Abstract
Background.
Treatments that target the vascular endothelial growth factor (VEGF) pathway have efficacy in colorectal cancer. We evaluated tolerability and efficacy of tivozanib (an oral VEGF receptor-1, -2, -3 inhibitor) plus everolimus (an oral mammalian target of rapamycin inhibitor).
Methods.
The phase Ib study followed a 3 + 3 dose-escalation design with three dose levels. The primary objective in the follow-on phase II study was improvement in 2-month progression-free survival (PFS) from 30% (historical benchmark) to 50% in patients with refractory, metastatic colorectal cancer.
Results.
Dose-limiting toxicities in the phase Ib study were grade 3 fatigue and dehydration. Oral tivozanib (1 mg daily for 3 of 4 weeks) and oral everolimus (10 mg daily continuously) were advanced to a 40-patient phase II study. The most common grade 3–4 adverse events were thrombocytopenia and hypophosphatemia. The 2-month PFS rate was 50%, with 20 of 40 patients having stable disease (SD). Seven (18%) patients were treated for ≥6 months. Median PFS and overall survival (OS) times were 3.0 months (95% confidence interval [CI]: 1.9–3.6 months) and 5.6 months (95% CI: 4.4–10.6 months), respectively. Patients who developed grade 1+ hypertension had increased SD rates (65.2% vs. 29.4%) and longer OS times (10.6 vs. 3.7 months).
Conclusions.
The oral combination of tivozanib and everolimus was well tolerated, with stable disease achieved in 50% of patients with refractory, metastatic colorectal cancer.
Author Summary
Discussion
Building on the known efficacy of antiangiogenic strategies in colorectal cancer and on preclinical studies of combined anti-angiogenic agents, we hypothesized that the combination of vascular endothelial growth factor (VEGF) receptor inhibition and mammalian target of rapamycin (mTOR) inhibition would be useful in patients with colorectal cancer. By independent radiologic review, 50% of 40 patients with refractory metastatic colorectal cancer had stable disease as their best response and nearly 20% of patients remained on study treatment for ≥6 months. The sample size of this single-arm trial was based on an increase in 2-month progression-free survival (PFS) rates from 30% to 50%, as a 2-month PFS of 50% was felt to be clinically relevant and worthy of further study.
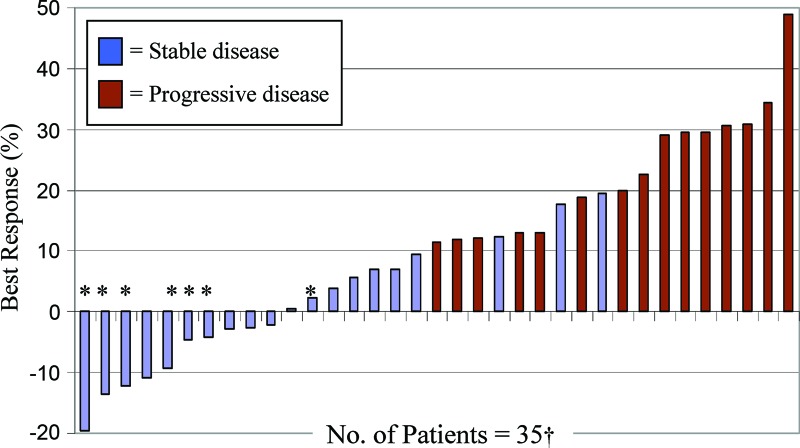
Figure 1.
Waterfall plot of best overall percentage change from baseline in target lesion measurement by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Six patients had progressive disease due to the development of new lesions rather than growth of the target lesions by ≥20%.
*Patients on study treatment ≥6 months (n = 7). The two patients with the largest tumor reduction by RECIST criteria, −19.6% and −13.5%, were still receiving study treatment at the data cutoff, with progression-free survival times of 13.1 and 12.9 months, respectively.
†Two patients withdrew consent and three patients developed symptomatic decline prior to objective evaluation.
The historical benchmark for a 2-month PFS rate of 30% was obtained from the best supportive care (BSC) arms in two randomized phase III trials of BSC versus BSC plus an anti-epidermal growth factor receptor (EGFR) monoclonal antibody. Notably, a recently reported phase III trial of patients with refractory metastatic colorectal cancer of BSC plus placebo versus BSC plus regorafenib demonstrated a similar 2-month PFS rate in the placebo arm, confirming the relative stability of this benchmark over time.
The disease control rate (DCR) of 50% and median PFS of 3.0 months for patients in the current trial compare favorably to trials in similar patient populations with refractory colorectal cancer, including a 99-patient phase II trial of everolimus alone (DCR: 25.3%; median PFS: 1.7 months), a 50-patient phase II trial of bevacizumab and everolimus (DCR: 46%; median PFS: 2.3 months), and the BSC plus regorafenib arm in the above-described trial (DCR: 44.8%; median PFS: 1.9 months). Nevertheless, cross-trial comparisons must be made with caution, given the potential for intrinsic differences in patient populations.
In exploratory analyses, we investigated whether patient outcomes appeared different by KRAS mutation status or development of hypertension. As with prior studies of bevacizumab in colorectal cancer, outcomes did not appear to differ by tumor KRAS mutation status. In contrast, we did note better outcomes in patients who developed grade 1+ hypertension while receiving tivozanib and everolimus. Development of hypertension has been associated with improved clinical outcome in other patient populations receiving antiangiogenesis agents, raising the possibility of hypertension as a predictive biomarker for antiangiogenenic agents in colorectal cancer.
In conclusion, tivozanib and everolimus were well tolerated by patients with refractory, metastatic colorectal cancer in this multicenter clinical trial. The phase II study met its primary endpoint, with 50% of patients achieving PFS at 2 months. Thus, modest efficacy was suggested for this drug combination in a patient population that greatly needs novel treatment programs.
Supplementary Material
Footnotes
ClinicalTrials.gov Identifier: NCT01058655 Sponsors: AVEO and Novartis Pharmaceuticals
Principal Investigator: Brian M. Wolpin IRB Approved: Yes
Disclosures
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.