Abstract
This commentary explores the challenges surrounding the development of a standard definition of lymphedema and method of quantification, proposes solutions, and calls for a collaborative effort among providers who care for patients with breast cancer.
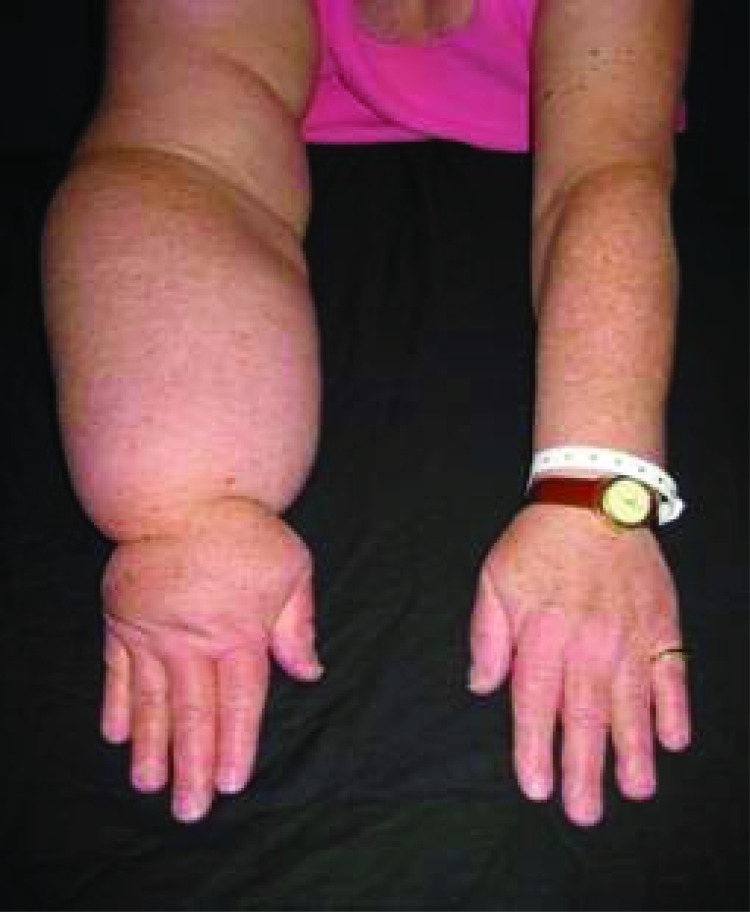
With an overall survival rate of 90% following treatment for breast cancer, post-treatment management has been increasingly focused on sequelae that negatively affect quality of life. Breast cancer-related lymphedema (BCRL) has long been recognized as a dreaded side effect of treatment; it can result in disfigurement, altered body image, pain, functional impairment, and emotional distress (Fig. 1) [1–3]. The risk of developing BCRL lasts for a lifetime, and the financial implications and burden of caring for this condition are well documented and significant [4, 5].
Figure 1.
This woman's right arm is 54% larger than her left, demonstrating the physical distortion of a limb with advanced lymphedema.
Despite the decreasing use of axillary dissection, lymphedema has been reported in patients with sentinel node biopsy, and many patients still require an axillary dissection [1, 6]. The literature frequently recommends regular screening for BCRL to allow for early detection, and this approach is now the official position of the National Lymphedema Network [7]. This is an exciting proposal and a worthy goal; however, much work needs to be done if screening and early detection are to be universally successful. Of particular importance is the need to achieve consensus regarding the most accurate method to quantify changes in arm volume, as well as to come to agreement on the definition of clinically significant lymphedema. This commentary explores the challenges surrounding the development of a standard definition of lymphedema and method of quantification, proposes solutions, and calls for a collaborative effort among providers who care for patients with breast cancer to resolve current controversies.
Multiple articles cite the inconsistencies in methods of quantification as impeding the reported incidence of BCRL [1–3, 8]. All approaches measure the arm on the side of surgery and the contralateral arm for comparison, but the methods are not interchangeable. Assessing limb size by circumferential measurement with a tape measure is an easy method to use in an office setting. Another technique is measuring limb volume by water displacement, which involves submerging the limb into a cylinder of water. Although water displacement has been reported to be a reliable method for quantifying limb volume, emptying, cleaning, and disinfecting the cylinder between patients can be messy and time consuming.
The perometer is a device that has been reported to be a valid and reliable tool to quantify limb volume. It contains a frame of infrared lights that takes readings of the limb at 0.5-cm increments. These measurements are converted to an overall arm volume by the software attached to the perometer [9–11]. Bioimpedance spectroscopy (BIS) is yet another method that has been found to be reliable for quantifying low-level edema by measuring the electrical impedance of interstitial fluid in the arms, with the outcome expressed as an impedance value in standard deviations from normal values [12]. With these variations in measurement methodology, it is not possible to accurately compare data. Standardization must be established if screening programs are to successfully report data that will contribute to advances in our knowledge of BCRL incidence.
This unresolved problem regarding quantifying lymphedema is further complicated as the differing methods express limb size changes as either an absolute difference or a relative volume difference between the two arms. When lymphedema is expressed as an absolute difference, a frequently used method to indicate the presence of lymphedema is to refer to a centimeter difference between arms. However, choice of measurement sites on the arm varies among examiners; some examiners take the reading at two points, whereas other examiners take circumferential measurements at multiple sites along the arm. The other method of reporting lymphedema as an absolute difference is by water displacement. With this method, the milliliter difference between arms is used as the indication of lymphedema. Not only are these two methods measuring different variables, measuring edema as an absolute volume difference can produce false-positive and false-negative results, depending on the shape and size of the limb [13].
Conversely, lymphedema can be quantified as a relative volume change by referring to a percent difference between the arms. This is the ideal method for lymphedema quantification because it is not dependent on body habitus [13]. Percent difference can be calculated by a device such as the perometer or can be mathematically calculated from circumferential measurements in the equation for a truncated cone to determine the raw values of the arms. BIS reports the presence of lymphedema as a ratio of impedance values between arms, usually with an impedance ratio greater than three standard deviations above normative data as an indication of lymphedema [14]. Measured impedance is often converted to a corresponding lymphedema index (referred to as L-Dex) which is a representation of extracellular fluid in the arms rather than overall arm volume. Regardless of the measurement method used (circumferences converted to percent or perometer), universally referring to lymphedema as a relative volume difference between arms would be a significant contribution in resolving one of the barriers in lymphedema research.
Another essential practice in screening for BCRL is to measure all patients at the time of diagnosis, prior to surgical intervention. This allows for the natural asymmetry that approximately 20% of patients may have between their arms to be accounted for when interpreting volume changes postoperatively. Without this baseline measurement to use as a reference point, postoperative changes cannot be accurately assessed. Universally integrating preoperative arm volume measurements and calculating a relative arm volume difference as the standard of care would be a large step in moving this field of research forward.
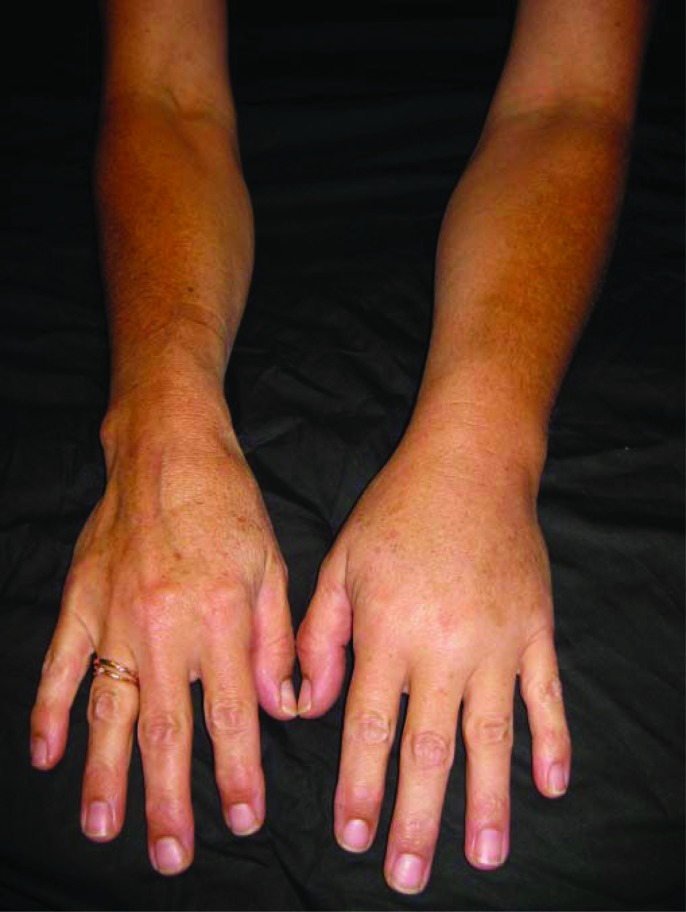
The lack of a universal definition of clinically significant lymphedema is an ongoing barrier to comparing data and conducting research investigating best management strategies. The most common absolute differences reported are either 2 cm or 200 mL when comparing limbs. Relative volume differences deemed to indicate lymphedema range from 3%–10% in the literature [2, 15–17]. Also worthy of consideration in determining the definition of clinically significant lymphedema is the correlation of symptoms, function, and pain in addition to objectively measured limb volume [18]. It is particularly important to take into account patient-reported symptoms when assessing for lymphedema, as growing evidence suggests that even small changes in the limb can be symptomatic (Fig. 2) [8]. Evidence that would clarify the level of edema that is symptomatic would be a significant contribution to the definition of clinical lymphedema and could be an outcome of a successful screening program.
Figure 2.
The left arm of this woman is 9% larger than the right and is symptomatic, illustrating a low-level swelling that is an impairment.
Screening for BCRL would allow for the study of its natural history and provide the opportunity to evaluate rates and risk factors for progression. It would be the mechanism to determine whether lymphedema can present as a transient occurrence, as has been suggested, and to identify patients who are at highest risk of progression. Screening will help to answer questions such as the following:
What relative volume difference is indicative of persistent lymphedema and warrants intervention?
Should treatment be initiated as soon as edema is identified or should clinicians monitor the patient to see if the trend progresses, as is often done for blood pressure screening?
Collaboration in conducting this type of research through proactive screening programs could lead to consensus regarding the definition of clinically significant lymphedema and to the most appropriate level of edema at which intervention should be initiated. Initiating treatment too early may add significant financial burden to patients and may negatively affect the health care system due to unnecessary use of resources. However, not intervening early enough may risk progression and the financial impact of managing established lymphedema. A review by Shih et al. of medical claims for 1,877 patients with breast cancer over 2 years reported that the cost for patients with lymphedema was twice as high as for survivors without lymphedema [5]. These expenses were attributed to episodes of lymphangitis, cellulitis, outpatient visits, increased imaging and mental health services. Stout et al. reported the annual per-patient cost of a surveillance model as $636.19 compared to $3,124.92 for the traditional model that addresses lymphedema at a later stage [4].
The lack of standardization is a significant barrier that, if continued, will limit the ability to move the knowledge in this field forward. Resolving these inconsistencies will facilitate the ability to compare data from multiple programs. Surveillance programs designed to collect reliable evidence regarding natural history, early detection, and appropriate interventions would greatly advance the care of survivors of breast cancer.
Our institution is conducting a clinical lymphedema screening trial in tandem with a prospective, randomized phase III trial investigating the efficacy of early detection and intervention, as well as optimal treatment strategies. Screening trial participants have newly diagnosed breast cancer and are prospectively screened for lymphedema via perometry and completion of a symptom, function, and quality-of-life questionnaire. Screening occurs preoperatively, at the completion of chemotherapy and/or radiation therapy, and every 3–8 months thereafter (Fig. 3). When a subject has a relative volume change ≥5% at two consecutive visits, they are eligible for the phase III trial to assess the outcome of early intervention.
Figure 3.
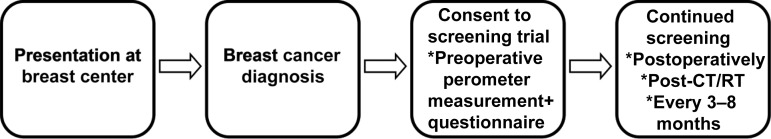
The lymphedema screening trial being conducted at Massachusetts General Hospital involves preoperative perometer measurements and completion of a symptom, function, and quality-of-life questionnaire. Screening continues postoperatively, at the completion of chemotherapy and/or radiation therapy, and every 3–8 months thereafter.
Abbreviations: CT, chemotherapy; RT, radiation therapy.
With multiple centers engaged in a collaborative effort, further research regarding effective interventions could be pursued. For example, although complete decongestive therapy has been cited as the standard of care for BCRL, there is no level 1 evidence to support this approach. Standardization of the method of quantification and definition of clinically significant lymphedema and the implementation of programs designed to generate level 1 evidence, would allow providers to base clinical decisions on solid evidence rather than anecdotal experience.
Author Contributions
Conception/Design: Jean O'Toole, Lauren S. Jammallo, Cynthia L. Miller, Melissa N. Skolny, Michelle C. Specht, Alphonse G. Taghian
Provision of study materials or patients: Jean O'Toole, Lauren S. Jammallo, Cynthia L. Miller, Melissa N. Skolny, Michelle C. Specht
Manuscript writing: Jean O'Toole, Lauren S. Jammallo, Cynthia L. Miller, Melissa N. Skolny, Michelle C. Specht, Alphonse G. Taghian
Final approval of manuscript: Alphonse G. Taghian
Disclosures
The authors indicated no financial relationships.
References
- 1.Bernas MJ, Askew RL, Armer JM, Cormier JN. Lymphedema: How do we diagnose and reduce the risk of this dreaded complication of breast cancer treatment? Curr Breast Cancer Rep. 2010;2:53–58. [Google Scholar]
- 2.Armer JM, Stewart BR. Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43:118–127. [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes S, Janda M, Cornish B, et al. Lymphedema secondary to breast cancer: How choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology. 2008;41:18–28. [PubMed] [Google Scholar]
- 4.Stout NL, Pfalzer LA, Springer B, et al. Breast cancer-related lymphedema: Comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92:152–163. doi: 10.2522/ptj.20100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J Clin Oncol. 2009;27:2007–2014. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 6.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102:111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Lymphedema Network. Position statement of National Lymphedema Network: Screening and measurement for early detection of breast cancer related lymphedema. [Accessed January 4, 2013]. Available at http://www.lymphnet.org/pdfDocs/nlnBCLE.pdf.
- 8.Cormier JN, Xing Y, Zaniletti I, et al. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009;42:161–175. [PMC free article] [PubMed] [Google Scholar]
- 9.Stanton AW, Northfield JW, Holroyd B, et al. Validation of an optoelectronic limb volumeter (Perometer) Lymphology. 1997;30:77–97. [PubMed] [Google Scholar]
- 10.Jain MS, Danoff JV, Paul SM. Correlation between bioelectrical spectroscopy and perometry in assessment of upper extremity swelling. Lymphology. 2010;43:85–94. [PMC free article] [PubMed] [Google Scholar]
- 11.Tierney S, Aslam M, Rennie K, et al. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412–417. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 12.Ward LC, Dylke E, Czerniec S, et al. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol. 2011;9:47–51. doi: 10.1089/lrb.2010.0014. [DOI] [PubMed] [Google Scholar]
- 13.Ancukiewicz M, Miller CL, Skolny MN, et al. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: The flaws in current studies and need for universal methodology. Breast Cancer Res Treat. 2012;135:145–152. doi: 10.1007/s10549-012-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes S, Sipio TD, Rye S, et al. Prevalence and prognostic significance of secondary lymphedema following breast cancer. Lymphat Res Biol. 2011;9:135–141. doi: 10.1089/lrb.2011.0007. [DOI] [PubMed] [Google Scholar]
- 15.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 16.Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol. 2010;49:166–173. doi: 10.3109/02841860903483676. [DOI] [PubMed] [Google Scholar]
- 17.Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: A meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–1972. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 18.Hormes JM, Bryan C, Lytle LA, et al. Impact of lymphedema and arm symptoms on quality of life in breast cancer survivors. Lymphology. 2010;43:1–13. [PubMed] [Google Scholar]