The outcomes of patients with locally advanced cervical cancer treated with three-dimensional image-guided brachytherapy after concomitant chemoradiation were evaluated. An excellent locoregional control rate with low treatment-related morbidity was observed, justifying the elimination of hysterectomy in the absence of obvious residual disease.
Keywords: Cervical cancer, Image-guided adaptive brachytherapy, Chemoradiation, Optimization, Dose escalation
Learning Objectives
Evaluate control rates of IGABT combined with CCRT for the treatment of locally advanced cervical cancer.
Describe survival outcomes in patients treated with IGABT combined with CCRT for locally advanced cervical cancer.
Describe toxicities in patients treated with IGABT combined with CCRT for locally advanced cervical cancer.
Abstract
Purpose.
To evaluate the outcomes of patients with locally advanced cervical cancer treated with three-dimensional image-guided brachytherapy (IGABT) after concomitant chemoradiation (CCRT).
Materials and Methods.
Data from patients treated with CCRT followed by magnetic resonance imaging-guided or computed tomography-guided pulsed-dose-rate brachytherapy, performed according to the Groupe Européen de Curiethérapie–European Society for Radiotherapy and Oncology guidelines, were reviewed. At first, stage I or II patients systematically underwent radical hysterectomy or were offered a randomized study evaluating hysterectomy. Then, hysterectomy was limited to salvage treatment.
Results.
Of 163 patients identified, 27% had stage IB, 57% had stage II, 12% had stage III, and 3% had stage IVA disease. The mean dose delivered (in 2-Gy dose equivalents) to 90% of the high-risk clinical target volume was 78.1 ± 9.6 Gy, whereas the doses delivered to organs at risk were maintained under the usual thresholds. Sixty-one patients underwent a hysterectomy. Macroscopic residual disease was found in 13 cases. With a median follow-up of 36 months (range, 5–79 months), 45 patients had relapsed. The 3-year overall survival rate was 76%. Local and pelvic control rates were 92% and 86%, respectively. According to the Common Toxicity Criteria 3.0, 7.4% of patients experienced late grade 3 or 4 toxicity. Most of those had undergone postradiation radical surgery (2.9% vs. 14.8; p = .005).
Conclusion.
IGABT combined with CCRT provides excellent locoregional control rates with low treatment-related morbidity, justifying the elimination of hysterectomy in the absence of obvious residual disease. Distant metastasis remains an important first relapse and may warrant more aggressive systemic treatment.
Implications for Practice:
Over the past 20 years, technical advances have led to dramatic changes in brachytherapy planning permitting three-dimensional image guidance and volume-based prescribing. Image-guided adaptive brachytherapy is about to become a gold standard in the treatment of locally advanced cervical cancer, pending the results of prospective studies. This series reports excellent locoregional control in patients treated with pulsed-dose rate brachytherapy following concomitant chemoradiation. Treatment-related morbidity was low, except in patients who underwent postradiation hysterectomy, justifying the abandon of hysterectomy in the absence of obvious residual disease.
Introduction
The treatment of patients with locally advanced cervical cancer (LACC) relies on a combination of concomitant chemoradiation (CCRT) and brachytherapy [1]. CCRT became the standard of care in 1999 with the publication of five randomized studies comparing chemoradiation with exclusive radiation therapy. A recent meta-analysis showed a 6% higher locoregional control rate with chemoradiation, which translated into a 5% gain in overall survival benefit. At the same time, technological advances took place in the field of brachytherapy. One has been the potential to integrate three-dimensional (3D) imaging into treatment planning. Delineation of clinical target volumes (CTVs) and organs at risk (OARs) therefore became possible with a prescription to these volumes instead of geometrically constructed points, such as point A. 3D-image guidance also allows for the adaptation of brachytherapy to the tumor at the time of brachytherapy, taking tumor regression resulting from external-bean radiation therapy (EBRT) into account. The Groupe Européen de Curiethérapie–European Society for Radiotherapy and Oncology (GEC-ESTRO) defined two CTVs based on their probability of involvement by the tumor, taking both the gross residual tumor at the time of brachytherapy and the initial tumor volume into consideration [2]. The other major advance was the availability of afterloaders, able to use miniaturized 192Ir sources. By modulating dwell times and positions, it became possible to optimize dosimetries by increasing the dose delivered to tumors while controlling the dose delivered to OARs.
These improvements allowed a move from two-dimensional (2D) brachytherapy to image-guided adaptive brachytherapy (IGABT). Previous reports, on a limited amount of patients, suggest that this move has improved the outcomes of LACC patients. In our institution, the use of this modern technique led us to abandon postradiation hysterectomy. The aim of this study is to report the clinical results of IGABT in a large monocentric cohort of patients and to show how we went from neoadjuvant to definitive radiation therapy.
Materials and Methods
Patients and Tumors
The data from all patients treated with IGABT for LACC from the launch of IGABT in our institution until the start of a prospective study, the International Study of MRI-Guided Brachytherapy in Locally Advanced Cervical Cancer (EMBRACE), were reviewed. Locally advanced disease was defined as tumor size >4 cm or stage IIA–IVA, Ib1 N+, according to the Fédération Internationale de Gynécologie et d'Obstetrique classification. Only patients treated with curative intent were included. Patients with para-aortic lymph node infiltration above L1 were excluded. In total, 231 patients were treated with pulsed-dose-rate (PDR) brachytherapy for cervical cancer during the inclusion period. Preoperative brachytherapy for stage 1B1 cervical cancer (n = 47) was excluded. Additionally, two palliative patients and 19 patients lost to follow-up were excluded, ultimately leaving 163 patients aged 26.5–82.7 years suitable for inclusion. Their characteristics are summarized in Table 1. Most were treated for stage 1B2 and stage II disease (81%). Forty percent were considered to have nodal involvement, which was diagnosed using either computed tomography (CT) scan, positron emission tomography (PET) scan, or surgical dissection. The histological subtypes were squamous cell carcinoma (87% of cases), adenocarcinoma (11% of cases), and adenosquamous (remaining 2.5% of cases). Sixteen patients (10%) had a cone biopsy before treatment. All patients had at least one pelvic magnetic resonance imaging (MRI) scan at diagnosis, and nearly 60% had a PET–CT scan in their work-up. Fifty percent of patients had a para-aortic dissection: half of these were done laparoscopically before treatment to determine the need for extended-field radiotherapy and the remaining were done postradiotherapy in combination with hysterectomy.
Table 1.
Patient characteristics
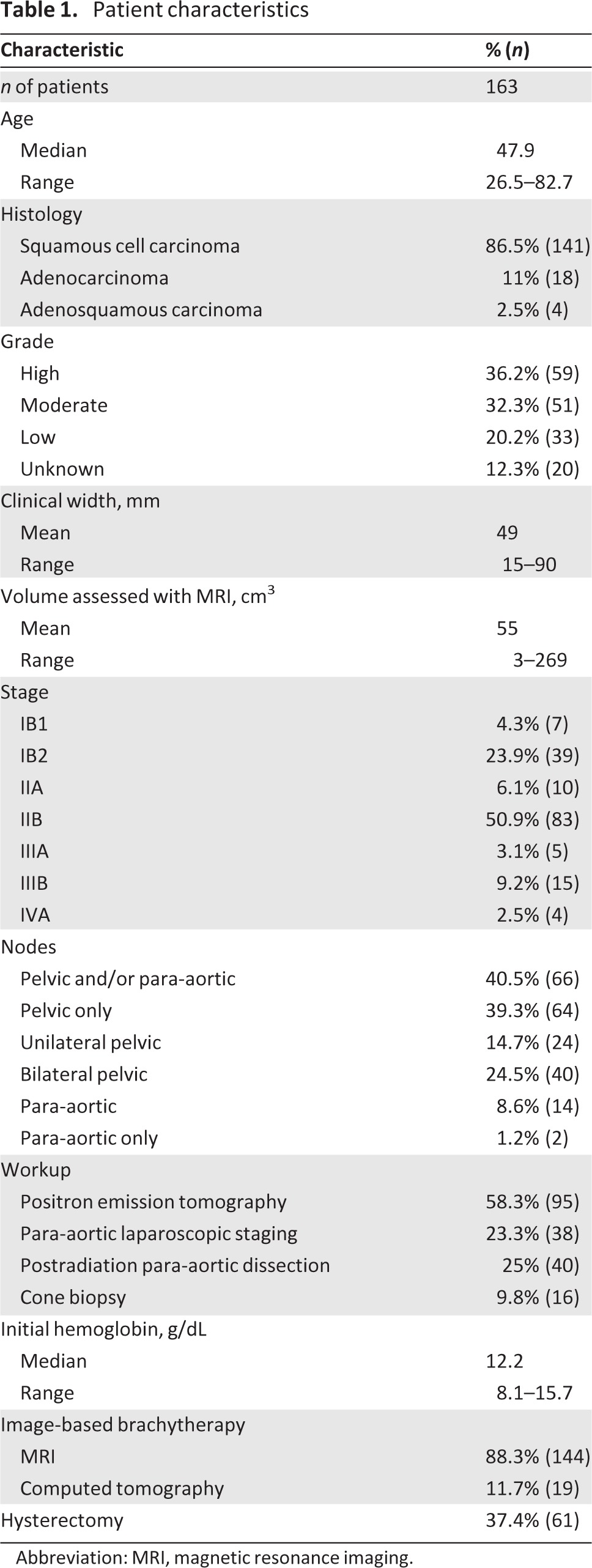
Abbreviation: MRI, magnetic resonance imaging.
Treatment Procedure
First, patients received CCRT at a dose of 45 Gy in 25 fractions of 1.8 Gy, five fractions per week. Treatment consisted of 3D conformal EBRT using a four-field box technique with high megavoltage photons from a linear accelerator (15–25 MV). The decision to treat the para-aortic lymph node area was made based on PET–CT and/or surgical dissection results when available; otherwise, it was based on nodal features on CT or MRI scans. Half of the patients had para-aortic lymph node dissection, either before treatment, in cases of definitive radiation therapy, or after treatment, when postradiation hysterectomy was scheduled, during the same general anesthesia. This decision was based on the significant false-negative rate in the initial workup. Even with PET–CT, which is considered to be the most effective noninivasive examination for regional extension, the false-negative rate is 8% [3]. However, the therapeutic impact of this dissection remains controversial. After completion of EBRT, patients received 15 Gy of PDR brachytherapy prescribed to the intermediate risk CTV (IR-CTV) using the PDR Selectron seed projector (Nucletron; Veenendaal, The Netherlands). To guarantee the best conformity to the tumor shape and personal anatomy, personalized vaginal molds were used for nearly all applications. This applicator requires a vaginal impression, which can easily be made without sedation during an outpatient clinic. The technique has been detailed elsewhere [4, 5]. Briefly, after a careful clinical examination under general anesthesia focused on the residual tumor, the cervical canal is gently dilated and a semiflexible tandem catheter is inserted. Then, the vaginal mold is inserted into the vagina so that it is flush with the cervix. Vaginal packing is unnecessary with molds. Brachytherapy planning is detailed in the next section.
In cases of pelvic or para-aortic node involvement, an EBRT boost was performed following brachytherapy, taking into account the contribution of brachytherapy evaluated from node delineation on brachytherapy planning images and dose-volume histogram generation to reach a total dose of 60 Gy on macroscopic nodes.
After completion of the treatment, patients were evaluated at 6 weeks with a clinical examination and MRI. In the initial time period of this study, patients with stage IB or IIA–IIB disease were systematically referred for a closure hysterectomy, even in cases with a complete response. Also during the same time period, in cases with a complete response (both MRI and gynecologic examinations), patients were offered a randomized study, gynecology (known as GYNECO) 2, evaluating hysterectomy versus no hysterectomy [6]. Then, in the second period after completion of that study, surgery was limited to salvage treatment.
Patients were followed up with every 4 months for 3 years, then every 6 months up to 5 years, and annually thereafter. For patients who had undergone hysterectomy, no specific follow-up investigations were performed in the absence of symptoms. For patients who did not have a hysterectomy, MRI was performed at least once a year.
IGABT Planning
After implantation, patients were transferred to MRI or CT scan. For MRI, a 1.5-Tesla machine was used. MRI-compatible dummy sources were inserted into the catheters to visualize the tip position of the sources with accuracy. Fast-spin echo T2-weighted axial, coronal, and sagittal images were acquired without contrast enhancement, with a 3-mm slice thickness, without gap, and with a matrix size of 256 × 224. The images were transferred to PLATO (Nucletron, Veenendaal, The Netherlands) or Oncentra Gyn (Nucletron) for delineation. Orthogonal x-ray imaging was also performed to check the imaging reconstruction of the catheters. When MRI was not available or in cases of refusal by the patient, a CT scan was used for planning purposes. In that situation, axial slices were acquired (1.5- to 3-mm thick) with iodine-based i.v. contrast to enhance the cervix. In both cases, the bladder catheter was left open during scanning as well as during treatment.
Uterine tandem and vaginal active lengths were determined according to delineation of target volumes and clinical examination. Optimization was performed manually with PLATO, following the GEC-ESTRO recommendations [2, 7]. The aim was to deliver at least 15 Gy to the IR-CTV and a minimum of 80 Gy to the high-risk CTV (HR-CTV). Dose constraints to OARs were 75 Gy (in 2-Gy dose equivalents [EQD2s]) to the maximal 2 cm3 of the rectum and 85 Gy for the bladder. By analogy with the rectum, the dose constraint to the sigmoid colon was 75 Gy. From our experience, additional constraints were applied: volume of tissue receiving 100% of the prescribed dose (V100%) of 60 Gy ≤250 cm3 and total reference air kerma ≤2 cGy/m2 when compatible with the probability of local control. At the end of the procedure, the prescription was adapted to respect a maximal dose rate of 0.6 Gy per pulse to OARs, based on results observed with low-dose-rate brachytherapy. Therefore, the fractionation varied from 30 pulses of 50 cGy/hour to 60 pulses of 25 cGy/hour. The delivery of brachytherapy was continuous, with hourly pulses, 24 hours a day, and the treatment was started on the same day as the applicator insertion. In cases of poor IR-CTV coverage, brachytherapy could be split into two fractions of 7.5 Gy to allow tumor regression (10 cases). The use of freehand interstitial needles was limited to two patients, to cover thick paravaginal involvement. All doses (adding EBRT and brachytherapy) were converted into EQD2 using an α/β ratio of three for the rectum, bladder, and sigmoid late reactions and 10 for the tumor, and using a half time repair of 1.5 hours.
Endpoints and Statistics
Overall disease-free survivals, as well as local and pelvic disease-free survivals, were defined from diagnosis to the date of last follow-up. Failures were defined as local irrespective of the localization, whether inside the CTVs (vagina, cervix, uterus, parametria), regional (pelvic or para-aortic lymph nodes), or distant metastasis (including ovaries and carcinomatosis). Local failures were divided into central (cervix, vagina, uterus) and lateral (parametria). Toxicities were graded according to the Common Toxicity Criteria 3.0.
Survival probabilities were calculated according to the Kaplan-Meier method and log rank test with TIGRE software (Benhamou and Wartelle statistics; Institut Gustave Roussy, Villejuif, France).
Results
One hundred sixty-three patients fulfilled the inclusion criteria. Their characteristics are summarized in Table 1. All patients received pelvic EBRT, with extended fields in the para-aortic area in 17.1% (n = 28) and in the inguinal area in 3% of cases. Concomitant chemotherapy was delivered to 90% of the patients, primarily with weekly cisplatin (91.2%). Forty-five Gy in 25 fractions at 1.8 Gy per fraction was delivered in 90% of cases. MRI was used to guide brachytherapy in 88.3% of cases, and CT was used for the remaining 11.7% of the cases.
Postradiation hysterectomy was performed in 61 cases (37%), and pathology was available for 59 cases. Complete histological response was achieved in 64.4% of cases, microscopic (≤1 cm) or indeterminate residual disease was observed in 13.6% of cases, and macroscopic residual disease (>1 cm) was observed in 22% of cases. Among the patients with macroscopic residual disease, 50% were initially assessed as stage IIB (8.4% of the included patients with IIB lesions), 14% were initially assessed as stage IIA (20% of all stage IIA patients), and the remaining 35% were initially assessed as stage IB (13.9% of all stage IB patients). Squamous cell carcinomas represented 57% of cases, 36% were adenocarcinomas, and 7% had adenosquamous histology. The median volume at diagnosis was 50.5 cm3 (range, 8.3–95.5 cm3), and 14% of patients had a cone biopsy before treatment (including a patient with minimal volume).
EQD2s to CTVs and OARs are summarized in Table 2, as well as doses to International Commission on Radiation Units and Measurements bladder and rectum points and to point A. The median IR-CTV and HR-CTV D90 (dose delivered in 90% of the indicated volume) values were 67.3 Gy (range, 50.3–95.9 Gy) and 78.6 Gy (range, 50.9–110.4 Gy), respectively. The median minimal doses to the maximal exposed 2 cm3 of the rectum, bladder, and sigmoid were: 58.8 Gy (range, 46.7–73.5 Gy), 67.9 Gy (range, 54.5–87.2 Gy), and 58.2 Gy (range, 47.8–77.1 Gy), respectively. The mean V100 (volume that receives 100% of prescribed dose) was 224.5 ± 62.6 cm3 (range, 125.3–490.3 cm3).
Table 2.
Dosimetric outcomes
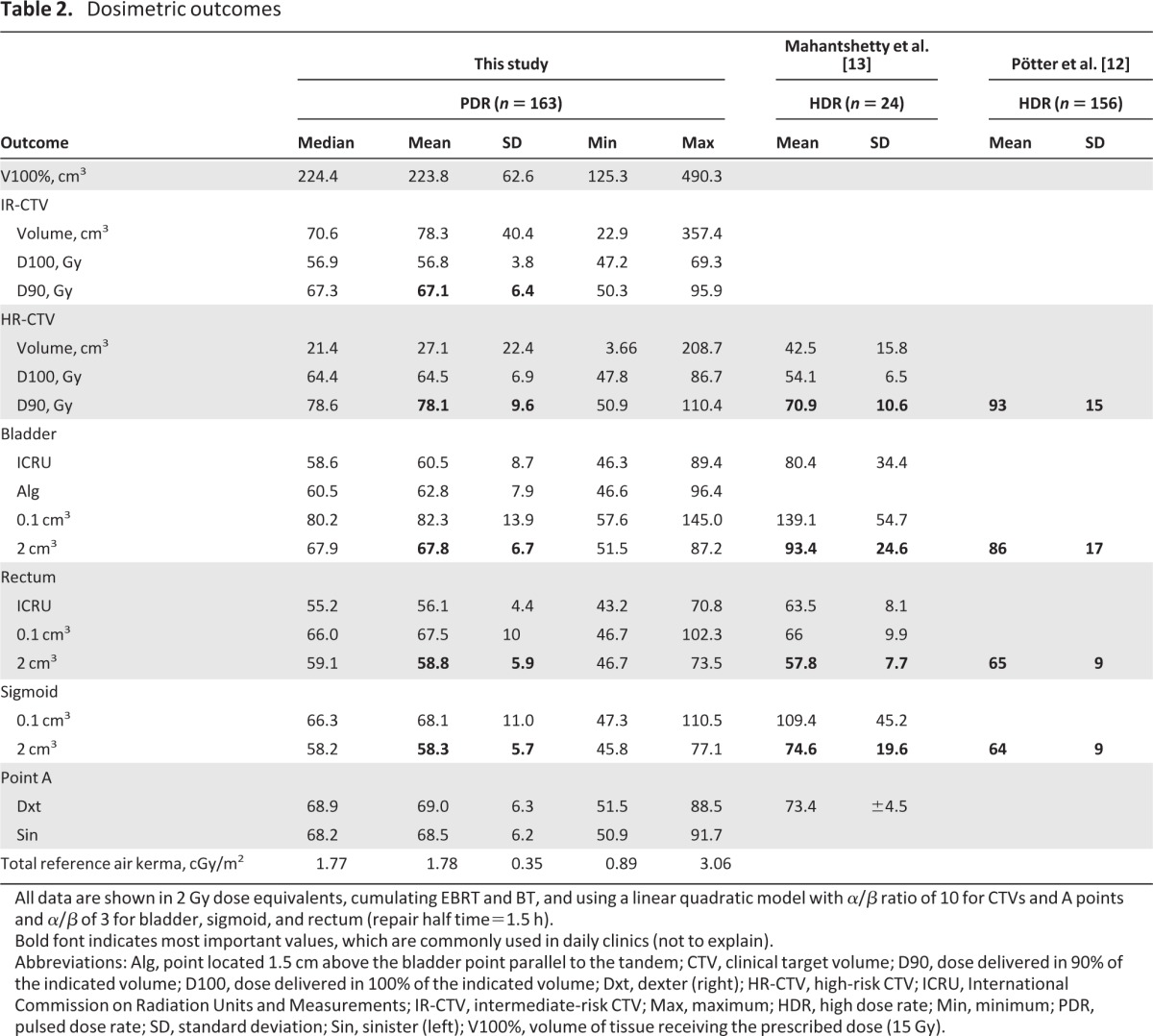
All data are shown in 2 Gy dose equivalents, cumulating EBRT and BT, and using a linear quadratic model with α/β ratio of 10 for CTVs and A points and α/β of 3 for bladder, sigmoid, and rectum (repair half time=1.5 h).
Bold font indicates most important values, which are commonly used in daily clinics (not to explain).
Abbreviations: Alg, point located 1.5 cm above the bladder point parallel to the tandem; CTV, clinical target volume; D90, dose delivered in 90% of the indicated volume; D100, dose delivered in 100% of the indicated volume; Dxt, dexter (right); HR-CTV, high-risk CTV; ICRU, International Commission on Radiation Units and Measurements; IR-CTV, intermediate-risk CTV; Max, maximum; HDR, high dose rate; Min, minimum; PDR, pulsed dose rate; SD, standard deviation; Sin, sinister (left); V100%, volume of tissue receiving the prescribed dose (15 Gy).
The median follow-up was 36.9 months (range, 6.7–85.5 months). At the time of analysis, 125 patients were alive and 38 had died, 35 as a result of their malignancy. Sixty-three events were recorded, involving 45 patients (Fig. 1). Among the 12 local relapses reported, five were central, three were lateral, and four were both lateral and central. Four patients had isolated local relapses. Of these, only one was eligible for curative salvage treatment (complete exenteration). That patient had a postradiation hysterectomy and relapsed in the vaginal cuff. The remaining three patients had pelvic side wall invasion and were unsuitable for curative surgery.
Figure 1.
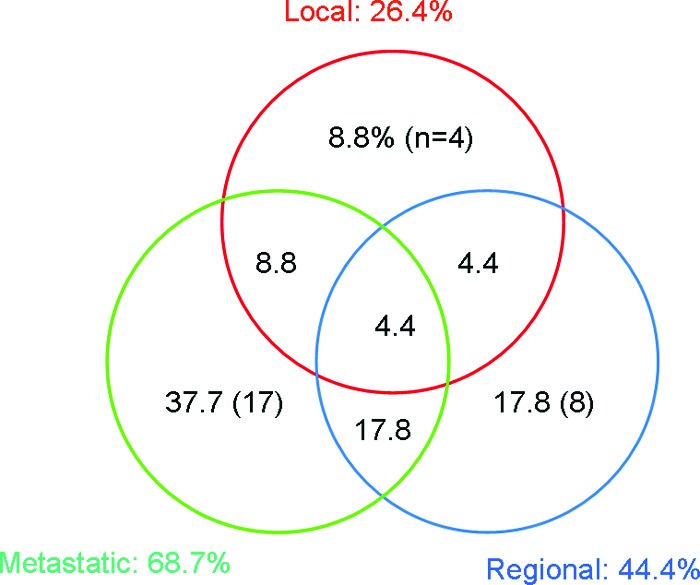
Pattern of relapses.
Nineteen regional events were reported: eight in pelvic nodes, eight in the para-aortic area, and three in the both pelvic and para-aortic areas. Thirty-two patients had distant metastasis, 68.7% of all relapses. Of these, half were isolated.
The 3-year overall and cancer-specific survival rates for the whole series were 76% and 78%, respectively. Local and pelvic control rates were 92% and 86%, respectively, at 3 years (Fig. 2). The local control rate for patients with stage I or stage II disease was 93% at 3 years, versus an 81% local control rate for patients with stage III and stage IV tumors (p = .202). The local control rate was 97% for tumors <5 cm width, 91% for tumors ≥5 cm to <6 cm, and 81% for tumors ≥6 cm (p = .036). The mean dose to 90% of the HR-CTV in patients who relapsed locally was 76.1 ± 3.9 Gy, versus 78.3 ± 9.9 Gy for the remaining patients (p = .46).
Figure 2.
Overall and disease-free survival (DFS) probabilities and local and pelvic control rates.
Three hundred six late toxicity events were reported, concerning 125 patients (76.7%) (Table 3). The majority of events were grade 1 or 2. One third of the patients reported grade 1 gastrointestinal disorders, mainly diarrhea, and slightly >20% reported grade 1 urinary toxicities. Grade 2 late gastrointestinal and urinary toxicities were reported in 10% and 8% of patients, respectively. Fourteen grade 3 or 4 events were reported, affecting 12 patients (7.4%). There were six grade 3 urinary events: four fistulae and two hydronephroses. Of the eight gastrointestinal events, two fistulae and six bowel obstructions were reported. Among the 12 patients who had at least one grade 3 or 4 toxicity, nine had a postradiation hysterectomy (75%). Other toxicities, such as hot flashes and limb edema, are reported as “other.”
Table 3.
Toxicities according to the Common Toxicity Criteria 3.0
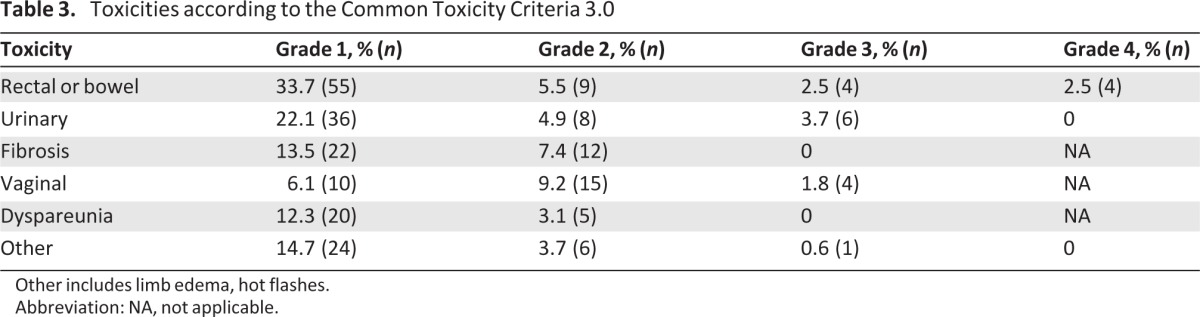
Other includes limb edema, hot flashes.
Abbreviation: NA, not applicable.
Discussion
CCRT is considered to be the standard local treatment for LACC. However, in certain countries, such as France, systematic postradiotherapy radical hysterectomy mainly for stage IB2 and stage II cervical cancer remains a common practice. The role of this surgery has been debated for a long time despite the lack of evidence that postradiation hysterectomy improves outcomes [8].
In recent years, the advent of CCRT and IGABT has dramatically increased complete histological response rates [9]. Classe et al. [10] reported a 38% complete histological response rate in a large multicentric series including stage IB to stage IVA lesions, whereas we report a 64% response rate in this series. In addition, in eight patients (13%), the specimen contained only microscopic disease or an uncertain remnant, and we can assume that these patients would have achieved a complete response later on. This high rate of complete histologic response was a strong argument to challenge the need for hysterectomy. Furthermore, the outcomes of patients with residual disease seem to be poor. The presence of residual disease in the cervix after hysterectomy is known to be predictive of relapse [11]. Furthermore, in another series of 10 patients with macroscopic residual disease after hysterectomy, we reported very poor outcomes, with most patients dying as a result of distant metastasis, which raises the relevance of achieving local control in this specific situation, especially when considering the high rate of severe morbidities related to surgery in irradiated tissues [12].
Another concern is morbidity. Postradiation surgery is difficult and clearly adds morbidity. In a review, the rate of grade 2 or 3 toxicities was evaluated as 15%–46% after chemoradiation, according to the Franco-Italian glossary [10]. In our series, the severe morbidity rate for patients who had a hysterectomy after IGABT following chemoradiation was nearly 15%, which is significantly higher than the rate for those who exclusively had radiation therapy (2.9%; p = .005). In the context in which hysterectomy did not show any superiority over exclusive radiotherapy, morbidity is a strong argument against systematic postradiation surgery. Some could argue that poor responders to EBRT could be candidates for immediate surgery instead of brachytherapy, but so far no evidence supports this idea. The GEC-ESTRO recommendations take into account the response to EBRT, and poor responders have larger CTVs to be covered at the time of brachytherapy. In this situation, interstitial brachytherapy appears to be effective at reaching the dose objectives by enlarging isodoses in the parametria, as shown in the series by Pötter et al. [13], in which local control remained high even for bulky lesions. The prospective study led by GEC-ESTRO aims to identify poor responders to adapt the brachytherapy plan.
To resolve the debate regarding the role of post-treatment hysterectomy, we designed a multicentric randomized study, GYNECO 2. The aim was to assess the addition of hysterectomy to the treatment of patients with stage I–II cervical cancer in complete remission following CCRT and brachytherapy. Unfortunately, the trial was closed early because of poor accrual, but 60 patients were included and no difference was reported between the two arms. Since that study closure, we have abandoned the use of hysterectomy in the absence of residual disease. Hysterectomy is still performed in selected cases of incomplete remission, after detailed clinical and radiologic evaluations, including MRI and PET.
Those three facts (the absence of scientific evidence that systematic hysterectomy could improve local control, high histological complete response rate after IGABT, and greater morbidity resulting from postradiation hysterectomy) ended the systematic use of that surgery. This is illustrated in Table 4, which shows the continuous decrease in the rate of hysterectomy over three time periods (2004–2006 [14], 2006–2009, and from 2009 onward), without a decrease in local control. At the same time, we were able to raise the dose delivered to tumors by 6 Gy for the HR-CTV while maintaining a similar or lower dose to OARs.
Table 4.
Comparison of dosimetric outcomes among three time periods
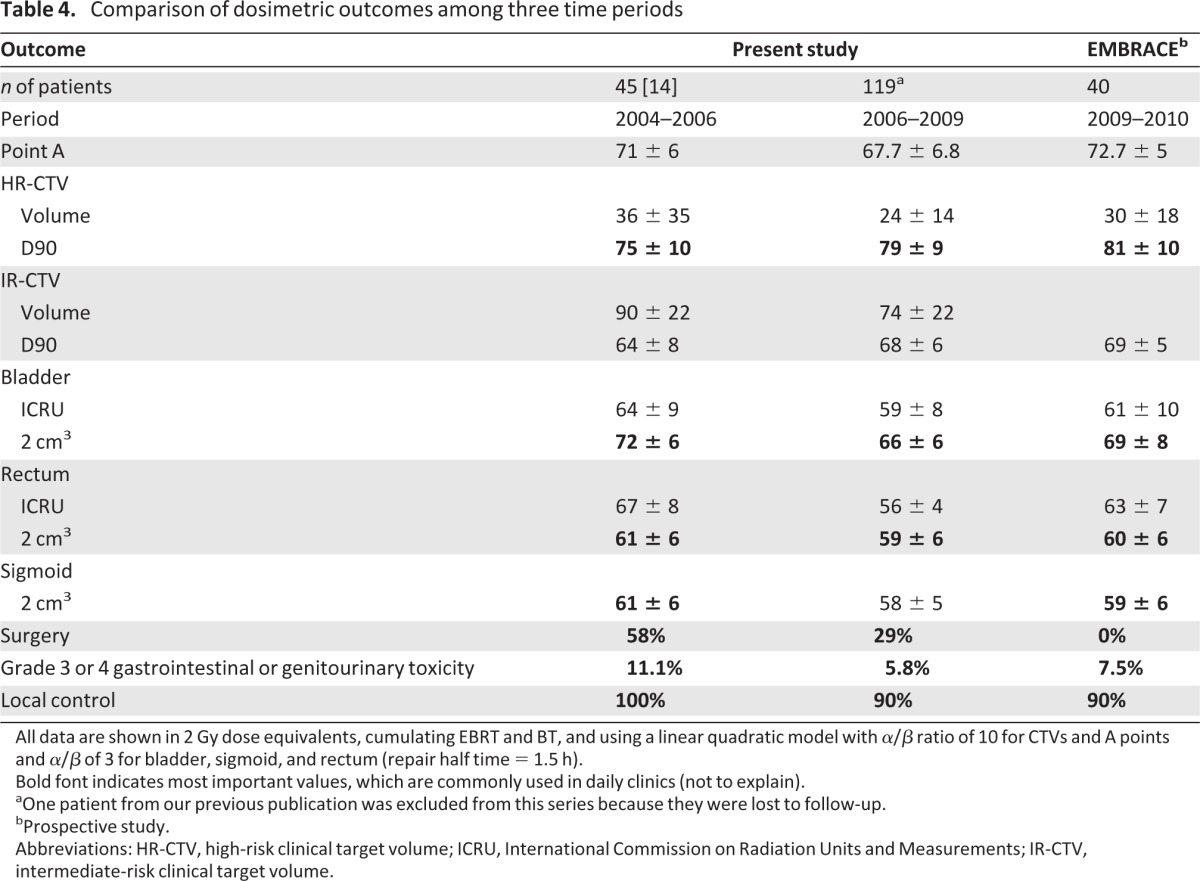
All data are shown in 2 Gy dose equivalents, cumulating EBRT and BT, and using a linear quadratic model with α/β ratio of 10 for CTVs and A points and α/β of 3 for bladder, sigmoid, and rectum (repair half time = 1.5 h).
Bold font indicates most important values, which are commonly used in daily clinics (not to explain).
aOne patient from our previous publication was excluded from this series because they were lost to follow-up.
bProspective study.
Abbreviations: HR-CTV, high-risk clinical target volume; ICRU, International Commission on Radiation Units and Measurements; IR-CTV, intermediate-risk clinical target volume.
The high rate of complete histological response led us to limit hysterectomy to salvage treatments for patients with residual disease. To improve the rate of complete response, interstitial brachytherapy is an option. The aim is to add interstitial needles to enlarge the isodoses and improve the coverage of distal invasion of the parametria. In the series by Pötter et al. [13], 47% of the patients were treated with this technique.
Several dosimetric studies have previously shown a clear advantage of IGABT in escalating doses delivered to tumors, but so far limited clinical data are available. The largest series on IGABT was published by Pötter et al. [13], with 156 patients treated with high-dose-rate (HDR) MRI-guided brachytherapy. They obtained complete remission in 97% of patients. The overall local control rate was 95% at 3 years, and it remained high at 92% for patients with bulky lesions >5 cm. Grade 3 or 4 toxicities were reported in 7.1% of patients, which could appear surprising in view of the doses delivered to OARs, which were higher than ours by 10–15 Gy to the maximal 2 cm3 of the OAR, but none of these patients had undergone hysterectomy. Mahantshetty et al. [15] reported promising results in a small cohort of 24 patients treated with MRI-based HDR brachytherapy. Despite the fact that half of the patients (n = 12) were diagnosed with stage IIIb lesions, only one patient relapsed in the cervix, after 24 months of follow-up [15].
However, higher level evidence is required. No randomized controlled trial comparing IGABT with conventional brachytherapy has been led. However, Pötter et al. [16] published a comparison of two cohorts of patients treated in 1998–2000 and 2000–2003. During the later treatment period, the investigators applied the concept of risk volumes with IGABT, whereas they did not in the previous period. They showed better local control during the later treatment period, which was significant for bulky lesions (>5 cm): 82%, versus 64% in the earlier period, with an absolute overall survival benefit for patients with a tumor >5 cm, 58% versus 28% (p = .003). They also reported a lower rate of severe late effects with the use of modern brachytherapy than in their earlier experience (6% versus 13%). Other evidence comes from the nonrandomized prospective French trial Soutien aux Technologies Innovantes et Couteuses (STIC) [17]. Three cohorts (preoperative brachytherapy for stage 1B1 lesions, radiotherapy followed by surgery, or exclusive radiation therapy for locally advanced disease) were reported, in which patients could be treated either using IGABT or using 2D low-dose-rate brachytherapy. In each group, the local control rate was higher, by 8.3% and 4.6% in the radiosurgery and exclusive radiotherapy groups, respectively. At the same time, grade 3 or 4 toxicity rates were lower in patients treated with IGABT, by 3.7% and 20.1%, respectively.
To provide a higher level of evidence, a large database, retro-EMBRACE, was created by pooling our data with Pötter's data and with data from other institutions such as Aarhus, Utrecht, Cambridge, and Mumbai. The aim is to provide mature data on outcomes and toxicities from a large series [18]. A higher level of evidence will be reached with the EMBRACE trial, a prospective study led by the GYN GEC-ESTRO members. Seven hundred patients have been included so far and 1,000 are awaited. Beyond outcomes, this study will also address several other unanswered questions, such as: what is the correlation between the dose delivered and tumor control or morbidity [19]?
With the use of such an effective local treatment, distant failures become the first failures, although CCRT led to better distant control. Among the studies included in the meta-analysis, two used adjuvant cycles of chemotherapy in addition to CCRT. Taken apart, they were highly positive, raising the question of the value of adjuvant chemotherapy [20]. Dueñas-González et al. [21] recently showed the superiority of cisplatin- and gemcitabine-based chemoradiation with two adjuvant cycles of gemcitabine and classic chemoradiation. Unfortunately, it is not possible to sort out the adjuvant effect of gemcitabine from its concomitant effect. However, the Gynecologic Oncology Group is starting a randomized study on adjuvant chemotherapy in high-risk patients (three cycles of carboplatin plus paclitaxel), the OUTBACK study [22]. Work on improving radiosensitization with new agents is also in progress. Targeted therapy (e.g., cetuximab) and gemcitabine are promising, but still in development. With that in mind, we are completing a phase I study evaluating a combination of cidofovir, an antiviral agent interfering with human papillomavirus DNA polymerase, shown to be a radiosensitizer, with platinum-based chemoradiation [23].
Conclusion
Image-guided PDR brachytherapy provides good local control after CCRT in patients with LACC with an acceptable level of late toxicity. These results led us to abandon systematic radical hysterectomy, which clearly adds unacceptable morbidity to patients free from residual disease, given the the high rate of complete response. Postradiation hysterectomy is therefore limited to patients with residual disease after radiation or with central relapses. The local control rate reported in this series, 92%, decreases with tumor width and could potentially be improved with the use of interstitial brachytherapy, which is indicated for poor responders after EBRT. The high rate of distant metastasis as the first relapse underlines the need for more aggressive systemic treatments such as adjuvant chemotherapy or the addition of novel systemic agents.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This work was presented at the European Multidisciplinary Cancer Congress, Stockholm, Sweden, September 23–28, 2011, and updated and presented at the World Brachytherapy Meeting, Barcelona, Spain, May 9–13, 2012.
Author Contributions
Conception/Design: Renaud Mazeron, Christine Haie-Meder
Provision of study material or patients: Renaud Mazeron, Isabelle Dumas, Jérôme Champoudry, Christine Haie-Meder
Collection and/or assembly of data: Renaud Mazeron, Jennifer Gilmore, Isabelle Dumas, Jérôme Champoudry, Anne Tailleur
Data analysis and interpretation: Renaud Mazeron, Jennifer Gilmore, Jennifer Goulart, Ben Vanneste, Philippe Morice, Christine Haie-Meder
Manuscript writing: Renaud Mazeron, Jennifer Gilmore, Jennifer Goulart, Christine Haie-Meder
Final approval of manuscript: Renaud Mazeron, Jennifer Gilmore, Isabelle Dumas, Jérôme Champoudry, Jennifer Goulart, Ben Vanneste, Anne Tailleur, Philippe Morice, Christine Haie-Meder
Disclosures
The authors indicated no financial relationships.
Section editors: Dennis Chi: None; Peter Harper: Sanofi, Roche, Imclone, Pfizer, GlaxoSmithKline, Lilly, Genentech (C/A); Lilly, Novartis, Sanofi, Roche (H)
Reviewer “A”: None
Reviewer “B”: Ethicon Inc. (H)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Haie-Meder C, Morice P, Castiglione M ESMO Guidelines Working Group. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v37–v40. doi: 10.1093/annonc/mdq162. [DOI] [PubMed] [Google Scholar]
- 2.Haie-Meder C, Pötter R, Van Limbergen E, Gynaecological (GYN) GEC-ESTRO Working Group et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Uzan C, Souadka A, Gouy S, et al. Analysis of morbidity and clinical implications of laparoscopic para-aortic lymphadenectomy in a continuous series of 98 patients with advanced-stage cervical cancer and negative PET-CT imaging in the para-aortic area. The Oncologist. 2011;16:1021–1027. doi: 10.1634/theoncologist.2011-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albano M, Dumas I, Haie-Meder C. [Brachytherapy at the Institut Gustave-Roussy: Personalized vaginal mould applicator: Technical modification and improvement] Cancer Radiother. 2008;12:822–826. doi: 10.1016/j.canrad.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Magné N, Chargari C, SanFilippo N, et al. Technical aspects and perspectives of the vaginal mold applicator for brachytherapy of gynecologic malignancies. Brachytherapy. 2010;9:274–277. doi: 10.1016/j.brachy.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Morice P, Rouanet P, Rey A, et al. Results of the GYNECO 02 study, an FNCLCC phase III trial comparing hysterectomy with no hysterectomy in patients with a (clinical and radiological) complete response after chemoradiation therapy for stage IB2 or II cervical cancer. The Oncologist. 2012;17:64–71. doi: 10.1634/theoncologist.2011-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Keys HM, Bundy BN, Stehman FB, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: A randomized trial of the Gynecologic Oncology Group. Gynecol Oncol. 2003;89:343–353. doi: 10.1016/s0090-8258(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 9.Tanderup K, Georg D, Pötter R, et al. Adaptive management of cervical cancer radiotherapy. Semin Radiat Oncol. 2010;20:121–129. doi: 10.1016/j.semradonc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Classe JM, Rauch P, Rodier JF, et al. Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: Morbidity and outcome: Results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer) Gynecol Oncol. 2006;102:523–529. doi: 10.1016/j.ygyno.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Morice P, Uzan C, Zafrani Y, et al. The role of surgery after chemoradiation therapy and brachytherapy for stage IB2/II cervical cancer. Gynecol Oncol. 2007;107(suppl 1):S122–S124. doi: 10.1016/j.ygyno.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Azria E, Morice P, Haie-Meder C, et al. Results of hysterectomy in patients with bulky residual disease at the end of chemoradiotherapy for stage IB2/II cervical carcinoma. Ann Surg Oncol. 2005;12:332–337. doi: 10.1245/ASO.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Pötter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chargari C, Magné N, Dumas I, et al. Physics contributions and clinical outcome with 3D-MRI-based pulsed-dose-rate intracavitary brachytherapy in cervical cancer patients. Int J Radiat Oncol Biol Phys. 2009;74:133–139. doi: 10.1016/j.ijrobp.2008.06.1912. [DOI] [PubMed] [Google Scholar]
- 15.Mahantshetty U, Swamidas J, Khanna N, et al. Reporting and validation of gynaecological Groupe Europeen de Curietherapie European Society for Therapeutic Radiology and Oncology (ESTRO) brachytherapy recommendations for MR image-based dose volume parameters and clinical outcome with high dose-rate brachytherapy in cervical cancers: A single-institution initial experience. Int J Gynecol Cancer. 2011;21:1110–1116. doi: 10.1097/IGC.0b013e31821caa55. [DOI] [PubMed] [Google Scholar]
- 16.Pötter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83:148–155. doi: 10.1016/j.radonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Charra-Brunaud C, Harter V, Delannes M, et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother Oncol. 2012;103:305–313. doi: 10.1016/j.radonc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Retro EMBRACE. Participants. [Accessed December 27, 2012]. Available at https://www.retroembrace.com/AboutParticipants.aspx.
- 19.EMBRACE. [Accessed December 27, 2012]. Available at https://www.embracestudy.dk/
- 20.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 21.Dueñas-González A, Zarba JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. Cisplatin and Radiation Therapy With or Without Carboplatin and Paclitaxel in Patients With Locally Advanced Cervical Cancer. [Accessed December 27, 2012]. Available at http://clinicaltrials.gov/ct/show/NCT01414608.
- 23.Abdulkarim B, Sabri S, Deutsch E, et al. Antiviral agent cidofovir restores p53 function and enhances the radiosensitivity in HPV-associated cancers. Oncogene. 2002;21:2334–2346. doi: 10.1038/sj.onc.1205006. [DOI] [PubMed] [Google Scholar]



