Proangiogenic cytokines such as vascular endothelial growth factor, platelet-derived growth factor, and fibroblast growth factor may play crucial roles in the treatment of hepatocellular carcinoma. Recent clinical and preclinical data along with ongoing studies are discussed in this review.
Keywords: Hepatocellular carcinoma, Vascular endothelial growth factor, VEGF, FGF, Angiogenesis
Abstract
Hepatocellular carcinoma (HCC) is a significant cause of death worldwide. HCC is a highly vascular tumor, and proangiogenic cytokines such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor may play crucial roles in this disease. Sorafenib, a multikinase inhibitor that blocks VEGF and PDGF signaling, was the first systemic therapy to demonstrate improved survival in patients with advanced HCC. Several other drugs targeting VEGF are in development. Because of the anticipation of eventual resistance to anti-VEGF therapies, drugs that also target alternative proangiogenic pathways are being investigated. Recent clinical and preclinical data along with ongoing studies are reviewed.
Implications for Practice:
Advanced hepatocellular carcinomas are refractory to most anticancer agents. Targeting of angiogenesis appears to be the most successful strategy to date for extending survival for patients with advanced HCC. This article discusses ongoing studies aimed at improving upon antiangiogenic therapy.
Introduction
Liver cancer is a major problem worldwide, accounting for more than 748,000 new cases and more than 695,000 deaths in 2008 [1]. The highest liver cancer rates are found in East and Southeast Asia. However, Western countries have seen an increase in the incidence of liver cancer in recent decades, likely due to increases in hepatitis C virus (HCV) infection and obesity—two of the most prominent risk factors for hepatocellular carcinoma (HCC) [2]. HCC accounts for 85%–90% of primary liver cancers. Most patients with HCC present with advanced-stage disease and consequently are not candidates for curative treatments, such as liver transplantation or surgical resection [3]. Even patients who undergo resection are likely to have recurrent disease, particularly those with larger tumors or tumors displaying vascular invasion [4]. Therefore, there is a clear need for development of additional agents in the management of this disease.
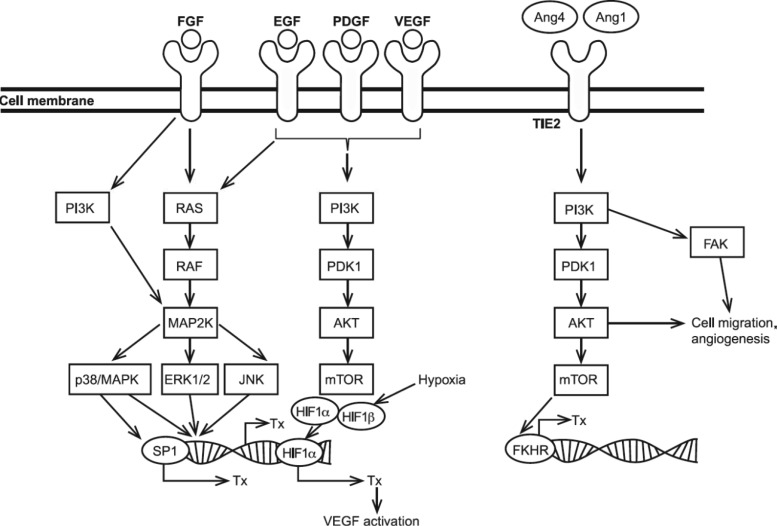
HCC is usually a highly vascular tumor, thus providing an attractive target for the development of new anticancer drugs. Tumor angiogenesis is a complex process that is regulated by many factors (Fig. 1) [5]. Vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (FGF) all appear to play important roles in angiogenesis in many cancers, including HCC [6]. For instance, VEGF and its receptors 1, 2, and 3 have been found to be overexpressed relative to normal liver tissue in HCC, and overexpression of VEGF has been correlated with poor prognosis [7, 8]. VEGF expression was also found to increase gradually during multistep hepatocarcinogenesis [9]. PDGF is required for the recruitment of smooth muscle cells, or pericytes, that surround new blood vessels [10]. Pericytes are considered to be supportive cells for endothelial cells; they play important roles in arteriogenesis, modulation of blood flow, and regulation of vascular permeability. Basic FGF stimulates endothelial cell migration, capillary branching, and the activity of proteases, which are also essential for angiogenesis [11]. FGF has also been shown in a murine model system to act synergistically with VEGF in the progression of HCC [12] and has been implicated in escape from VEGF inhibition, as discussed herein.
Figure 1.
Alternative angiogenic signaling. All transcriptions noted can lead to angiogenesis.
Abbreviations: EGF, epidermal growth factor; FGF, fibroblast growth factor; HIF, hypoxia inducible factor; mTOR, mammalian target of rapamycin; PDGF, platelet-derived growth factor; SP1, specificity protein-1; Tx, transcription; VEGF, vascular endothelial growth factor.
Sorafenib
Sorafenib is an oral multitargeted tyrosine kinase inhibitor (TKI) that inhibits VEGF receptors (VEGFR)-1, VEGFR-2, VEGFR-3, PDGF receptors (PDGFR)-α, PDGFR-β, c-KIT, and B-Raf, and to a lesser degree, many other kinases [13]. Sorafenib has been shown to promote apoptosis in HCC cell lines and inhibit angiogenesis in HCC xenografts [14]. Sorafenib was originally approved by the U.S. Food and Drug Administration (FDA) for treating patients with advanced-stage renal cell carcinoma; it has since been approved in many countries for treating patients with advanced HCC [13].
Approval of sorafenib in the setting of HCC was based on results of the Sorafenib Hepatocellular carcinoma Assessment Randomized Protocol (SHARP) study—a multicenter, phase III, double-blind, placebo-controlled trial that compared sorafenib to placebo in 602 patients with locally advanced or metastatic HCC and predominantly Child-Pugh class A liver disease [15]. Approximately one third of the patients included in this study underwent prior embolization, but no prior systemic therapy was allowed. Median overall survival (OS) was significantly longer in the sorafenib group compared with the placebo group: 10.7 versus 7.9 months, respectively (hazard ratio [HR]: 0.69, 95% confidence interval [CI]: 0.55–0.87; p < .001). Median time to progression (TTP), according to Response Evaluation Criteria in Solid Tumors, was also significantly longer in the sorafenib group compared with the placebo group: 5.5 versus 2.8 months, respectively (HR: 0.58, 95% CI: 0.45–0.74; p < .001). This benefit was achieved in spite of a radiographic response rate of only 2%. The main treatment-related side effects were diarrhea (39% of patients), hand-foot syndrome (21%), fatigue (22%), and rash (16%). The magnitude of benefit of sorafenib in the SHARP trial was confirmed in another phase III study conducted in the Asia-Pacific region (NCT00492752; ClinicalTrials.gov), in which the hazard ratio was remarkably similar, but control and experimental arms had lower survival due to a preponderance of high-risk factors such as poor liver function and extrahepatic disease [16].
Because both of these randomized trials were restricted to patients with Child-Pugh A liver disease, questions have remained about use of sorafenib in patients with more advanced liver disease, for whom prognosis is known to be significantly worse [5]. The Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of its Treatment with Sorafenib (GIDEON) study, initiated at the time of sorafenib's approval to monitor the safety of the real-world use of sorafenib, is an ongoing global, noninterventional, prospective study of patients with advanced HCC treated with sorafenib [17]. A recent interim analysis revealed that treatment-related adverse effects were similar in patients with Child-Pugh A and B liver disease; however, a greater percentage of patients with Child-Pugh B liver dysfunction had to discontinue treatment due to adverse effects (38% vs. 23%). In the intention-to-treat population (1,614 patients), preliminary OS was 10.5 months in the Child-Pugh A group and 4.8 months in the Child-Pugh B group. This study suggests that it may be safe to use sorafenib in patients with a higher degree of liver dysfunction if close attention is paid to side effects, but median survival is very short in spite of sorafenib use. Further study is warranted before use of sorafenib in the Child-Pugh B population is considered standard.
Because many patients' tumors are initially refractory to sorafenib and all tumors eventually develop secondary resistance, there has been interest in combining sorafenib with conventional chemotherapy in an effort to improve outcomes. A phase II trial was conducted comparing doxorubicin alone versus doxorubicin plus sorafenib in patients with advanced HCC with Child-Pugh A liver dysfunction and no prior chemoembolization [18]. At the time of trial design and accrual, doxorubicin was an accepted control treatment for randomized trials in HCC. Compared with doxorubicin monotherapy, the sorafenib-doxorubicin combination was associated with significantly increased median TTP as evaluated by radiologic progression (6.4 vs. 2.8 months; p = .02), progression-free survival (PFS; 6.0 vs. 2.7 months; p = .006), and OS (13.7 vs. 6.5 months; p = .006). This magnitude of benefit seems greater than that seen with sorafenib alone in the SHARP study but, due to the lack of a sorafenib monotherapy group, potential synergism between sorafenib and doxorubicin could not be confirmed. The study investigators appropriately concluded that the combination of sorafenib and doxorubicin should not yet be adopted into routine clinical use. However, the results of this trial served as the basis of an ongoing phase III Alliance (formerly Cancer and Leukemia Group B) trial comparing sorafenib plus doxorubicin with sorafenib alone (NCT01015833; ClinicalTrials.gov).
The efficacy of sorafenib in the advanced setting has led to the thought that antiangiogenic therapy could augment the efficacy of embolic therapy, particularly given findings that embolization results in transient but significant increases in serum VEGF [19]. The Sorafenib or Placebo in Combination with Transarterial Chemoembolization (SPACE) trial was a large randomized phase II trial (307 patients) that randomized patients to sorafenib 400 mg twice daily or placebo with drug-eluting bead transarterial chemoembolization (DEB-TACE) [20]. The primary endpoint was time to radiologic progression and OS was a secondary endpoint. For the sorafenib arm, the HR for TTP was 0.797 (95% CI: 0.588–1.080; p = .072) and the HR for OS was 0.898 (95% CI: 0.606–1.330; p = .295). The authors concluded that the study met its primary endpoint of improving TTP. However, the study was unusual in that a p value of .15 was considered significant based on the trial's design. Overall survival data were immature. These results do not suggest a large benefit of sorafenib in this setting, but they do support the continuation of more definitive studies, such as the ongoing Eastern Cooperative Oncology Group 1208 trial (transarterial chemoembolization with sorafenib versus transarterial chemoembolization alone; NCT01004978; ClinicalTrials.gov) in patients with liver-only disease.
Emerging Antiangiogenic Therapies for HCC
Phase III Trial of Sunitinib Versus Sorafenib
Several other drugs that target angiogenesis have been studied or are being evaluated in phase III trials for advanced HCC (Tables 1, 2). One agent to report in a phase III trial against sorafenib has been sunitinib. Sunitinib is an oral multitargeted TKI with activity against VEGFR-1, VEGFR-2, PDGFR-α, PDGFR-β, c-KIT, FLT3, and various other kinases [21]. The kinase inhibition spectrum of sunitinib is broader than sorafenib and broader than most other kinase inhibitors studied to date [22].
Table 1.
Randomized phase III antiangiogenic therapy trials
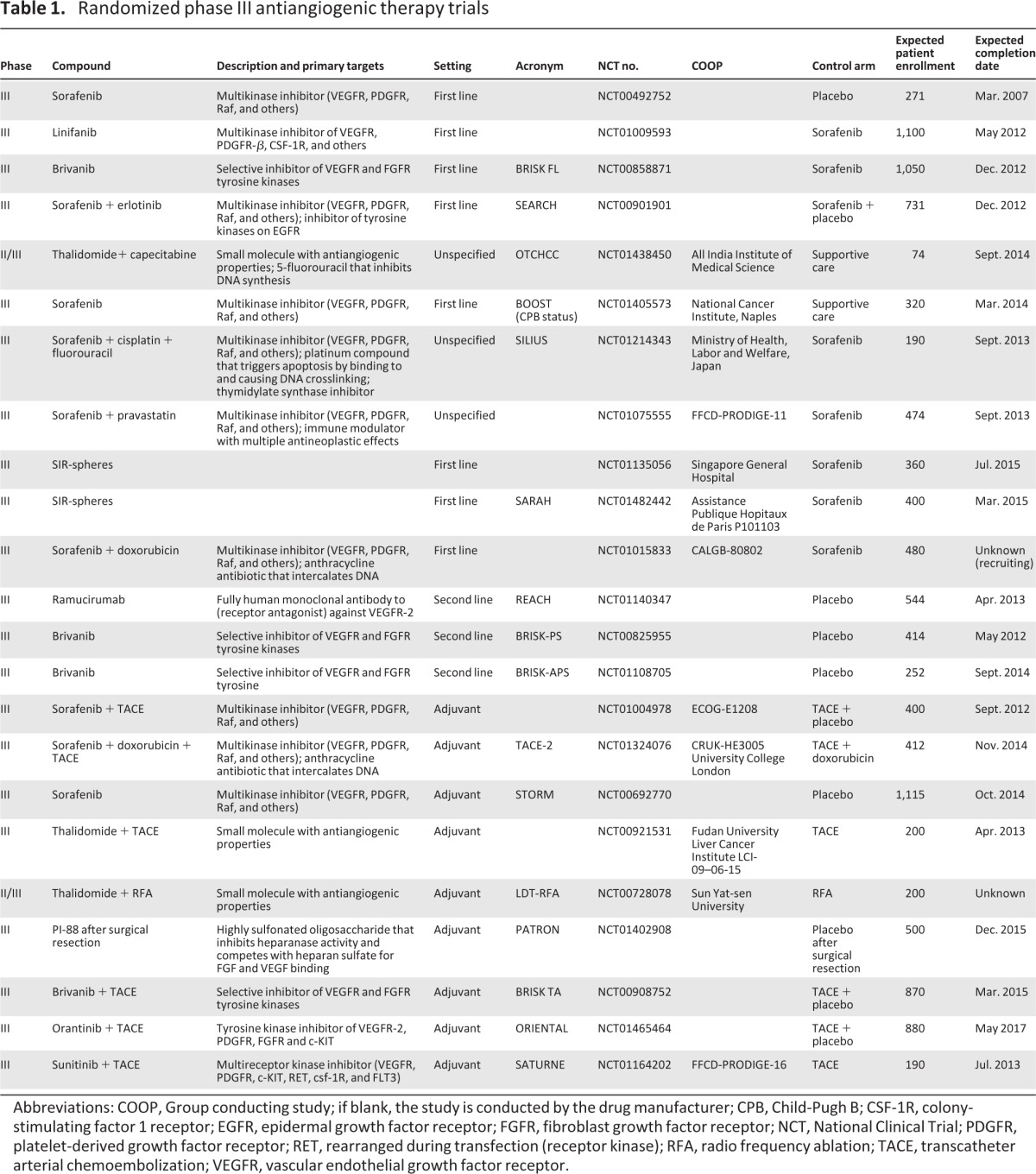
Abbreviations: COOP, Group conducting study; if blank, the study is conducted by the drug manufacturer; CPB, Child-Pugh B; CSF-1R, colony-stimulating factor 1 receptor; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; NCT, National Clinical Trial; PDGFR, platelet-derived growth factor receptor; RET, rearranged during transfection (receptor kinase); RFA, radio frequency ablation; TACE, transcatheter arterial chemoembolization; VEGFR, vascular endothelial growth factor receptor.
Table 2.
Randomized phase II antiangiogenic therapy trials
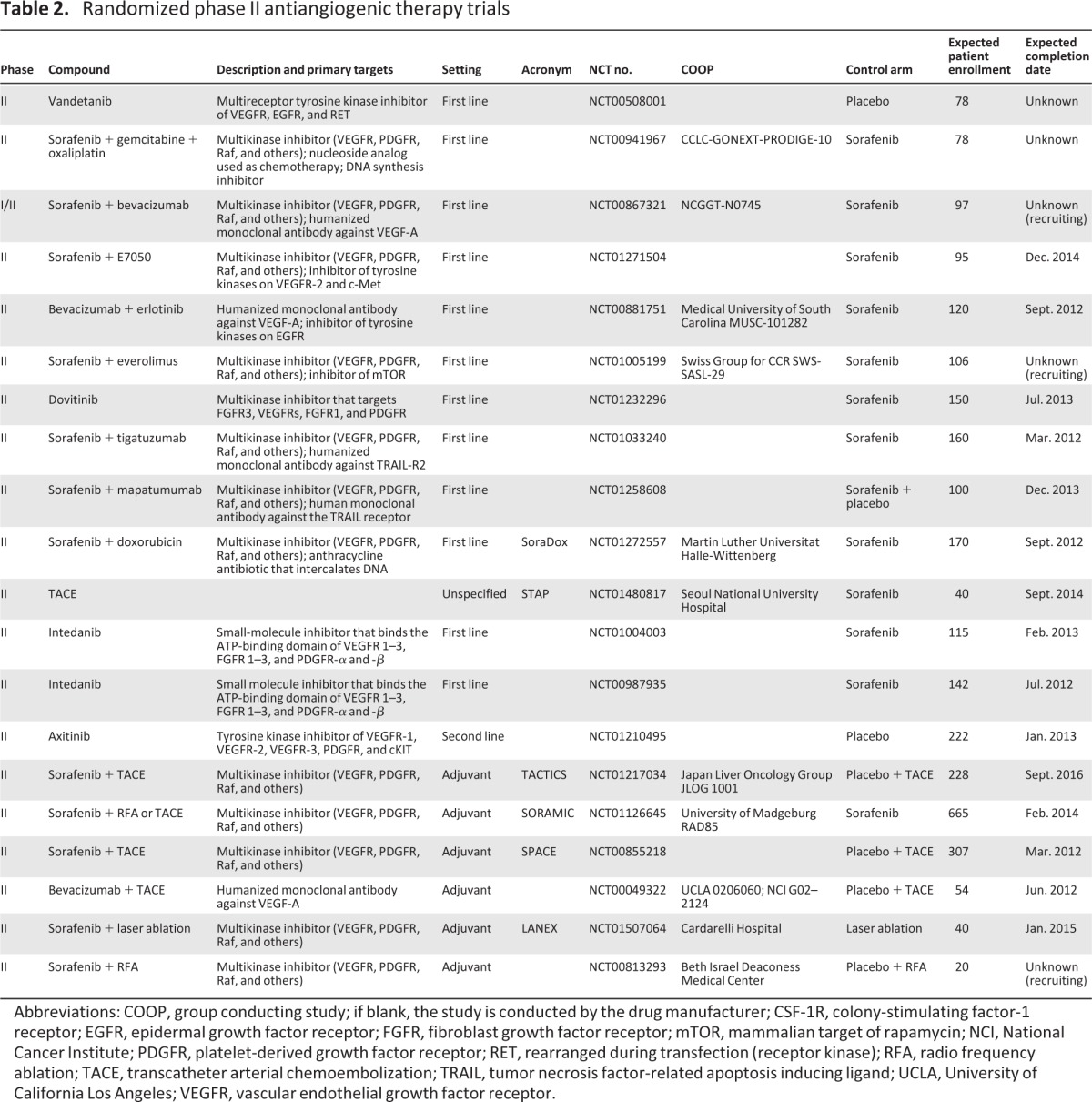
Abbreviations: COOP, group conducting study; if blank, the study is conducted by the drug manufacturer; CSF-1R, colony-stimulating factor-1 receptor; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; mTOR, mammalian target of rapamycin; NCI, National Cancer Institute; PDGFR, platelet-derived growth factor receptor; RET, rearranged during transfection (receptor kinase); RFA, radio frequency ablation; TACE, transcatheter arterial chemoembolization; TRAIL, tumor necrosis factor-related apoptosis inducing ligand; UCLA, University of California Los Angeles; VEGFR, vascular endothelial growth factor receptor.
Based on promising phase II results, a phase III study (SUN 1170) comparing sorafenib with sunitinib was performed [23]. More than 1,000 patients with Child-Pugh A liver disease and advanced HCC were randomized between sorafenib and sunitinib. Median OS was 10.0 months for sorafenib versus 8.1 months for sunitinib (HR: 1.31, 95% CI: 1.13–1.52; p = .0019) [23]. As such, the study did not meet prespecified endpoints of superiority or noninferiority for sunitinib. Interestingly, PFS was 3.0 versus 3.6 months (HR: 1.13, 95% CI: 0.98–1.29; p = .1386) in the sorafenib and sunitinib arms, respectively. Furthermore, serious adverse events were more common in the sunitinib group than in the sorafenib group. This study was therefore stopped early due to safety concerns with sunitinib and statistical inferiority in OS of patients taking sunitinib compared with sorafenib. As such, further development of sunitinib in HCC is unlikely.
Given the activity seen with bevacizumab, it is somewhat surprising that phase III trials have not yet occurred for HCC. This may in large part be due to concerns over variceal hemorrhage. In light of this risk, the use of screening upper endoscopy along with primary preventive strategies may help minimize the risk of such fatal hemorrhages.
Summary of Phase II/III Trials
A relatively large number of phase II studies have now been conducted with alternative antiangiogenic agents. Some of these alternative therapies could hold advantages over sorafenib due to differential selectivity of drug targets or could be useful in patients who have failed sorafenib.
Bevacizumab is a recombinant humanized antibody against VEGF isoform A (which acts primarily through VEGF receptors 1 and 2) that is currently FDA-approved for treating patients with metastatic colorectal cancer and advanced nonsquamous non-small cell lung cancer, metastatic kidney cancer, and glioblastoma multiforme [24]. In a phase II study enrolling 46 patients with unresectable HCC and Child-Pugh A or B liver dysfunction and no extrahepatic metastases, bevacizumab was given intravenously (IV) every 2 weeks at a dose of 5 (n = 12) or 10 mg/kg (n = 34) until disease progression [25]. The primary objective of this study was to determine whether 6-month PFS was greater than 60% in the population treated with bevacizumab (an endpoint used because response is considered to be unlikely with angiogenesis-targeting agents). The study found that 65% of patients were progression-free at 6 months (95% CI: 51%–79%). Median PFS was 6.9 months (95% CI: 6.5–9.1 months) and median OS was an encouraging 12.4 months (95% CI: 9.4–19.9 months). Grade 3 or 4 adverse events included hypertension (15%), major bleeding (11%, including 1 fatality due to variceal bleeding), and thrombosis (6%). As a secondary endpoint, significant reductions in tumor enhancement by dynamic contrast enhanced magnetic resonance imaging were documented but did not correlate with outcome. Other phase II studies of bevacizumab have shown similar activity [26–28]. Given the activity seen with bevacizumab, it is somewhat surprising that phase III trials have not yet occurred for HCC. This may in large part be due to concerns over variceal hemorrhage. In light of this risk, the use of screening upper endoscopy along with primary preventive strategies may help minimize the risk of such fatal hemorrhages [29].
A subsequent single-arm phase II trial enrolled 40 patients with advanced HCC with Child-Pugh A and Child-Pugh B liver dysfunction to receive bevacizumab (10 mg/kg every 14 days) and daily erlotinib (150 mg), an oral epidermal growth factor receptor (EGFR) inhibitor [30]. The result for the primary endpoint of the study (PFS at 16 weeks) was 62.5%, a figure considered positive per the trial design. Median PFS was a highly encouraging 9.0 months (95% CI: 26–45 weeks) and median OS was 15.7 months (95% CI: 48–78 weeks). The major adverse effects were fatigue (20%) and hypertension (15%). Two patients did develop life-threatening gastrointestinal bleeding and one eventually died. A large randomized phase II trial of this combination versus sorafenib is currently ongoing to determine whether the regimen should proceed to a phase III trial (NCT00881751; ClinicalTrials.gov).
VEGFR-2 signaling mediates most of the known cellular processes enacted by VEGF, including the promotion of angiogenesis in tumors [31]. Signaling through VEGFR-1, in contrast, appears to be only weakly involved in mediating the angiogenic effects of VEGF [32]. No drugs that specifically inhibit VEGFR-2 have been evaluated in patients with HCC until recently. Ramucirumab is a recombinant fully human monoclonal antibody that binds the extracellular domain of VEGFR-2. In contrast to other anti-VEGF drugs, such as bevacizumab, ramucirumab binds VEGFR-2 with high affinity and specificity, preventing all VEGF ligands from binding to VEGFR-2 [33]. A phase II study of ramucirumab was conducted in 42 previously untreated patients with Child-Pugh A or Child-Pugh B liver dysfunction [34]. Seventy-six percent of these patients had extrahepatic disease and 49% had HCV infection. Ramucirumab was given IV every 2 weeks until disease progression. Median PFS was 4.2 months for Child-Pugh A patients and 2.8 months for Child-Pugh B patients. The most common serious adverse events were hypertension (12%), fatigue (10%), and bleeding (7%). An ongoing phase III trial is evaluating the use of ramucirumab in patients with HCC in the second-line treatment setting after sorafenib (NCT0114034; ClinicalTrials.gov).
Linifanib is an orally available TKI with potent activity against VEGFR-1, -2, and -3 and PDGFR-β in kinase assays and a much more limited kinase inhibition spectrum than sorafenib or sunitinib [35]. In a multicenter phase II trial of linifanib with 44 patients with advanced HCC with Child-Pugh A or B liver dysfunction, median OS was 10.4 months (95% CI: 8.4–14.9) in the Child-Pugh A group (n = 38) and 2.5 months (95% CI: 1.1–4.5) in the Child-Pugh B group (n = 6) [36]. The most common serious side effects were hypertension (18%) and fatigue (14%). These results suggested that linifanib is clinically active in patients with advanced HCC and Child-Pugh A liver dysfunction. A phase III study comparing linifanib to sorafenib in patients with advanced HCC and Child-Pugh A status has completed enrollment and results are expected within the next year (NCT01009593; ClinicalTrials.gov).
Cediranib is another potent oral TKI that (relatively) specifically targets VEGFR-1 and -2 [37]. In a phase II study of daily oral cediranib enrolling 28 patients with unresectable HCC, median OS was 5.8 months (95% CI: 3.4–7.3 months) and the median TTP was 2.8 months (95% CI: 2.3–4.4 months) [37]. Twenty-six patients (93%) experienced a grade 3 or greater treatment-related adverse event; these included fatigue, anorexia, hypertension, and elevated alanine aminotransferase. Because of these toxicities, cediranib at the doses and schedule given in this trial does not appear to be as effective or safe as other agents in the class for patients with HCC.
Brivanib is an oral, selective, dual inhibitor of FGF receptor (FGFR) and VEGFRs that has demonstrated potent antitumor and antiangiogenic effects in preclinical models of various tumor types, including HCC [38, 39]. Brivanib has also been associated with delayed tumor growth and increased survival in an HCC xenograft model of acquired resistance to sorafenib [40]. A phase II study was completed in patients with advanced HCC with Child-Pugh A and B liver status who did not receive prior systemic therapy (first-line therapy cohort; n = 55) [41] or who received prior treatment with at least one regimen of antiangiogenic therapy, primarily sorafenib (second-line therapy cohort; n = 46) [42]; 64% of these patients were Asian. In the first-line therapy cohort, median OS was 10 months (95% CI: 6.8–15.2) and median PFS was 2.7 months (95% CI: 1.4–3.0), based on modified World Health Organization tumor response criteria [41]. Interestingly, results for the second-line therapy cohort were nearly as good as those for the untreated patients, with median OS of 9.79 months (95% CI: 5.52–13.17) [42]. This suggests that brivanib may in fact help reverse one or more mechanisms of resistance to sorafenib, as will be discussed further below. The most common treatment-related side effects were elevated liver enzymes (87.3% of patients experienced elevated aspartate aminotransferase and 85.5% of patients experienced elevated alanine aminotransferase), proteinuria (65.5%), fatigue (45.5%), hypertension (45.5%), nausea (38.2%), and diarrhea (41.8%) [41].
Brivanib has been or is being investigated in several phase III trials, including first-line treatment with brivanib versus sorafenib (BRISK-FL; NCT00858871; ClinicalTrials.gov), second-line treatment with brivanib after progression on sorafenib (BRISK-PS; NCT00825955; ClinicalTrials.gov), second-line treatment with brivanib after progression on sorafenib in patients from the Asia-Pacific region (BRISK-APS; NCT01108705; ClinicalTrials.gov), and transarterial chemoembolization with or without brivanib (BRISK-TA; NCT00908752; ClinicalTrials.gov). Results from the second-line study (after sorafenib failure) have been presented in abstract form [43]. BRISK-PS randomized 395 patients in a 2:1 fashion between brivanib 800 mg/day orally and placebo. Patients entering the study were required to have been treated with sorafenib for at least 14 days and then progressed with or became intolerant to sorafenib. The primary endpoint was overall survival and the study was statistically powered to detect an HR of 0.67. The study was conducted worldwide, and patient demographics reflected a slight preponderance of Asian HCC characteristics. The results demonstrated an HR of 0.89 with a difference in median overall survival of 1.2 months favoring the brivanib arm that did not reach statistical significance. Notably, there was increased incidence of portal vein thrombosis with brivanib (31% in the brivanib arm vs. 18% in the placebo arm). In spite of the study not meeting its primary endpoint, a difference of 1.5 months and an HR of 0.56 for TTP appears to confirm that brivanib has some activity in this patient population. These results do not rule out the possibility that brivanib will meet its planned endpoints in other trials.
Phase I/II Trials
In the past, pharmaceutical companies did not sponsor trials, especially with investigational agents, in patients with liver disease and HCC. Currently, a large number of antiangiogenic agents with the potential to affect tumor growth are being explored in clinical trials, with many in earlier stages of development. Some of the most promising agents include inhibitors of the mammalian target of rapamycin (mTOR) and multiple growth factor signaling pathways.
The mTOR signaling pathway is broadly involved in the translation of proteins, including proangiogenic factors (including VEGF). Inhibition of mTOR has been explored as a potential therapeutic strategy in renal cell carcinoma, a cancer that is clearly driven by VEGF production [44, 45]. Importantly, mTOR inhibition has been effective in tumors that are refractory to VEGF pathway inhibitors.
A phase I/II study has been completed using the oral mTOR inhibitor everolimus (at a dose of 10 mg/kg/day in phase II) in 28 patients with advanced HCC and Child-Pugh A and B liver dysfunction [46]. In all, 71% of the patients in this study had received prior therapy, including sorafenib. Median OS was 8.4 months (95% CI: 3.9–21.1) and median PFS was 3.8 months (95% CI: 2.1–4.6). Fatigue, hyperglycemia, and anemia were the most common side effects associated with everolimus. There is an ongoing global, randomized, double-blinded, placebo-controlled phase III trial investigating the use of everolimus in patients after the failure of sorafenib therapy (NCT01035229; Clinicaltrials.gov).
TSU-68 is an oral TKI that targets VEGFR-2, PDGFR, and FGFR [47]. A phase I/II study was performed with TSU-68 in 35 patients with advanced HCC with Child-Pugh A and B liver dysfunction [47]. None of these patients had been treated with sorafenib and 83% were HCV positive. The median OS was 13.1 months (95% CI: 6.9–26.6), although TTP was discordant at a median of 2.1 months (95% CI: 1.2–2.9). The main treatment-related adverse events associated with TSU-68 were diarrhea, anorexia, and abdominal pain. Based on the promising activity shown in this phase I/II trial, a phase III trial is currently recruiting participants.
Resistance to Antiangiogenic Agents
The approval of sorafenib marked a major milestone in the treatment of advanced HCC and has opened the door for the development of additional antiangiogenic agents. However, antiangiogenic agents have not been as successful as initially imagined, not only in HCC but also in other solid tumors [48, 49]. Tumors typically respond initially to anti-VEGF therapies with stability (rarely shrinkage), then quickly become resistant.
Several mechanisms have been proposed to explain how cancers resist VEGF inhibition. There appears to be adaptive or evasive resistance to VEGF inhibition along with intrinsic resistance [48]. The upregulation of alternative proangiogenic signaling pathways within tumors is thought to circumvent VEGF inhibition; a well-described alternative signaling pathway involves FGF and the FGFRs [50]. In a preclinical pancreatic neuroendocrine tumor model, FGF levels were noted to be much higher in tumors treated with VEGF inhibitors when compared with untreated tumors [51]. Furthermore, VEGF inhibition with concurrent FGF blockade showed decreased tumor growth compared to VEGF inhibition alone. A clinical study of 5-FU, leucovorin, and irinotecan (FOLFIRI) plus bevacizumab in patients with metastatic colon cancer showed that FGF levels were elevated in patient plasma both prior to and after disease progression [52]. Several other cytokines and angiogenic factors were also significantly elevated in patient plasma (compared to baseline) before measurable radiographic progression, including hepatocyte growth factor, placental growth factor, stromal-derived factor-1, and macrophage chemoattractant protein-3 [52]. Recently, upregulation of the FGF-8 subfamily has been shown to promote tumor cell survival in HCC [53].
Because of structural similarities in the kinase domains of several growth factor receptors, some TKIs have multiple-receptor specificity. It is therefore not surprising that ongoing clinical trials include agents such as brivanib, dovitinib, and BIBF 1120 that target both FGFR and VEGFR. These studies may provide interesting information about whether dual inhibition of FGFR and VEGFR is more effective in the front-line setting or around the time of progression.
VEGF and VEGFR-targeting therapy has represented the first real success in HCC in many years, reinvigorating research in this deadly and difficult malignancy. The success of targeting VEGF receptors now needs to be followed by better understanding of mechanisms of intrinsic and acquired resistance to these agents. As our understanding evolves, next-generation anti-VEGFR agents and agents targeting alternative proangiogenic pathways hold significant promise for the treatment of HCC.
Acquired resistance to VEGF inhibition may also be related to changes in vascular stromal cells [54]. In a mouse xenograft model of non-small cell lung adenocarcinoma, increased expression of components of the EGFR and FGFR signaling pathways were evident in the gene expression profile of the stromal cell compartment [54]. In bevacizumab-resistant tumors, pericytes expressed higher levels of EGFR than control (non-bevacizumab resistant) tumors. Furthermore, bevacizumab-resistant tumors exhibited an increase in pericyte coverage of vessels—a necessary step in angiogenesis. Targeting both the EGFR and VEGF pathways with bevacizumab and erlotinib in mouse xenografts significantly delayed the onset of therapeutic resistance compared with inhibition of either pathway alone [54]. This suggests that tumors may switch from VEGFR- to EGFR-driven angiogenesis as resistance occurs, and it may explain the efficacy of the bevacizumab/erlotinib combination mentioned earlier.
Future Directions for Antiangiogenic Therapy
Proteins other than growth factors play key roles in tumor angiogenesis and may eventually serve as therapeutic targets in the quest to prevent resistance to antiangiogenic treatment. For example, hypoxia inducible factor (HIF)-1α, a proangiogenic transcription factor upstream of VEGF and FGF, may participate in resistance to VEGF inhibition [55]. Increased tumor hypoxia due to VEGF inhibition has been shown to lead to increased expression of HIF-1α. In a non-HCC mouse xenograft model, the addition of topotecan, a potent HIF-1α inhibitor, to bevacizumab treatment led to significant reduction in tumor growth compared to bevacizumab or topotecan treatment alone [55]. Similarly, specificity protein (SP)-1 is another transcription factor that is involved in the expression of VEGF and other proangiogenic mediators [56]. Inhibition of SP-1 with mithramycin A appears to have antiangiogenic and antitumor activity in mouse pancreatic cancer xenografts [56]. These preclinical studies suggest that targeting transcription factors such as HIF-1α and SP-1 (with drugs) would be a worthwhile strategy to investigate in tumors with robust angiogenesis.
Blood vessel integrity and normalization are emerging as critical parts of understanding angiogenesis in cancer [57]. The angiopoietin (ANGPT)-TIE (tyrosine kinase with immunoglobulin-like and EGF-like domains) system is thought to be intimately involved in vascular maintenance. ANGPTs are ligands that bind to TIE-2 receptors on vascular endothelial cells [58], influencing the maintenance of existing blood vessels and maturation of new vessels. To take advantage of this interaction, several drugs targeting the ANGPTs and TIE2 are currently in development for solid tumors and may represent an additional opportunity to target tumor vasculature [58].
VEGF and VEGFR-targeting therapy has represented the first real success in HCC in many years, reinvigorating research in this deadly and difficult malignancy. The success of targeting VEGF receptors now needs to be followed by better understanding of mechanisms of intrinsic and acquired resistance to these agents. As our understanding evolves, next-generation anti-VEGFR agents and agents targeting alternative proangiogenic pathways hold significant promise for the treatment of HCC.
At present, the lack of good radiographic and biologic biomarkers remains an additional challenge for the development of effective therapies for HCC. However, using newer imaging modalities to detect early biologic changes is an active area of research. For example, a small retrospective study demonstrated that baseline positron emission tomography-computed tomography (PET-CT) scans may predict OS and PFS for patients who undergo treatment with sorafenib [59]. Imaging techniques such as computed tomography or magnetic resonance perfusion imaging and PET-CT hold promise in assessing responses in HCC and will aid the development of biologic therapies in the future [60].
Acknowledgments
We thank Cailin Moira Wilke, Ph.D., of StemScientific (funded by Bristol-Myers Squibb) for providing assistance with editing and generation of figures.
Author Contributions
Conception/Design: Keeran R. Sampat, Bert O'Neil
Collection and/or assembly of data: Keeran R. Sampat, Bert O'Neil
Manuscript writing: Keeran R. Sampat, Bert O'Neil
Final approval of manuscript: Keeran R. Sampat, Bert O'Neil
Disclosures
Bert O'Neil: Bayer, Bristol-Myers Squibb, Genentech (C/A); Bayer, Novartis, GlaxoSmithKline (RF). The other author indicated no financial relationships.
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Nathan H, Schulick RD, Choti MA, et al. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 5.Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: Consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández M, Semela D, Bruix J, et al. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Dhar DK, Naora H, Yamanoi A, et al. Requisite role of VEGF receptors in angiogenesis of hepatocellular carcinoma: A comparison with angiopoietin/Tie pathway. Anticancer Res. 2002;22:379–386. [PubMed] [Google Scholar]
- 8.Tseng PL, Tai MH, Huang CC, et al. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349–357. doi: 10.1002/jso.21109. [DOI] [PubMed] [Google Scholar]
- 9.Park YN, Kim YB, Yang KM, et al. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- 10.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 11.Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8:483–489. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- 12.Yoshiji H, Kuriyama S, Yoshii J, et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology. 2002;35:834–842. doi: 10.1053/jhep.2002.32541. [DOI] [PubMed] [Google Scholar]
- 13.Nexavar [package insert] Wayne, NJ: Bayer Healthcare Pharmaceuticals; 2011. [Google Scholar]
- 14.Huynh H, Ngo VC, Koong HN, et al. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Cell Mol Med. 2009;13:2673–2683. doi: 10.1111/j.1582-4934.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 16.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 17.Marrero JA, Lencioni R, Kudo M, et al. Global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafenib (GIDEON) second interim analysis in more than 1,500 patients: Clinical findings in patients with liver dysfunction. J Clin Oncol. 2011;29(suppl 15):4001. [Google Scholar]
- 18.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 19.Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. ASCO Meeting Abstracts. 2012;30:LBA154. [Google Scholar]
- 21.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 22.Weis SM, Cheresh DA. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 23.Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2011;29(suppl 15) Abstract 4000. [Google Scholar]
- 24.Avastin [package insert] South San Francisco, CA: Genetech Inc; 2009. [Google Scholar]
- 25.Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun W, Sohal D, Haller DG, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer. 2011;117:3187–3192. doi: 10.1002/cncr.25889. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CH, Yang TS, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981–986. doi: 10.1038/sj.bjc.6605580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]
- 29.Vlachogiannakos J, Goulis J, Patch D, et al. Primary prophylaxis for portal hypertensive bleeding in cirrhosis. Aliment Pharmacol Ther. 2000;14:851–860. doi: 10.1046/j.1365-2036.2000.00778.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–850. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]
- 31.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34:1785–1788. doi: 10.1248/bpb.34.1785. [DOI] [PubMed] [Google Scholar]
- 33.Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu AX, Finn RS, Mulcahy MF, et al. A phase II study of ramucirumab as first-line monotherapy in patients (pts) with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2010;28(suppl 15):4083. [Google Scholar]
- 35.Wong CI, Koh TS, Soo R, et al. Phase I and biomarker study of ABT-869, a multiple receptor tyrosine kinase inhibitor, in patients with refractory solid malignancies. J Clin Oncol. 2009;27:4718–4726. doi: 10.1200/JCO.2008.21.7125. [DOI] [PubMed] [Google Scholar]
- 36.Toh H, Chen P, Carr BI, et al. Linifanib phase II trial in patients with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2010;28(suppl 15):4038. [Google Scholar]
- 37.Alberts SR, Fitch TR, Kim GP, et al. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: A phase II north central cancer treatment group clinical trial. Am J Clin Oncol. 2012;35:329–333. doi: 10.1097/COC.0b013e3182118cdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huynh H, Ngo VC, Fargnoli J, et al. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 39.Bhide RS, Lombardo LJ, Hunt JT, et al. The antiangiogenic activity in xenograft models of brivanib, a dual inhibitor of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinases. Mol Cancer Ther. 2010;9:369–378. doi: 10.1158/1535-7163.MCT-09-0472. [DOI] [PubMed] [Google Scholar]
- 40.Tovar V, Cornella H, Villanueva A, et al. FGF signaling dysregulation in HCC and role in the development of acquired resistance to anti-angiogenic therapies. Paper presented at: 62nd Annual Meeting of the American Association for the Study of Liver Diseases; November 4–8, 2011; San Francisco, CA. [Google Scholar]
- 41.Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973–1983. doi: 10.1158/1078-0432.CCR-10-2011. [DOI] [PubMed] [Google Scholar]
- 42.Finn RS, Kang YK, Mulcahy M, et al. Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2090–2098. doi: 10.1158/1078-0432.CCR-11-1991. [DOI] [PubMed] [Google Scholar]
- 43.Llovet JM, Decaens T, Raoul J-L, et al. Brivanib versus placebo in patients with advanced hepatocellular carcinoma (HCC) who failed or were intolerant to sorafenib: results from the phase 3 BRISK-PS study. J Hepatol. 2012;56(suppl 2):S549. [Google Scholar]
- 44.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 45.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 46.Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–5102. doi: 10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanai F, Yoshida H, Tateishi R, et al. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2011;67:315–324. doi: 10.1007/s00280-010-1320-2. [DOI] [PubMed] [Google Scholar]
- 48.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10:992–1000. doi: 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 50.Lieu C, Heymach J, Overman M, et al. Beyond VEGF: Inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17:6130–6139. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gauglhofer C, Sagmeister S, Schrottmaier W, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53:854–864. doi: 10.1002/hep.24099. [DOI] [PubMed] [Google Scholar]
- 54.Cascone T, Herynk MH, Xu L, et al. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest. 2011;121:1313–1328. doi: 10.1172/JCI42405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapisarda A, Hollingshead M, Uranchimeg B, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. 2009;8:1867–1877. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan P, Wang L, Wei D, et al. Therapeutic inhibition of Sp1 expression in growing tumors by mithramycin a correlates directly with potent antiangiogenic effects on human pancreatic cancer. Cancer. 2007;110:2682–2690. doi: 10.1002/cncr.23092. [DOI] [PubMed] [Google Scholar]
- 57.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 58.Huang H, Bhat A, Woodnutt G, et al. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Park JY, Kim do Y, et al. Prognostic value of 18F-FDG PET for hepatocellular carcinoma patients treated with sorafenib. Liver Int. 2011;31:1144–1149. doi: 10.1111/j.1478-3231.2011.02541.x. [DOI] [PubMed] [Google Scholar]
- 60.Jiang T, Zhu AX, Sahani DV. Established and novel imaging biomarkers for assessing response to therapy in hepatocellular carcinoma. J Hepatol. 2012;58:169–177. doi: 10.1016/j.jhep.2012.08.022. [DOI] [PubMed] [Google Scholar]