This retrospective study examines the incidence and causes of drug-induced pulmonary toxicity and classifies high-resolution computed tomography findings for antitumor-therapy associated pulmonary toxicity based on characteristic patterns and pathological considerations, with a special focus on gemcitabine-induced pulmonary toxicity.
Keywords: Pulmonary toxicity, Gemcitabine, Hypersensitivity pneumonitis, High-resolution CT, Antitumor drugs, Lung pathology
Abstract
Background.
Gemcitabine (GEM) is widely used as a chemotherapeutic agent. However, pulmonary toxicity has been rarely observed with GEM use. This article aims to determine the incidence and causes of drug-induced pulmonary toxicity, and to classify the high-resolution computed tomography (HRCT) findings for antitumor therapy-associated pulmonary toxicity based on characteristic patterns and pathological considerations, with a special focus on GEM-associated pulmonary toxicity (GAPT).
Methods.
Medical records of all patients with drug-induced pulmonary toxicity seen at Kyorin University hospital between April 2006 and December 2011 were retrospectively reviewed. The study examined correlations between HRCT and the assessed pathological or clinical findings, with a specific focus on antitumor drugs.
Results.
We identified 66 patients with drug-induced pulmonary toxicity. Among the antitumor drugs, GEM was the primary offending agent (n = 8) for pulmonary toxicity followed by docetaxel and gefitinib. HRCT patterns for the eight GAPT patients included the non-specific interstitial pneumonia (NSIP; n = 5) and the hypersensitivity pneumonitis (HP)-like pattern (n = 3). In contrast, four patients in the study were found to have the HP-like pattern, with three cases associated with GEM and one case associated with imatinib mesylate. The transbronchial lung biopsy or video-assisted thoracic surgery specimens for these patients showed granuloma or organizing tissue with a random distribution that was independent of the respiratory bronchiole. These results appeared to correspond to the HRCT-determined centrilobular nodules.
Conclusion.
GEM was the leading cause of drug-induced pulmonary toxicity in the patients examined in this study. This toxicity appears as NSIP or an HP-like pattern during HRCT examinations. This HP-like pattern may be useful for diagnosing GEM-induced pulmonary toxicity, as well as demonstrating granuloma or organizing tissue during lung pathology examinations.
Implications for Practice:
Gemcitabine (GEM) is widely used as a chemotherapeutic agent in the treatment of non-small cell lung cancer and pancreatic cancer. It has also been actively used for breast, urothelial, and ovarian cancers. Because pulmonary toxicity has been rarely observed with GEM use, there are few relevant HRCT findings; thus, both the lung pathology and clinical findings have yet to be examined in detail. This study showed that GEM-associated pulmonary toxicity (GAPT) was divided into either NSIP pattern or HP-like pattern on HRCT. We successfully compared the HP-like pattern and pathological findings in patients with GAPT. Based on the pathological findings of the specimens obtained from transbronchial lung biopsy or video-assisted thoracic surgery, HP-like pattern depicted as HRCT-determined centrilobular nodule are corresponded to the granuloma or organizing tissue with a random distribution that was independent of the respiratory bronchiole.
Introduction
Gemcitabine (GEM) is a major chemotherapeutic agent with tolerable side effects, such as nausea, vomiting, skin rash, fever, peripheral edema, and myelosuppression, which is the most common dose-limiting toxicity [1]. However, there have been few reports published on GEM-associated pulmonary toxicity (GAPT), which may be the reason for the low incidence or possible underestimation of this side effect. Because of this lack of previous data, it can be difficult to make GAPT diagnoses based on high-resolution computed tomography (HRCT) findings. In the current study, we examined eight patients with GAPT using HRCT and then correlated the results with transbronchial lung biopsy (TBLB) pathology findings.
Patients and Methods
This retrospective study was approved by the Ethics Board of Kyorin University (Mitaka, Tokyo, Japan). This single-institution study retrospectively assessed 66 patients who were diagnosed with drug-induced pneumonia. All patients were referred to the pulmonary disease center in our hospital in Mitaka City, Tokyo, Japan, between April 2006 and December 2011. The study additionally focused on 28 patients with antitumor-therapy associated pulmonary toxicity.
To be enrolled in the study, patients had to have the presence of abnormal lung lesions after commencing antitumor drug treatment. Additionally, all patients had to have infectious pneumonia, cardiogenic pulmonary edema, and diffuse alveolar hemorrhage clinically ruled out as being a possible cause. The HRCT findings determined at the time of the diagnosis were classified as nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), hypersensitivity pneumonitis (HP)-like pattern, and diffuse alveolar damage (DAD) pattern. The classification of the interpretations followed the Glossary of Terms for Thoracic Imaging proposed by the Fleischner Society [2]. We also assessed the involved area in 28 cases that were associated with antitumor drugs, with the specific drug responsible for the toxicity determined in each of the cases.
The upper zone was defined as the part of the lung above the level of the tracheal carina, whereas the lower zone was defined as the part of the lung below the level of the inferior pulmonary vein. The middle zone was defined as the portion of the lung between the upper and lower zones. The proportion of the involved area for each zone was assessed in accordance with a previously described method [3]. Briefly, visual scores were defined as follows: grade 0, no opacity; grade 1, <5% opacity; grade 2, 5%–24% opacity; grade 3, 25%–49% opacity; grade 4, 50%–74% opacity; and grade 5, >75% opacity. We also analyzed the correlation between the total score and the associated laboratory data. Although laboratory data were used at the time of diagnosis, the coefficient of correlation assessed between the total score and the serum data was used for all subsequent analyses. Pulmonary pathologists and radiologists with >15 years of experience who were blinded to the clinical findings of the patients independently reviewed the biopsied specimens or HRCT findings, with decisions interpreted by consensus.
Statistical Analyses
Data were statistically analyzed using Pearson's χ2 test or the Mann-Whitney test and SPSS version 19. Statistical significance was defined as a p value of <.05 in paired two-sided tests.
Results
Drugs Associated with Pulmonary Toxicity in the Study Patients
A total of 66 patients were found to have drug-associated pulmonary toxicity. The offending drugs were classified in accordance with their mechanism and included antitumor drugs (n = 28, 42%), antirheumatic drugs (n = 16, 24%), antiarrhythmic drugs (n = 6, 9%), nonsteroidal anti-inflammatory drugs (n = 4, 6%), kampo medicine (n = 3, 5%), and others (n = 9, 14%). Thus, the data from our hospital indicated that antitumor drugs were the most common cause of pulmonary toxicity.
Clinical Demographics of 28 Patients with Antitumor Drug-Associated Pulmonary Toxicity
Table 1 presents the clinical demographics for the 28 patients who had pulmonary toxicity associated with antitumor drugs. Patient age (mean ± SD) was 69.4 ± 9.4 years and there was a 5:4 male-to-female ratio. GEM was the leading cause of the drug-associated pulmonary toxicity (n = 8, 28.6%), followed by docetaxel (n = 4, 14.3%) and gefitinib (n = 4, 14.3%).
Table 1.
Patient demographics for antitumor drugs associated with pulmonary toxicity.
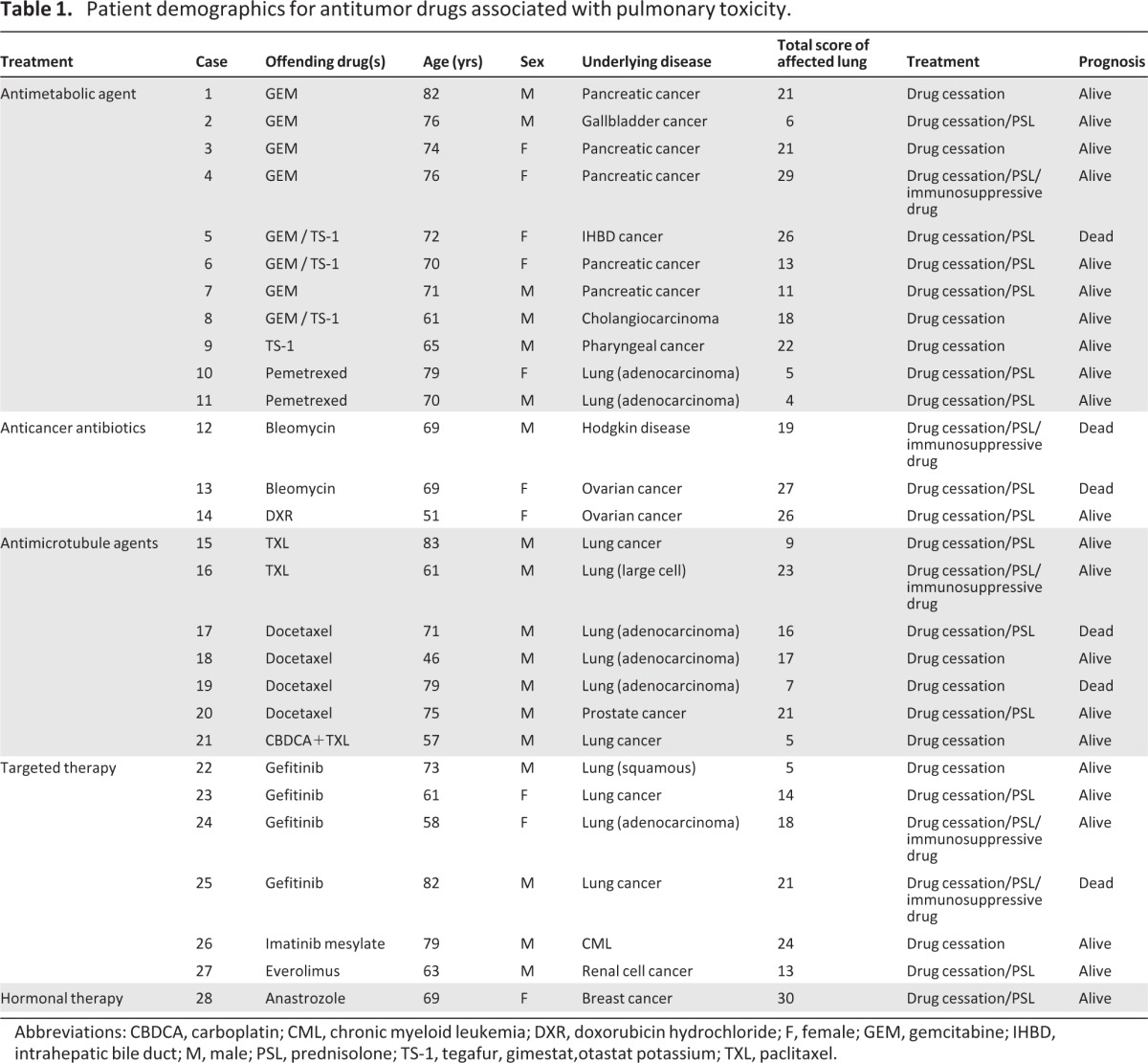
Abbreviations: CBDCA, carboplatin; CML, chronic myeloid leukemia; DXR, doxorubicin hydrochloride; F, female; GEM, gemcitabine; IHBD, intrahepatic bile duct; M, male; PSL, prednisolone; TS-1, tegafur, gimestat,otastat potassium; TXL, paclitaxel.
During the study period, a total of 454 patients were treated with GEM. Out of these patients, eight (1.7%) had GAPT, with five being treated for pancreatic cancer, one for intrahepatic bile duct cancer, one for cholangiocarcinoma, and one for gallbladder cancer. However, GAPT did not occur in any of the patients with non-small cell lung cancer. The treatments for the 28 patients who had pulmonary toxicity associated with antitumor drugs were drug cessation only (n = 9, 32.1%), followed by drug cessation plus prednisolone (n = 14, 50%), and drug cessation plus prednisolone with immunosuppressive drugs (n = 5, 17.9%). The survival rate was 78.6% (n = 22) during the investigational period, with six patients (21.4%) dying from drug-induced pulmonary toxicity.
Note that the patient with chronic myeloid leukemia and drug-induced pneumonitis associated with imatinib mesylate was previously reported in one of our earlier studies [4].
Relevance Between the Total Score of the Affected Lung Area and the Laboratory Findings in Patients with Antitumor-Associated Pulmonary Toxicity
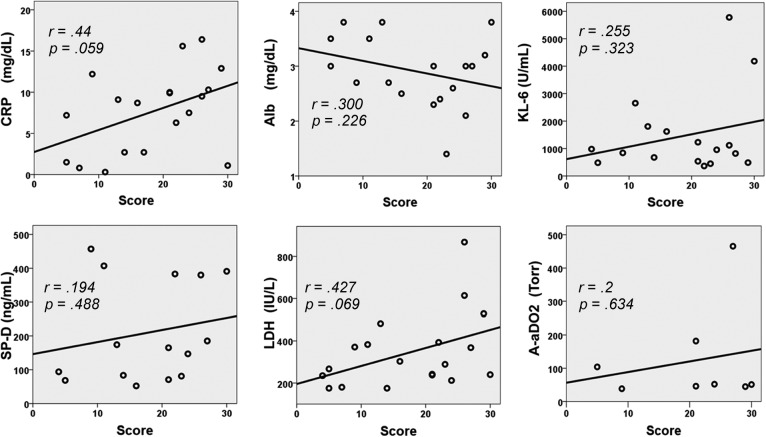
No apparent associations were found between the total score of the affected lung and the antitumor drug groups (antimetabolic agents, anticancer antibiotics, antimicrotubule agents, targeted therapies, and hormonal therapy). Figure 1 shows the relevance between the total score of the affected lung area and the laboratory findings for the patients with antitumor-associated pulmonary toxicity. Although the coefficient of correlation between the total score (Table 1, right column) and the serum C-reactive protein (CRP) or serum lactate dehydrogenase (LDH) appeared to be positive, the results were found not to be statistically significant (CRP: r = 0.44, p = .059; LDH: r = 0.427, p = .069). In addition, the other laboratory data also did not show any apparent correlation with the total score.
Figure 1.
Relevance between the total score of the affected lung area and the laboratory findings in patients with antitumor-associated pulmonary toxicity. Although serum C-reactive protein (CRP) and lactate dehydrogenase (LDH) appeared to have a positive coefficient of correlation, the results were not statistically significant (CRP: r = 0.44, p = .059, LDH: r = 0.427, p = .069). All other data exhibited no correlation with the total score.
Abbreviations: A-aDO2, alveolar-arterial oxygen difference; Alb, albumin; CRP, C-reactive protein; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; SP-D, surfactant protein-D.
Classification of Antitumor Drug-Associated Pulmonary Toxicity in Accordance to the HRCT Pattern
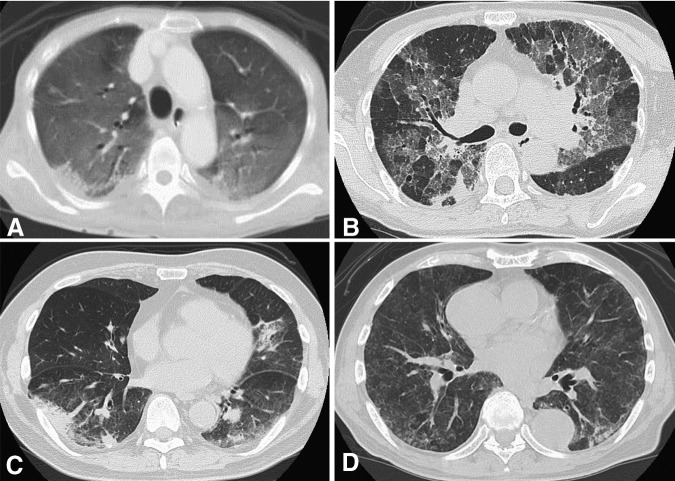
HRCT findings were separated into four pattern groups that included NSIP, HP, DAD, and OP; the numbers of patients in each category were 20 (71%), 4 (14%), 3 (11%), and 1 (4%), respectively. Representative HRCT patterns are presented in Figure 2. Of the four patients exhibiting the HP-like pattern, three were associated with GEM (cases 1, 3, and 6), whereas one was associated with imatinib mesylate (case 26). For the three patients exhibiting the DAD pattern, two were associated with bleomycin (cases 12 and 13), whereas one was associated with gefitinib (case 25). The OP pattern, which was only found in case 27, was associated with everolimus.
Figure 2.
High-resolution computed tomography (HRCT) patterns for the 28 patients were classified into four patterns, which included (A) diffuse alveolar damage (case 12), (B) nonspecific interstitial pneumonia (case 5), (C) organizing pneumonia (case 27), and (D) hypersensitivity pneumonitis-like pattern (case 26). HRCT pattern definitions were as follows: (A): Diffuse hazy increased opacity of the lung, with preservation of bronchial and vascular margins. (B): Ground-glass opacities with reticulation, traction bronchiectasis, or bronchiolectasis, with little or no honeycombing. (C): Consolidation appears as a homogeneous increase in the pulmonary parenchymal attenuation that obscures the margins of the vessels and airway walls. (D): Random distribution of tiny centrilobular nodules.
Correlation Between the HRCT-Determined HP-Like Pattern and the Lung Pathology
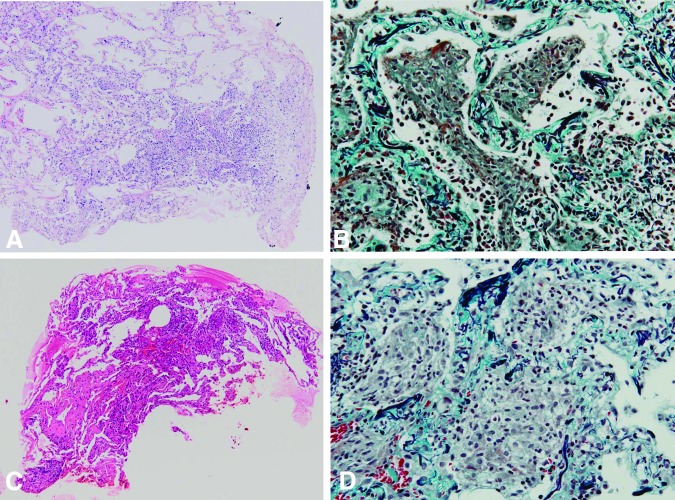
This study focused on the HP-like pattern, which showed scattered centrilobular nodules in a random distribution. As reported in our previous study [4], we performed video-assisted thoracic surgery in case 26 and determined that the nodules in the biopsied specimen contained a patchy distribution of epithelioid cell granulomas, with their transitional form exhibiting intra-alveolar organization. Of special note in this case, the lung pathology also showed that the lesions were distributed independent of the respiratory bronchioles. As previously mentioned, the HRCT for cases 1 and 3 exhibited the HP-like pattern; similar to case 26, the TBLB specimens for both of these cases clearly demonstrated exudative tissue within the alveolar area (Fig. 3A–D).
Figure 3.
Transbronchial lung biopsy pathology evaluation in patients with the hypersensitivity pneumonitis-like pattern determined by high-resolution computed tomography. Staining by hematoxylin and eosin (A) or by Elastica-Masson (B) in case 1 demonstrated alveolar air spaces that were filled with an exudative fluid and which contained mild inflammatory cells with desquamated tracheal epithelial cells. In case 3, both hematoxylin and eosin (C) and the Elastica-Masson stain (D) showed exudative fluid in the alveolar air spaces, along with an abundant accumulation of alveolar macrophages.
GAPT Findings
Of the eight patients with GAPT in this case series, five had pancreatic cancer (Table 2). Among these eight cases, the HRCT pattern was determined to be NSIP in five patients (cases 2, 4, 5, 7, and 8), whereas three patients had the HP-like pattern (cases 1, 3, and 6). In case 8, the TBLB pathology evaluation revealed a granuloma formation that was located apart from the respiratory bronchiole (figure not shown), which mimics the pathology seen for the HP-like pattern (Fig. 3). The average number of GEM doses was 9.5 ± 5.6 (mean ± SD), with the number of doses given ranging from 4 to 21 over a duration of 42 to 205 days after commencing the treatment. None of the patients had any risk factors for GAPT, such as exposure to asbestos or radiation [5]. All patients exhibited an improvement of their pulmonary toxicity after cessation of drug administration or after treatment with prednisolone or an immunosuppressive drug. However, patient 5 died due to progression of intrahepatic bile duct cancer.
Table 2.
Clinical characteristics of eight patients associated with gemcitabine-induced lung disease

Abbreviations: F, female; GEM, gemcitabine; HP, hypersensitivity pneumonitis; HRCT, high-resolution computed tomography; IHBD, intrahepatic bile duct; M, male; NSIP, nonspecific interstitial pneumonia; PSL, prednisolone; TBLB, transbronchial lung biopsy.
Discussion
The present study showed that antitumor agents were the most common cause of drug-induced pulmonary toxicity in our hospital. The incidence of drug-induced pulmonary toxicity has been shown to differ from drug to drug, with reports of incidence varying over a wide range. For example, drug-induced pulmonary toxicity has been reported for antimetabolic agents: GEM, 0.02%–0.27%; pemetrexed, 0.5% [6]; anticancer antibiotics (for bleomycin, 20% [7]; for doxorubicin, rare but exact incidence remains unknown); antimicrotubule agents (paclitaxel, 0.73%–12%; docetaxel, 7%–26%), and targeted therapy (gefitinib, 1%–2%) [8]. Among these, GEM has been most frequently found to be associated with the toxicity, with the HRCT showing NSIP or HP-like patterns in these cases. At present, however, the mechanism of pulmonary toxicity remains unclear.
Incidence of GAPT has been shown to vary from 0.02% to 0.27% [9], whereas severe cases have been reported to be 0.002% [9]. In contrast to these previous results, our study indicated there was a somewhat higher incidence (1.7%). Although the exact reason for our findings remains unknown, the fact that our study included many patients with digestive organ cancer might have influenced the current results. Because these pathological assessments have rarely been reported [10], a full evaluation of the significance of the correlations between the HRCT findings and the lung pathology has yet to be undertaken.
We previously reported on the significance of the HRCT results showing the presence of the HP-like pattern in a 79-year-old man being treated with imatinib mesylate for his chronic myelocytic leukemia [4]. Specimens were previously obtained in this subject by using video-assisted thoracic surgery. The HRCT-determined centrilobular nodules were identified as the HP-like pattern, and they corresponded to a random distribution of granuloma or organizing tissue that was not relevant to the respiratory bronchiole. When we performed TBLB in two of the three cases shown to have the HP-like pattern in the current study (cases 1 and 3), the exudative inflammation that was found within the alveolar area seemed to correspond to the HRCT-determined centrilobular nodules.
Although the present study is somewhat limited due to only having lung pathology from two cases, the HP-like pattern may indeed be able to provide clinical clues for diagnosing GAPT in the future. A further limitation in this retrospective study was that it was conducted by a single institution with only a small number of patients with GAPT. To definitively clarify the current findings, a larger number of cases will need to be examined in a future study.
Our HRCT results also showed that there was no significant correlation between the total affected lung area in patients with antitumor-associated pulmonary toxicity and the serum laboratory data (Fig. 2). Thus, placing a greater emphasis on interpretations of the HP-like pattern may be beneficial during patient evaluations, especially in cases where GAPT is suspected.
In previous case series reviews, several studies have reported that the average number of doses of GEM before the onset of symptoms ranged from 3.08 [11] to 5.4 [12]. In our present study, we found a much larger number of administrations, with an average dose number of 9.5 (Table 2). Interestingly, because we found that GAPT mainly occurred in patients with pancreatic cancer, the differences reported for the treatment doses might be related to the type of cancer for the patients enrolled in the studies.
Acknowledgments
Masaki Tamura and Takeshi Saraya contributed equally to this work.
Author Contributions
Conception and design: Masaki Tamura, Takeshi Saraya, Hajime Takizawa, Hajime Goto
Provision of study materials or patients: Masaki Tamura, Takeshi Saraya
Collection and/or assembly of data: Masaki Tamura, Takeshi Saraya
Data analysis and interpretation: Masaki Tamura, Takeshi Saraya, Masachika Fujiwara, Sayuki Hiraoka, Takuma Yokoyama, Kinuko Yano, Haruyuki Ishii, Junji Furuse, Tomoyuki Goya, Hajime Takizawa, Hajime Goto
Manuscript writing: Masaki Tamura, Takeshi Saraya
Final approval of manuscript: Takeshi Saraya
Disclosures
The authors indicated no financial relationships.
References
- 1.Saif MW. Pulmonary toxicity associated with gemcitabine. JOP. 2010;11:189–190. [PubMed] [Google Scholar]
- 2.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 3.Akira M, Inoue G, Yamamoto S, et al. Non-specific interstitial pneumonia: Findings on sequential CT scans of nine patients. Thorax. 2000;55:854–859. doi: 10.1136/thorax.55.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koide T, Saraya T, Nakamoto K, et al. [A case of imatinib mesylate-induced pneumonitis based on the detection of epithelioid granulomas by video-assisted thoracoscopic surgery biopsy in a patient with chronic myeloid leukemia] Nihon Kokyuki Gakkai Zasshi. 2011;49:465–471. [PubMed] [Google Scholar]
- 5.Barlesi F, Doddoli C, Gimenez C, et al. [Acute pulmonary toxicity due to gemcitabine: A role for asbestos exposure?] Rev Mal Respir. 2003;20:201–206. [PubMed] [Google Scholar]
- 6.Ohe Y, Ichinose Y, Nakagawa K, et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res. 2008;14:4206–4212. doi: 10.1158/1078-0432.CCR-07-5143. [DOI] [PubMed] [Google Scholar]
- 7.Jules-Elysee K, White DA. Bleomycin-induced pulmonary toxicity. Clin Chest Med. 1990;11:1–20. [PubMed] [Google Scholar]
- 8.Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528–538. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 9.Roychowdhury DF, Cassidy CA, Peterson P, et al. A report on serious pulmonary toxicity associated with gemcitabine-based therapy. Invest New Drugs. 2002;20:311–315. doi: 10.1023/a:1016214032272. [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Ahmed I, Steinberg H, et al. Gemcitabine-induced pulmonary toxicity: Case report and review of the literature. Am J Clin Oncol. 2002;25:96–100. doi: 10.1097/00000421-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Barlesi F, Villani P, Doddoli C, et al. Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol. 2004;18:85–91. doi: 10.1046/j.0767-3981.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 12.Maniwa K, Tanaka E, Inoue T, et al. An autopsy case of acute pulmonary toxicity associated with gemcitabine. Intern Med. 2003;42:1022–1025. doi: 10.2169/internalmedicine.42.1022. [DOI] [PubMed] [Google Scholar]