Recurrence risk perceptions after 5 years were evaluated in women with a history of ductal carcinoma in situ. Many women were found to harbor inaccurate perceptions of their risk for future breast cancer events even 5 years after diagnosis.
Keywords: Carcinoma, Intraductal, Noninfiltrating, Survivors, Anticipation, Psychological, Anxiety
Learning Objectives
Identify predictors of excessive risk perception in women with a distant history of DCIS.
Explain the importance of educating women with a history of DCIS about reasonable assessments of their risk for future breast cancer.
Abstract
Introduction.
Previous research has demonstrated that many women with ductal carcinoma in situ (DCIS) overestimate their risk for future breast cancer at the time of diagnosis and soon thereafter. This study aims to evaluate risk perceptions after 5 years.
Patients and Methods.
In a longitudinal cohort study, we mailed long-term follow-up surveys to 315 women who had previously responded to a survey 18 months after they were diagnosed with DCIS, excluding those who had experienced recurrence and those not treated at our institution. We evaluated risk perceptions with items used previously in the cohort.
Results.
One hundred ninety-three women (61%) responded. The median time since diagnosis was 5.9 years. We excluded 12 because of recurrence. Of the 181 remaining, 32% perceived at least a moderate 5-year risk for developing DCIS again, 43% perceived at least a moderate lifetime risk for developing DCIS again, 27% perceived at least a moderate 5-year risk for invasive breast cancer, 38% perceived at least a moderate lifetime risk for invasive breast cancer, and 24% perceived at least a moderate risk for DCIS spreading to other body parts. In a multivariate model, worse financial status and higher perceived risk in the previous survey were the only predictors of at least a moderate perception of risk for DCIS spreading.
Conclusion.
Women with a history of DCIS continue to harbor inaccurate perceptions of their risk for future breast cancer events even 5 years after diagnosis.
Implications for Practice:
Five years after being diagnosed with ductal carcinoma in situ (noninvasive breast cancer), many women overestimate their risks of having breast cancer in the future. This may cause unnecessary distress and impair quality of life in these survivors, who actually have a low likelihood of experiencing future breast cancer events (particularly life-threatening ones such as breast cancer that spreads to other parts of the body). This study found that financial discomfort and less education were associated with certain heightened perceived risks, suggesting that medical professionals may more effectively communicate accurate risk data to more affluent and more educated women, or that these women might have more access to other reliable sources of information. Clinicians should inquire and attempt to correct the frequently inaccurate perceptions of risk harbored by many long-term survivors of ductal carcinoma in situ.
Introduction
Ductal carcinoma in situ (DCIS) is noninvasive breast cancer that, after appropriate treatment, carries a relatively low risk for local recurrence and very small risk for distant spread [1, 2]. Recurrence rates are 5%–32%, depending on the grade of the DCIS, the treatment received, and the length of follow-up. These are primarily local recurrences. Only 1%–2% of women develop metastatic disease after treatment for DCIS [2, 3]. In a recent study of 1,701 DCIS patients, the 12-year rates of in-breast recurrence (ipsilateral or contralateral) after lumpectomy were 32% for those who received no adjuvant therapy, 24% for those who received tamoxifen alone, 13% for those who received radiation alone, and 10% for those who received radiation plus tamoxifen. Half of these recurrences were invasive disease [3]. According to an abstract presented by Cuzick and colleagues in 2009, the risk for recurrence of DCIS or invasive cancer 5–10 years after the initial diagnosis of DCIS was only 5%–8%. Race and ethnicity do not appear to impact the risk for recurrence after treatment for DCIS [4], but younger age has been found to correlate with a greater risk for local recurrence. A recent evaluation of 2,037 DCIS patients revealed a 5-year local recurrence rate of 10.1% in women aged <40 years and a rate of only 3.2% in older women [5]. Another study of DCIS patients after lumpectomy and radiation demonstrated that only 1.4% experienced ipsilateral breast recurrence 5–10 years after diagnosis, 3.9% experienced contralateral recurrence, and none experienced distant disease [6]. After mastectomy, the rate of new breast cancer diagnosis is even lower (∼1% over 10 years) [7].
In 800 breast cancer patients, mental health-related quality of life (HRQoL) and psychiatric symptoms were comparable between women with DCIS and those with invasive disease at 1 month, 6 months, and 12 months after diagnosis, despite the substantially better prognosis of those with DCIS [8]. Despite the low risk for recurrence, soon after a diagnosis of DCIS, many women believe that their risk for local or distant recurrence of breast cancer is substantial, and inaccurate risk perceptions have been associated with greater anxiety [9]. Little is known about whether or not these risk perceptions and anxiety decrease over time. Given that the rates of recurrence are lower 5–10 years after diagnosis than they are during the first 5 years, we would expect that patient perceptions of risk would be lower later in follow-up.
To test this, we evaluated risk perceptions among women who had been diagnosed with DCIS ∼5 years prior. In earlier reports from this cohort, we showed that, soon after diagnosis, 28% of women perceived at least a moderate likelihood of DCIS spreading to other places in their bodies [9]. Anxiety level was strongly associated with perceived risk, and risk perceptions did not change substantially in an 18-month follow-up. In a separate analysis focusing on predictors of anxiety and depression 9 months after diagnosis, we found that high financial status was associated with less anxiety and depression [10]. Here, we report on risk perceptions and psychological states in these same women later in the survivorship period.
Materials and Methods
As described previously, consecutive women with DCIS diagnosed within the prior 6 months were identified from October 15, 2000 to May 3, 2004 via pathology report review in 9 participating hospitals in eastern Massachusetts [9]. The study received institutional review board approval through each participating hospital. Patients were eligible if they had a diagnosis of DCIS without microinvasive or invasive disease, had no breast surgery earlier than 3 months prior to enrollment (because the goal was to collect the initial survey data close to the diagnosis), and were able to understand written and spoken English or Spanish. After obtaining passive physician permission to contact potential participants (i.e., physicians were offered the opportunity to refuse participation for any reason for each individual patient), we mailed a consent form to each eligible woman. Those who consented were surveyed by mail at the time of enrollment and at 9 and 18 months after diagnosis. The original consent form did not include a later survey; eligible participants who had completed the 18-month survey were mailed a letter requesting participation in a long-term follow-up survey ∼5 years after diagnosis. Here, we describe the results of this long-term follow-up survey (which was included in the mailing with the letter). This analysis was restricted to women cared for at the Dana-Farber/Harvard Cancer Center institution (Massachusetts General Hospital, Dana-Farber Cancer Institute, Brigham and Women's Hospital, or Beth Israel Deaconess Medical Center) to ensure access to complete data on recurrences via medical record review.
Measures
Surveys included 225 items pertaining to sociodemographics, treatments, risk perceptions, exercise, medical history, sexual functioning, body image, and quality of life. Psychological state was assessed using the Hospital Anxiety and Depression Scale (HADS) and the Impact of Event Scale (IES), and HRQoL was assessed using the Physical and Mental Health Component Summary (PCS and MCS) scales of the Medical Outcomes Short Form Survey (SF-36). These methods were described previously [9, 10]. There were five items about qualitative risk perception, all of which had been used in the prior surveys from this study:
In your opinion, how likely is it that: (a) Your DCIS will spread to other places in your body in your lifetime? (b) You will develop DCIS again within the next 5 years compared with other women your age? (c) You will develop DCIS again within your lifetime? (d) You will develop invasive breast cancer within the next 5 years compared with other women your age? and (e) You will develop invasive breast cancer within your lifetime?
Respondents were asked to select among five qualitative options for each question (“very unlikely,” “unlikely,” “moderate chance,” “likely,” or “very likely”) and also to estimate these risks quantitatively as a percent likelihood on a scale of 0–100.
Statistical Analysis
Descriptive statistics were used to characterize the population. Univariate comparisons of sociodemographic, disease, and treatment characteristics of responders to nonresponders used Fisher's exact or Wilcoxon rank-sum testing to assess biases among the responders. To assess factors that were most influential in determining survey response, a multivariate logistic regression model was fit to sociodemographic, disease, and treatment characteristics. Forward, backward, and stepwise variable selection methods were employed to assess model consistency, with p ≤ .05 used as the criterion for variable inclusion. Based on medical record review and survey data, responders who had experienced a recurrence were not included in subsequent analyses. Missing data were not imputed for covariates in any of the models. Continuous variables were recoded as categorical variables and a category of “missing/unknown” was created for each covariate with missing data.
We described the proportion of patients who chose each qualitative answer for each of the five risks and the medians and ranges for those participants' estimated quantitative perceived risks. Each of the five perceived risks was modeled using multivariate logistic regression, with risk dichotomized as (a) “moderate,” “likely,” or “very likely” versus (b) “very unlikely” or “unlikely.” If a risk perception outcome was missing at any point, it was categorized in the lower group to avoid overestimating risk perception. Independent variables considered for inclusion in each multivariate model were age at baseline (≤65 years vs. >65 years), race (white vs. nonwhite), education (at least college graduate vs. less than college graduate), DCIS grade (1, 2, 3, or missing), presence of comedonecrosis (yes vs. no), marital status (married or living as married vs. other), employment status (full time vs. not full time), financial status (money for special things vs. no money for special things), comorbidities that interfere with activities (yes vs. no), HADS anxiety score (≥11 vs. <11), IES score (≥26 vs. <26), mastectomy (yes vs. no), radiation treatment (yes vs. no), time since DCIS diagnosis, and the respective risk perception recorded at the 18-month assessment. The HADS depression score was not included in the models because of a very low incidence of scores consistent with depression (≥11). Demographic and treatment data (e.g., marital status, employment status, comorbidities, financial status, HADS anxiety, IES, mastectomy) from the long-term follow-up survey were used in the models to reflect the most current patient status. Standard model-building techniques were used to determine factors associated with each of the five perceived risks. First, univariate comparisons were examined and variables were recoded for simplicity. Variables with a univariate p value ≤ .2 were selected for inclusion into each multivariate logistic regression model. Mastectomy status was forced into each multivariate model because mastectomy reduces the true risk for DCIS recurrence so substantially. Forward, backward, and stepwise variable selection methods were employed to assess model consistency, with p ≤ .05 used as the criterion for variable inclusion. An odds ratio and 95% Wald confidence interval (CI) were calculated for each significant predictor in the models. The C-index assessed the discriminatory ability of each model. In an exploratory analysis, we refit models with age divided at the median of 54 years to more carefully assess the influence of age on risk perception.
To assess whether or not perception of the risk for DCIS spreading throughout the body was an important predictor of HRQoL, norm-based scores of the PCS and MCS scales in long-term follow-up were modeled separately using multivariate linear regression. By construct, each score is normalized to have a mean value of 50 and a standard deviation (SD) of 10 using published U.S. population norms. Age, race, education, DCIS grade, comedonecrosis, marital status, employment, financial status, comorbidities, HADS score, IES score, mastectomy, radiation, and time since DCIS diagnosis were included in the models as possible confounders. All statistical testing was two sided with statistical significance defined as a p value ≤ .05. There were no adjustments for multiple comparisons. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Study Population
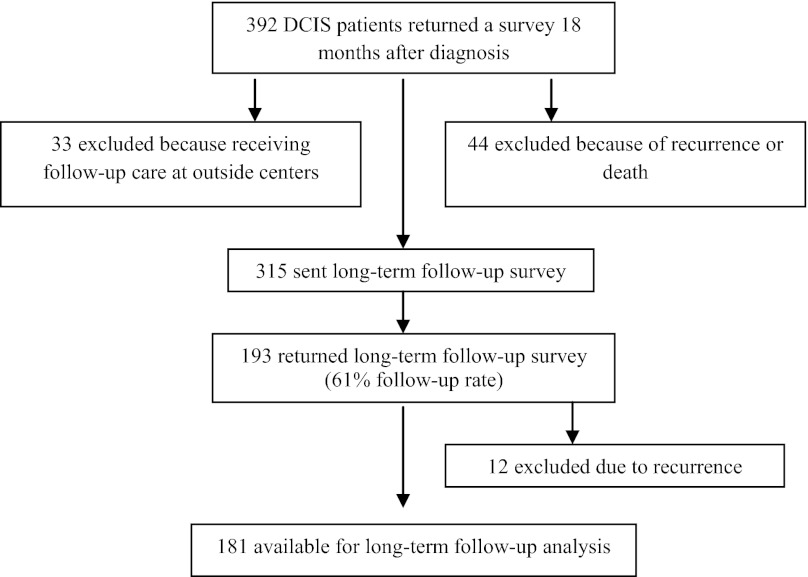
Eight hundred sixteen women with newly diagnosed DCIS were originally identified for this study and 764 were invited to participate. Four hundred eighty-seven responded to the initial survey, 426 responded to the 9-month survey, and 392 responded to the 18-month survey. We excluded 33 because they were not Dana-Farber/Harvard Cancer Center patients, precluding medical record review. We subsequently also excluded 44 after medical record review revealed breast cancer recurrence or death. We mailed the long-term follow-up survey to 315 women; 14 (4%) declined participation, 108 (34%) did not respond, and 193 (61%) returned the survey (Fig. 1). Of the women who responded to the follow-up survey, 12 reported that they had had a recurrence (6 invasive and 6 DCIS alone). These women were excluded from further analysis, yielding a final sample size of 181.
Figure 1.
Flow diagram of participants.
Abbreviation: DCIS, ductal carcinoma in situ.
Eligible respondents and nonrespondents were compared using data from their enrollment surveys. Nonrespondents were younger, less likely to be college graduates, less likely to be financially comfortable, less likely to have received radiation, and more likely to have had a mastectomy. At trend level, a higher proportion of nonrespondents had reported that they were “likely” to develop invasive disease within 5 years at enrollment (Table 1).
Table 1.
Eligible respondents versus nonrespondents

Table 1.
(Continued)

aResults presented in Table 1 are based on data from the baseline survey.
Abbreviations: HADS, Hospital Anxiety and Depression Scale; IES, Impact of Event Scale; MCS, Mental Component Summary; PCS, Physical. Component Summary; SF-36, Medical Outcomes Short Form Survey.
Risk Perceptions
Overall, 24% of the participants perceived their risk for DCIS spreading to other places in their body to be at least moderate, 32% perceived at least a moderate risk for developing DCIS again within 5 years, 43% perceived at least a moderate lifetime risk for developing DCIS again, 27% perceived at least a moderate risk for developing invasive breast cancer within 5 years, and 38% perceived at least a moderate lifetime risk for developing invasive breast cancer (Table 2). Compared with the surveys conducted at enrollment and after 18 months, the proportion of women who perceived their risk to be at least moderate decreased for all outcomes other than spread to other parts of the body (Table 3). The median reported quantitative risks were 5% for DCIS recurrence within 5 years, 5% for invasive cancer within 5 years, 10% for DCIS over a lifetime, 10% for invasive cancer over a lifetime, and 9% for spread of DCIS to other parts of the body.
Table 2.
Qualitative and quantitative risk perceptions in long-term follow-up

Abbreviation: DCIS, ductal carcinoma in situ.
Table 3.
Proportion reporting at least moderate risk perception over time
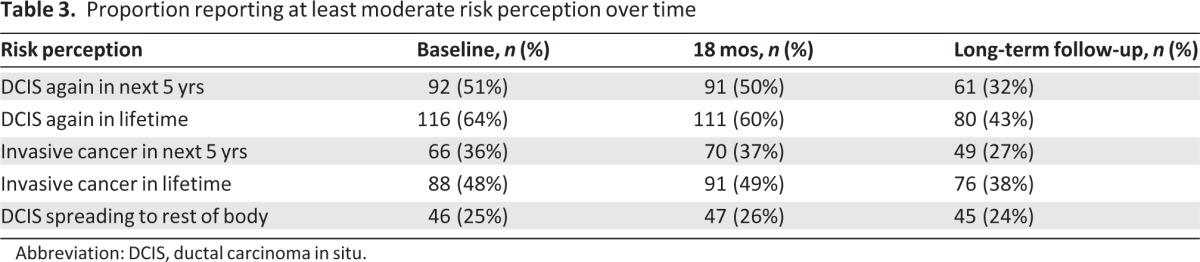
Abbreviation: DCIS, ductal carcinoma in situ.
Results of multivariate modeling for each of the five perceived risks are presented in Table 4. In all models, the factor most strongly associated with perceived risk in long-term follow-up was risk perception at 18 months. In fact, this was the only factor statistically significantly associated with the perceived risk for DCIS recurring within 5 years, DCIS recurring within a lifetime, and invasive cancer recurring within 5 years. Perception of developing invasive breast cancer within a lifetime was also associated with socioeconomic factors. Women who were financially comfortable were less likely to perceive moderate or greater risk, as were women who were at least college graduates. Perceptions of DCIS spreading throughout the body were also associated with financial status: women who were financially comfortable were less likely to have a moderate or greater perceived risk for this event. When these models were refit using an age cutoff at the median of 54 years, the results were unchanged except for the perceived risks for DCIS recurrence within 5 years or within a lifetime, both of which were found to be greater in those aged ≤54 years old than in older women (p = .04 and p = .005 for 5-year and lifetime risks, respectively).
Table 4.
Logistic regression models of moderate or greater perceived risks in long-term follow-up (with age dichotomized at 65 years)

ap values (two sided) were calculated using χ2 tests of parameters from a logistic regression model. No adjustment was made for multiple testing.
Abbreviations: CI, confidence interval; DCIS, ductal carcinoma in situ; OR, odds ratio.
Anxiety and Depression
The proportion of these women who met the criteria for anxiety based on HADS score (≥11) was low and declined only slightly over time. Depression, defined as a HADS score ≥11, was only identified in one of the 181 eligible respondents. There was no association between any of the measures of risk perception and anxiety as measured using the HADS. The proportion of participants with an IES score ≥26, reflecting intrusive and avoidant thoughts about DCIS, decreased substantially over time, from 23.8% at enrollment to 3.9% at 5 years.
Quality of Life
Norm-based SF-36 scores in long-term follow-up could be calculated for 177 of the 181 respondents. The average PCS score was 47.6 (SD, 6.7; median, 48.7; range, 15.7–57.8). The average MCS score was 46.2 (SD, 5.8; median, 47.2; range, 23.6–60.3). Compared with the average score of 50 in the general population, these differences approximate a minimum clinically significant difference of three points for these measures [11]. Only 22% of evaluable women had a PCS score <50 in long-term follow-up, whereas 54% had a PCS score <50 at baseline (Table 5). Controlling for other factors, women who felt that they had at least a moderate risk for DCIS spreading had a PCS score that was three points lower than those who perceived their risk to be lower (95% CI, 0.9–5.3; p =.005). MCS score was not significantly associated with elevated perceived risk for DCIS spreading.
Table 5.
Psychological state and QoL over time after DCIS diagnosis
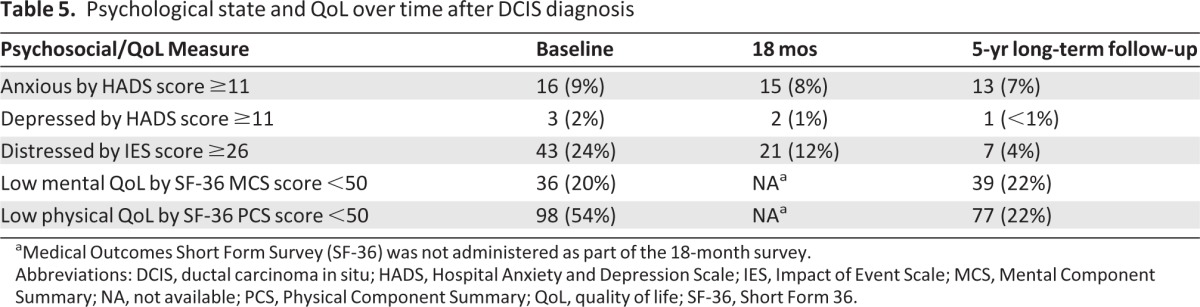
aMedical Outcomes Short Form Survey (SF-36) was not administered as part of the 18-month survey.
Abbreviations: DCIS, ductal carcinoma in situ; HADS, Hospital Anxiety and Depression Scale; IES, Impact of Event Scale; MCS, Mental Component Summary; NA, not available; PCS, Physical Component Summary; QoL, quality of life; SF-36, Short Form 36.
Discussion
Inaccurate perceptions of the risk for a disease may lead to suboptimal health-related decision-making and health behaviors and may have a negative impact on psychosocial and disease outcomes. Risk perceptions in women with DCIS have received more attention over the past several years because it is recognized that there is anxiety surrounding this relatively low-risk disease [9, 12]. There have even been calls to modify the nomenclature to remove the word “carcinoma” from DCIS in an effort to make perceptions of the disease more accurate [13–15]. In the present study, we demonstrated that, although a greater proportion of women accurately perceive their risks 5 years after diagnosis than at earlier time points, one quarter of women still indicated that their risk for developing distant spread of breast cancer was at least moderate. The disparity between the appropriately decreasing perception of local recurrence risk and stably high perception of risk for distant spread of disease raises a question as to whether or not respondents understand the meaning of this question, and if so, what drives this heightened risk perception specifically.
Given that <1% of women actually develop metastatic breast cancer following a DCIS diagnosis [16, 17], our data suggest that many women continue to vastly overestimate this risk in long-term follow-up. This may reveal poor communication or confusion on the part of providers about risks after a DCIS diagnosis [18], or it may reflect the fact that additional factors impede accurate risk perception (e.g., patient anxiety). The content of discussions between the participants in this study and their providers was unknown at the time of diagnosis and over time as new data emerged about the low risk for recurrence after DCIS. In women at risk for breast cancer, one study found that individualized risk counseling was ineffective at reducing excessive risk perceptions [19], suggesting that misinformation may not be the source of heightened risk perception. Interventions that target anxiety (e.g., antianxiolytic medications and therapy) might help modify inaccurate risk perceptions, but in our study, the HADS anxiety score in long-term follow-up was not associated with a greater perception of risk for any of the five potential future breast cancer events, unlike earlier in the survivorship period [9]. In long-term follow-up, a heightened perception of each risk at the prior time point, financial discomfort, and less education were associated with certain heightened perceived risks. Physicians may more effectively communicate risk data to more affluent and more educated women, or these women might have more access to other sources of information (e.g., websites). Financial barriers to health care use may increase perceived risks if health care providers are a source of reassurance for DCIS patients or if antianxiolytic medications reduce risk perceptions in some of those who can more easily afford them. Interestingly, no other patient or tumor characteristic was found to be associated with heightened perception of the risk for any of the five breast cancer events. Although the elevated risk for recurrence shown in young women during the first 5 years after diagnosis may continue in later years [5], age was not found to predict risk perceptions in this survey when age was dichotomized at 65 years as planned a priori. However, the finding from our exploratory analysis that women aged ≤54 years may perceive greater risks suggests that younger women may be aware of their higher risks; also, these perceptions may be mediated by and/or contributing to the heightened levels of distress that have been consistently demonstrated in younger survivors. Race was not found to be a predictor of risk perceptions, though this was not unexpected given prior research showing no prognostic implication of race for DCIS patients [4]. However, given that there were only nine nonwhite participants, the study was not adequately powered to detect such an association. Furthermore, demographic and treatment history differences between responders and nonresponders to the long-term follow-up survey may impede generalizability for the population of DCIS patients as a whole.
Conclusion
In conclusion, this study suggests that, even 5 years after diagnosis, some women with a history of DCIS harbor inaccurate perceptions of their risk for future breast cancer events. Physical HRQoL appeared to be worse in women who reported a heightened perceived risk for DCIS spreading to other parts of their body. Excessive perception of risk may be either a cause or an effect of a worse quality of life. Research on interventions to improve risk perception accuracy is warranted.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
Funding was provided by the Dana-Farber/Harvard Cancer Center Specialized Program of Research Excellence in Breast Cancer (5 P50 CA89393–03). The principal investigator for this grant was James D. Iglehart, M.D. However, the National Cancer Institute was not involved in the study design; collection, analysis, or interpretation of the data; manuscript preparation; or the decision to submit the manuscript for publication. No one other than the authors of the study contributed to the writing of this manuscript.
Author Contributions
Conception/Design: Kathryn J. Ruddy, Karen M. Emmons, Jane C. Weeks, Eric P. Winer, Ann H. Partridge
Provision of study material or patients: Eric P. Winer, Ann H. Partridge
Collection and/or assembly of data: Meghan E. Meyer, Ann H. Partridge
Data analysis and interpretation: Kathryn J. Ruddy, Anita Giobbie-Hurder, Ann H. Partridge
Manuscript writing: Kathryn J. Ruddy, Karen M. Emmons, Jane C. Weeks, Eric P. Winer, Ann H. Partridge
Final approval of manuscript: Kathryn J. Ruddy, Meghan E. Meyer, Anita Giobbie-Hurder, Karen M. Emmons, Jane C. Weeks, Eric P. Winer, Ann H. Partridge
Disclosures
The authors indicated no financial relationships.
Section editors: Gabriel Hortobágyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe (C/A); Novartis (RF); Taivex (O); founder and member of the board of directors for Citizen's Oncology Foundation; Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Sanofi, Ortho-Biotech, Amgen, Bristol-Myers Squibb (C/A), (H)
Reviewer “A”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Sanders ME, Schuyler PA, Dupont WD, et al. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 2.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailes AA, Kuerer HM, Lari SA, et al. Impact of race and ethnicity on features and outcome of ductal carcinoma in situ of the breast. Cancer. 2013;119:150–157. doi: 10.1002/cncr.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarado R, Lari SA, Roses RE, et al. Biology, treatment, and outcome in very young and older women with DCIS. Ann Surg Oncol. 2012;12:3777–3784. doi: 10.1245/s10434-012-2413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kestin LL, Goldstein NS, Lacerna MD, et al. Factors associated with local recurrence of mammographically detected ductal carcinoma in situ in patients given breast-conserving therapy. Cancer. 2000;88:596–607. doi: 10.1002/(sici)1097-0142(20000201)88:3<596::aid-cncr16>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Jebsen PW, Karesen R, et al. Ductal carcinoma in situ of the breast—a review of diagnosis, treatment and outcome in a hospital-based Norwegian series. Acta Oncol. 2000;39:131–134. doi: 10.1080/028418600430662. [DOI] [PubMed] [Google Scholar]
- 8.Lauzier S, Maunsell E, Levesque P, et al. Psychological distress and physical health in the year after diagnosis of DCIS or invasive breast cancer. Breast Cancer Res Treat. 2010;120:685–691. doi: 10.1007/s10549-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 9.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: Longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100:243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 10.de Moor JS, Partridge AH, Winer EP, et al. The role of socioeconomic status in adjustment after ductal carcinoma in situ. Cancer. 2010;116:1218–1225. doi: 10.1002/cncr.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute; 1994. pp. 1–188. [Google Scholar]
- 12.Ernster VL, Barclay J, Kerlikowske K, et al. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160:953–958. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 13.Graff S. Ductal carcinoma in situ: Should the name be changed? J Natl Cancer Inst. 2010;102:6–8. doi: 10.1093/jnci/djp497. [DOI] [PubMed] [Google Scholar]
- 14.Farante G, Zurrida S, Galimberti V, et al. The management of ductal intraepithelial neoplasia (DIN): Open controversies and guidelines of the Istituto Europeo di Oncologia (IEO), Milan, Italy. Breast Cancer Res Treat. 2011;128:369–378. doi: 10.1007/s10549-010-1124-4. [DOI] [PubMed] [Google Scholar]
- 15.Tavassoli FA. Breast pathology: Rationale for adopting the ductal intraepithelial neoplasia (DIN) classification. Nat Clin Pract Oncol. 2005;2:116–117. doi: 10.1038/ncponc0109. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 17.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 18.Partridge A, Winer JP, Golshan M, et al. Perceptions and management approaches of physicians who care for women with ductal carcinoma in situ. Clin Breast Cancer. 2008;8:275–280. doi: 10.3816/CBC.2008.n.032. [DOI] [PubMed] [Google Scholar]
- 19.Lerman C, Lustbader E, Rimer B, et al. Effects of individualized breast cancer risk counseling: A randomized trial. J Natl Cancer Inst. 1995;87:286–292. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]