD8+ T cells in sites where it is appropriate to prevent reinfection. Our results suggest that CD1c+ DCs in the mucosal sites might represent the master regulator of CD103 expression on CD8+ T cells thereby controlling their accumulation in the tissue.
The expression of CD103 on CD8+ T cells appears to mostly depend upon TGF-β1 (Parker et al., 1992; Rihs et al., 1996). Graft-versus-host disease with mice engineered to lack TGF-β receptor signaling demonstrated that the effector CD8+ T cells infiltrating the intestinal epithelium did not express CD103 and showed a lower pathogenic potential (El-Asady et al., 2005). Therefore our demonstration that human CD1c+ DCs utilize membrane-bound TGF-β1 to drive CD103 expression on proliferating CD8+ T cells, both in the allogeneic and autologous influenza-specific T cell responses, expands these data to the human setting. Indeed, flow cytometry analysis revealed that CD1c+ DCs but not CD141+ DCs express membrane TGF-β1 despite the fact that both subsets express transcripts involved in the production of TGF-β1. Interestingly, transcriptional profiling of DC subsets revealed that CD1c+ DCs in steady state display an array of transcripts coding molecules involved in the activation and cleavage of latent TGF-β1 in humans. These include furin and MMP9 and several molecules interacting with TSP-1 including CD36 (Crawford et al., 1998; Silverstein et al., 1992; Wang et al., 2009, 2010). CD36 binds TSP-1 which then binds latent TGF-β1 (Yehualaeshet et al., 2000). The lack of CD36 might explain why CD141+ DCs are less potent at driving CD103 expression on CD8+ T cells despite the local availability of human TGF-β1 stored in extracellular matrix or available in the serum. These unique molecular portraits of CD1c+ and CD141+ DCs are preserved across different tissues in both humans and humanized mice thereby suggesting that the capacity to regulate CD103 expression on CD8+ T cells represents an intrinsic feature of CD1c+ DCs rather than imprinting by tissue microenvironment.
Our results show that both CD1c+ DCs and CD141+ DCs are capable of influenza vaccine antigen presentation and that each subset generates CD8+ T cells with unique phenotypic and functional properties. These results further our understanding of the functional specializations of human DC subsets and their underlying molecular mechanisms. Earlier studies of human cutaneous DCs have demonstrated their phenotypic and functional heterogeneity (Morelli et al., 2005; Nestle et al., 1993; Valladeau and Saeland, 2005; Zaba et al., 2007). Our studies with human LCs and interstitial DCs concluded their specialization in priming CD8+ T cell immunity and humoral immunity, respectively (Caux et al., 1997; Klechevsky et al., 2008). They stressed the role of IL-15 in the priming of cytotoxic effectors and the role of interstitial DC-derived IL-12 in the priming of follicular helper T cells (Schmitt et al., 2009) and in the differentiation of plasma cells (Dubois et al., 1998). Recent studies have analyzed lymph-node-resident and skin-migratory DC subsets in the human (Chu et al., 2012; Haniffa et al., 2012; Segura et al., 2012). Both CD1c+ and CLEC9A-expressing CD141+ DCs isolated from human LNs were able to cross-present long peptides (requiring processing) and short peptides of melanoma-tissue-derived antigen (MART-1) to T cell lines (Segura et al., 2012). In agreement with earlier observations, blood DC subsets required activation via Toll-like receptor ligands to cross-present long peptides (Crozat et al., 2010; Jongbloed et al., 2010). Our results described herein bring a new perspective to the specialization of human DC subsets and reveal a novel function for the CD1c+ DC subset in driving peripheral T cell homeostasis in the steady state and after vaccination with attenuated virus. These results have implications for the design of novel vaccines aimed at promoting long-lived T cell immunity at epithelial interfaces. Furthermore, they strengthen the rationale for targeting specific DC subsets to generate desired types of immune responses.
Experimental Procedures
DC purification
Humanized mice were generated on NOD-SCID B2m−/− mice background as previously described (Yu et al., 2008). All protocols were approved by the Institutional Review Board (IRB, 097-053 and 099-076) and the Institutional Animal Care and Use Committee (A01-005) at Baylor Research Institute. At 4 weeks post transplant, humanized mice were either mock inoculated with PBS or vaccinated with LAIV (1/5 of human dose) via intranasal inoculation and harvested according to the experimental design. After flushing out the blood, the lungs were processed to prepare single-cell suspensions: they were digested with 2 mg/mL collagenase D (Roche Diagnostics, Indianapolis, IN) for 30 minutes at 37°C; suspensions were made and the debris was removed by filtering through a 70 μm cell strainer. Spleens and LNs were digested for 30 minutes using the same method. Cells were first treated with murine and human Fc blocker and then stained on ice with fluorochrome-conjugated specific Abs. DCs were sorted with FACSAria using Diva software (BD). Detailed antibodies and reagents were provided in the supplemental material.
Human lung tissues were obtained from consented patients undergoing lung resection surgery at The Mount Sinai Medical Center (New York, NY). Tissues were taken from non-involved lung areas in patients with pulmonary resection surgeries for either pulmonary metastatic disease or primary non-small cell lung cancer. All protocols were approved by IRB (08-1236-0001 through -0005) at MSSM. Care was taken to obtain lung tissue as distant as possible from any primary lesions and anatomic abnormalities and in a way to proportionally represent subpleural (distal) and deeper (proximal) anatomic regions of lung. Human lung tissue was processed analogously to the humanized mice protocol. Alternatively, DCs were first enriched using human Pan-DC Pre-Enrichment Kit (StemCell Technologies) before staining for cell sorting by flow cytometry.
PBMCs were isolated from leukapheresis products using Ficoll-Paque Plus density gradient centrifugation (Stemcell Technologies, Vancouver, Canada). DCs were first enriched using human Pan-DC Pre-Enrichment Kit before staining for cell-sorting by flow cytometry.
Autologous T cell assay
CD8+ T cells or total T cells were isolated from PBMCs autologous to CD34+ HPCs using human T cell enrichment kits (StemCell Technologies) following the manufacturer’s protocol. Isolated CD8+ T cells or total T cells had a purity >95%. T cells were labeled with 1 μM CFSE (Invitrogen) for 10 min at room temperature and cocultured with sorted DCs for 8 days in complete RPMI with 10% AB serum. Samples were stained with fluorochrome-conjugated Abs for surface markers and APC-conjugated FluM1 or NS1-HLA-A*0201 tetramer at room temperature for 30 min, acquired on an LSRII (BD), and analyzed with FlowJo software (Tree Star, Ashland, OR). In experiments with in vitro loading, sorted CD1c+ and CD141+ DC subsets were loaded with LAIV (moi=2) for 1 hour before cultured with T cells.
Allogeneic mixed lymphocyte reaction
Naive T cells were isolated from PBMCs of healthy volunteers using human naïve T cell enrichment kits (StemCell Technologies) and further sorted for CD45RA+CCR7+ T cells (>99% purity). CFSE-labeled naive T cells (100,000 cells) were cocultured with sorted DCs for 6 days. In TGF-β blocking experiments, DCs were first treated with anti-TGF-β antibodies (1D11, R&D Systems) for 30 min at 37°C and TGF-β RI kinase inhibitor II was added in the beginning of the coculture.
Microarrays
Total RNA was extracted using the RNAqueous-Micro Kit (Life technologies). Biotinylated, amplified cRNA targets were then prepared, hybridized to Illumina Human HT-12 V4 BeadChips and scanned following the manufacturer’s instructions (Illumina). GenomeStudio (Illumina) was then used to perform quality control, generate signal intensity values, and perform normalization by subtracting background and scaling average signal intensity for each sample to the global average signal intensity for all samples. Scaled and background subtracted data were analyzed using GeneSpring GX v 7.3.1 (Agilent Technologies). All signal values <10 were then set to a threshold level of 10. Next, per-gene normalization to the median intensity of all the samples was applied. Probes were filtered by ‘present’ call requiring signal intensity detection p-value less than 0.01 in at least one of all the samples for each tissue type. Raw microarray data has been deposited with GEO (Accession number GSE43184).
Stamper-Woodruff binding assay
The technique was modified from previous reports (Woodruff et al., 1977). We added 20,000 CFSE-labeled CD8+ T cells in 200 μl to the frozen human lung sections that were acetone-fixed and rinsed previously. T cells were allowed to settle for 1 hour at 37°C and unbound cells were subsequently removed by extensive washing with PBS. The sections were then further fixed with 4% paraformaldehyde, and stained with anti-cytokeratin-Alexa flour 568. Each experiment used sections from a single human lung using sequential sections. If antibody blocking was performed, T cells were pre-incubated with 20 μg/ml of anti-CD103 Abs or isotype control for 30 min before adding to the tissue sections. The binding capacity of T cells was measured by the numbers of CFSE+ cells in a 0.1 mm2 cytokeratin+ epithelial area using a fluorescence microscope (Olympus, Japan) with MetaMorph software (Molecular Devices, Sunnyvale, CA).
In vivo human lung graft experiment
H1650 (ATCC) human lung cancer cells (10 ×106) were harvested from cultures and injected subcutaneously into the flanks of sublethally irradiated NOD/SCID IL-2Rγ chain−/− mice. At day 3, CFSE-labeled CD8+ T cells were injected directly into human epithelial grafts and analyzed three days later for the presence of T cells by flow cytometry. If Ab blocking was performed, T cells in the human epithelial grafts were treated daily with 50 μg of anti-CD103 Abs or isotype control. In some experiments, both sorted DCs (3 ×105) and CFSE-labeled naïve T cells (3 ×106) were injected into human epithelial grafts, and the T cell phenotypes were analyzed at day 6.
Statistical analysis
Nonparametric tests were used for all group analyses. Differences in variables between any 2 groups were analyzed using the Mann-Whitney U test or two-tailed t-test. Differences between any 3 or more groups were analyzed by analysis of variance (ANOVA). Two-way ANOVA with Bonferroni post-tests was used to compare data with two variances. All statistics and graphs were done with Prism software (GraphPad, La Jolla, CA).
Supplementary Material
Highlights.
CD1c+ and CD141+ DC subsets populate human lungs and the lungs of humanized mice.
Both DCs acquire influenza antigens in vivo and expand specific CD8+ T cells.
Human CD1c+ DCs expand CD103+CD8+ mucosal T cells in vitro and in vivo.
CD1c+ DCs expand CD103+CD8+ T cells via membrane bound TGF-β.
Acknowledgments
We thank our patients and healthy volunteers; Drs. Chin, Weiser, Krellenstein, Kaufman and Flores (Cardiothoracic Surgery); Dr. Harkin (Department of Medicine); and Drs. Gil, Beasley and the entire staff (Department of Pathology), MSSM; Dr. Fay, the Clinical Core, the Apheresis Core, the Flow Cytometry Core, the Imaging Core, the Genomic Core and the Animal Facility team at BIIR. CB thanks Dr. M. Padilla and the late Dr. A. S. Teirstein of MSSM. Supported by: Lucille A. Fennessy Pulmonary Research Fund, the Vivian Richenthal Institute for Pulmonary and Critical Care Research, and NIH training grants (T32AI007623-10, and 5T32CA078207-12) CB; the NIH grants to KP: U19AI057234, U19AI089987 and UO1AI095611 and to MM: R01HL086899, R01AI095611, and UO1AI095611.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–568. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Thompson-Snipes L, Zurawski S, Blanck JP, Cao Y, Clayton S, Gorvel JP, Zurawski G, Klechevsky E. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood. 2012;119:5742–5749. doi: 10.1182/blood-2011-08-371245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Chu CC, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, Barinaga G, Grys K, Sharif-Paghaleh E, Karagiannis SN, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med. 2012;209:935–945. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol. 2005;32:177–184. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
- Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Bradstreet TR, Kc W, Hildner K, Herzog JW, Sim J, Russell JH, Murphy TL, Unanue ER, Murphy KM. Batf3-dependent CD11b(low/−) peripheral dendritic cells are GM-CSF-independent and are not required for Th cell priming after subcutaneous immunization. PLoS One. 2011;6:e25660. doi: 10.1371/journal.pone.0025660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glader PS, Lofdahl CG, von Wachenfeldt KA. alphaEbeta7 expression on CD8+ T-cells in COPD BAL fluid and on TGF-beta stimulated T-cells in vitro. Lung. 2005;183:123–138. doi: 10.1007/s00408-004-2528-x. [DOI] [PubMed] [Google Scholar]
- Godar S, Horejsi V, Weidle UH, Binder BR, Hansmann C, Stockinger H. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta1. Eur J Immunol. 1999;29:1004–1013. doi: 10.1002/(SICI)1521-4141(199903)29:03<1004::AID-IMMU1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, et al. Human Tissues Contain CD141(hi) Cross-Presenting Dendritic Cells with Functional Homology to Mouse CD103(+) Nonlymphoid Dendritic Cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O’Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Rubin JP, Erdos G, Tkacheva OA, Mathers AR, Zahorchak AF, Thomson AW, Falo LD, Jr, Larregina AT. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–7915. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Zheng X-G, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–6545. [PubMed] [Google Scholar]
- O’Doherty U, Steinman RM, Peng M, Cameron PU, Gezelter S, Kopeloff I, Swiggard WJ, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, Gatlin J, Blanck JP, Melkus MW, Clayton S, Ueno H, Kraus ET, Cravens P, Bennett L, Padgett-Thomas A, et al. Human dendritic cell subsets in NOD/SCID mice engrafted with CD34+ hematopoietic progenitors. Blood. 2003;102:3302–3310. doi: 10.1182/blood-2003-02-0384. [DOI] [PubMed] [Google Scholar]
- Parker CM, Cepek KL, Russell GJ, Shaw SK, Posnett DN, Schwarting R, Brenner MB. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1924–1928. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet B, de Bree GJ, Smids-Dierdorp BS, van der Loos CM, Remmerswaal EB, von der Thusen JH, van Haarst JM, Eerenberg JP, ten Brinke A, van der Bij W, et al. CD8 T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011;121:2254–2263. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs S, Walker C, Virchow JC, Jr, Boer C, Kroegel C, Giri SN, Braun RK. Differential expression of alpha E beta 7 integrins on bronchoalveolar lavage T lymphocyte subsets: regulation by alpha 4 beta 1-integrin crosslinking and TGF-beta. Am J Respir Cell Mol Biol. 1996;15:600–610. doi: 10.1165/ajrcmb.15.5.8918367. [DOI] [PubMed] [Google Scholar]
- Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F, Fink MJ, St Angelo ET, Mehrara B, Heller G, et al. Human Langerhans cells use an IL-15R-alpha/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood. 2012;119:5182–5190. doi: 10.1182/blood-2011-09-382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012 doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SK, Brenner MB. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995;7:335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL, Baird M, Lo SK, Yesner LM. Sense and antisense cDNA transfection of CD36 (glycoprotein IV) in melanoma cells. Role of CD36 as a thrombospondin receptor. J Biol Chem. 1992;267:16607–16612. [PubMed] [Google Scholar]
- Smyth LJ, Kirby JA, Cunningham AC. Role of the mucosal integrin alpha(E)(CD103)beta(7) in tissue-restricted cytotoxicity. Clin Exp Immunol. 2007;149:162–170. doi: 10.1111/j.1365-2249.2007.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. Decisions About Dendritic Cells: Past, Present, and Future. Annu Rev Immunol. 2011 doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, Oh S, Fay J, Pascual V, Banchereau J, Palucka K. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- van der Aar AM, de Groot R, Sanchez-Hernandez M, Taanman EW, van Lier RA, Teunissen MB, de Jong EC, Kapsenberg ML. Cutting edge: virus selectively primes human langerhans cells for CD70 expression promoting CD8+ T cell responses. J Immunol. 2011;187:3488–3492. doi: 10.4049/jimmunol.1101105. [DOI] [PubMed] [Google Scholar]
- van der Vlist M, de Witte L, de Vries RD, Litjens M, de Jong MA, Fluitsma D, de Swart RL, Geijtenbeek TB. Human Langerhans cells capture measles virus through Langerin and present viral antigens to CD4(+) T cells but are incapable of cross-presentation. Eur J Immunol. 2011;41:2619–2631. doi: 10.1002/eji.201041305. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen Y, Lv L, Chen J. Silencing CD36 gene expression results in the inhibition of latent-TGF-beta1 activation and suppression of silica-induced lung fibrosis in the rat. Respir Res. 2009;10:36. doi: 10.1186/1465-9921-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen Y, Lv L, Chen J. Inhibition of latent transforming growth factor-beta1 activation by lentivirus-mediated short hairpin RNA targeting the CD36 gene in NR8383 cells. Mol Biol Rep. 2010;37:1649–1655. doi: 10.1007/s11033-009-9579-2. [DOI] [PubMed] [Google Scholar]
- Yehualaeshet T, O’Connor R, Begleiter A, Murphy-Ullrich JE, Silverstein R, Khalil N. A CD36 synthetic peptide inhibits bleomycin-induced pulmonary inflammation and connective tissue synthesis in the rat. Am J Respir Cell Mol Biol. 2000;23:204–212. doi: 10.1165/ajrcmb.23.2.4089. [DOI] [PubMed] [Google Scholar]
- Yu CI, Gallegos M, Marches F, Zurawski G, Ramilo O, Garcia-Sastre A, Banchereau J, Palucka AK. Broad influenza-specific CD8+ T-cell responses in humanized mice vaccinated with influenza virus vaccines. Blood. 2008;112:3671–3678. doi: 10.1182/blood-2008-05-157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Carrington EM, van Nieuwenhuijze A, Bedoui S, Seah S, Xu Y, Wang N, Mintern JD, Villadangos JA, Wicks IP, Lew AM. GM-CSF increases cross-presentation and CD103 expression by mouse CD8(+) spleen dendritic cells. Eur J Immunol. 2011;41:2585–2595. doi: 10.1002/eji.201141540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.