This study investigated factors correlated with cancer-related fatigue before surgery and just before subsequent adjuvant therapy in patients with breast cancer. Results suggest that worsening fatigue after surgery for breast cancer is associated with a decrease in physical functioning and an increase in psychological distress.
Learning Objectives
Describe the effect of worsening fatigue after breast cancer surgery on physical functioning and psychological distress.
Better identify women at risk for developing cancer-related fatigue.
Direct target interventions to patients most in need.
Abstract
Purpose.
Fatigue is one of the most frequent symptoms in patients with cancer. However, the precise determinants of fatigue are still unknown. This study was conducted to investigate factors correlated with cancer-related fatigue before surgery and just before subsequent adjuvant therapy.
Methods.
Patients completed the Multidimensional Fatigue Inventory (MFI-20), the European Organization for Research and Treatment of Cancer 30-item quality-of-life questionnaire before and after surgery, the Trait Anxiety Inventory and the Life Orientation Test before surgery, and the State Anxiety Inventory before the start of adjuvant therapy. Multiple regression analysis of determinants of change in MFI-20 total score after surgery was conducted.
Results.
A series of 466 eligible patients with stage I–III breast cancer with planned surgery were recruited. An increase in MFI-20 total score after surgery was significantly correlated with higher preoperative fatigue and lower role functioning before surgery; a decrease in role functioning, physical functioning, and cognitive functioning after surgery; an increase in insomnia after surgery; and a higher state anxiety after surgery. Disease stage, lymph node metastases, surgical procedure, and demographic characteristics (e.g., age, marital status, having children, educational level) were not correlated with fatigue in multivariate analysis.
Conclusion.
These results suggest that worsening fatigue after surgery for breast cancer is associated with a decrease in physical functioning and an increase in psychological distress rather than with the cancer characteristics. Therefore, screening measures should be implemented at the time of diagnosis—before starting treatment—to identify psychologically vulnerable patients and to offer them professional support.
Implications for Practice:
To decrease fatigue after surgery, it is necessary to account for the level of cancer-related fatigue, to assess the presence of correlates, and to provide guidelines when developing intervention strategies for helping breast cancer patients to prevent and to limit this symptom. In addition, screening measures should be implemented at the time of diagnosis, before starting breast cancer treatment, to identify psychologically vulnerable patients and to offer them professional support.
Introduction
Breast cancer is the most common malignant disorder among women. Approximately 350,000 new cases of breast cancer are registered in Europe annually [1]. An early mammography screening program, early diagnosis, and improvement in therapeutic strategies have increased the life expectancy of these patients. Ensuring the well-being of patients has become one of the aims of treatment. Quality of life (QoL), along with other patient-reported outcomes, has become one of the primary outcomes to assess the efficiency of the new cancer therapies [2].
Surgical treatment is usually the initial treatment for invasive early stage of breast cancer. Surgical options include breast-conserving therapy or mastectomy. Sentinel lymph node biopsy has become an alternative procedure to axillary node dissection [3, 4]. Patients undergoing surgery have been observed to experience significant QoL changes and have been found to become more anxious and to develop sleep difficulties [5, 6]. Several studies have compared QoL associated with breast-conserving surgery versus mastectomy and conflicting results have been reported [7–9]. Nevertheless, two of the most commonly reported consequences of breast cancer surgery are pain and postoperative fatigue [6, 10], which could impair QoL. Although postsurgical pain has been the focus of many studies, fatigue has been more rarely investigated [11, 12].
Fatigue is considered to be a multidimensional concept with several modes of expression: physical (e.g., diminished energy), cognitive (e.g., diminished concentration or attention), and affective (e.g., decreased motivation or interest). It involves chronic exhaustion and diminished capacity for physical activity that is not relieved by rest [13].
Several factors could contribute to cancer-related fatigue, including direct effects of cancer, adverse effects of cancer treatment, psychological factors such as personality traits (e.g., optimism, anxiety, depression), comorbid physical symptoms, comorbid medical conditions, and lifestyle factors, such as physical activity practice [14–16]. The majority of studies examining determinants of cancer-related fatigue have focused on adjuvant therapy or breast cancer survivors [17]. To our knowledge, no study to date has investigated determinants of fatigue before surgery as predictors of fatigue evolution after surgery. Furthermore, no study has examined the relationship between personality factors (optimism and trait anxiety) before treatment and fatigue in patients with breast cancer having surgery. Nevertheless, understanding specific predictors of change in cancer-related fatigue is a necessary step for developing effective interventions to reduce and/or alleviate fatigue. Furthermore, identifying a comprehensive set of factors before surgery and before subsequent adjuvant therapy would enable clinicians to better identify women at risk for developing cancer-related fatigue for targeted interventions.
In the present study, we tested the following research hypotheses:
An increase in cancer-related fatigue after surgery might be associated with a decrease in most postoperative functioning domains of QoL.
A lower level of optimism before surgery might be associated with an increase in postoperative cancer-related fatigue.
Higher preoperative trait anxiety might be associated with worse fatigue after surgery.
Lifestyle factors, such as physical activity, might be related to a reduced risk of developing fatigue after surgery [18–20].
Thus, the aim of this study was to identify factors associated with change in cancer-related fatigue before subsequent adjuvant therapy in patients with breast cancer in a longitudinal cohort study.
Methods
The present study is part of the FATSEIN study (“FATigue dans le cancer du SEIN”), an investigation of patients with breast cancer [21]. This prospective longitudinal study was designed to examine cancer-related fatigue in women who received adjuvant therapy for early-stage breast cancer. Protocol details have been previously published and extensively described elsewhere [21].
Eligibility criteria included the following: women newly diagnosed with invasive breast cancer, undergoing breast surgery as primary treatment, 18 years of age or older, no history of other cancers, able to read and write French, able to provide informed consent, no other major disabling medical or psychiatric conditions that would confound evaluation of QoL and fatigue, no previous chemotherapy or radiotherapy, no metastases, and no inflammatory breast cancer.
Sampling
All patients who met the eligibility criteria were informed about the study on the day preceding surgery (at baseline T0). FATSEIN study's inclusion was conducted between August 2008 and January 2011 in three French cancer care centers. For this study, we examined the data collected on two occasions: T0, the day preceding surgery (at baseline); and T1, after surgery and just before subsequent adjuvant treatment.
Data Collection
At inclusion, patients completed the Multidimensional Fatigue Inventory (MFI-20), the European Organization for Research and Treatment of Cancer 30-item QoL questionnaire (EORTC QLQ-C30), the Life Orientation Test (LOT), and the Trait Anxiety Inventory (STAI-Trait). Before adjuvant therapy, patients completed again the MFI-20, the EORTC QLQ-C30, and the STAI-State.
Fatigue Outcome
Fatigue was assessed by a 20-item questionnaire, the MFI-20 [22]. It consists of four scales based on different dimensions: general/physical fatigue, reduced activity, reduced motivation, and mental fatigue. Each item of each subscale was answered on a 5-point Likert scale (1: true to 5: not true). The score of each four-item subscale was obtained as the sum of the scores on four items. Each subscale and the total score of the MFI-20 are standardized on a scale from 0 to 100, with higher scores indicating more fatigue.
In the absence of established guidelines and to interpret the meaningful clinical change in MFI-20 scores, we used the minimal clinically important difference as defined for the EORTC QLQ-C30 scale [23]. A mean change/difference of 5–10 in the MFI-20 score is considered to be a small change, 10–20 a moderate change, and >20 a very large change.
Other Variables
Quality of Life
The EORTC QLQ-C30 questionnaire is a well-validated QoL instrument [24]. The questionnaire consists of 30 items on five functional scales that evaluate physical, role, emotional, cognitive, and social functioning; nine symptom scales that measure fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, and diarrhea; as well as financial difficulties and a global QoL scale. Scores are standardized to 0–100 scales, in which higher scores are indicative of a higher level of functioning or a higher level of symptom disturbance.
Optimism
Optimism was measured using the LOT, a 12-item questionnaire [25]. Participants indicated the extent of their agreement with each item from 1 (strongly disagree) to 5 (strongly agree). After reversing scoring for negatively worded items, scores can be summed up to yield an overall score. The score is standardized on a scale from 0 to 100. Higher scores indicate greater optimism and lower scores indicate lesser optimism (referred to as pessimism).
Anxiety
The STAI is a 40-item instrument measuring transient (STAI Y-A state anxiety) and enduring levels of anxiety (STAI Y-B trait anxiety) [26]. According to the developers, state anxiety may fluctuate over time and can vary in intensity, in contrast with trait anxiety, which denotes “relatively stable individual differences in anxiety proneness” and refers to a general tendency to respond with anxiety to perceived threats in the environment [26]. The trait version (STAI Y-B) consists of 20 statements that assess how patients feel “generally.” Patients respond to each item on a 4-point Likert scale (almost never, sometimes, often, and almost always). For the state version (STAI Y-A), patients indicate for 20 items on 4-point Likert scale (not at all, somewhat, moderately so, very much so) the extent to which they are currently experiencing each symptom of anxiety. In the present study, only trait anxiety was assessed postsurgery. State anxiety was assessed at each presurgery measurement. Each subscale STAI is standardized on a scale from 0 to 100, with higher scores indicating a higher level of anxiety.
Data concerning sociodemographics, medical history, type of surgery, tumor size on pathological examination, and adjuvant treatment were obtained from medical records. Information on physical activity was collected at each assessment. Patients were asked if they practiced a physical activity (yes/no). Daily help (and the number of hours per week) was also collected at each assessment.
Statistical Analyses
Descriptive statistics (frequencies, percentages, means, standard deviations, and ranges) were generated to characterize the study sample. At baseline, patient's sociodemographic and clinical characteristics and questionnaire scores were compared according to patients' participation (patients who participated vs. those that withdrew). The Wilcoxon nonparametric test was used to evaluate group scores differences. The percentage of missing questionnaires and missing items for fatigue's questionnaire was provided. Only women who provided information in T0 and in T1 could be included for the comparison.
The differences between MFI-20 total scores over time for cancer-related fatigue and between each QoL score over time were calculated. The Wilcoxon nonparametric test was used to evaluate differences. In addition, we assessed the number of clinically relevant changes in fatigue and in QoL (differences of 10 points or more) from T0 to T1. Bivariate associations between presurgery predictor variables and change in cancer-related fatigue and between change in QoL scores and change in cancer-related fatigue were examined using the Pearson correlation coefficient.
A multicolinearity analysis was completed to determine whether candidate variables were strongly associated with each other. The variance inflation factors (VIF) were calculated. A VIF >4 was generally considered to indicate severe multicolinearity. The global QoL scale of QLQ-C30 has been shown to be a significant predictor; intuitively, it is expected to be most affected by multicolinearity [27, 28]. QLQ-C30 fatigue scale and MFI-20 scales might also cause multicolinearity problems. Therefore, these variables were not included in the multivariate analysis, except for cancer-related fatigue at baseline. Other significant variables (p ≤ .1) were included in the model. Stepwise regression analysis was used to determine the independent variables that contributed significantly to variance in change in cancer-related fatigue. The variables that were significantly different between centers were forced into the multivariate analyses. Sequential bootstrap resampling was implemented to evaluate stability of the model. Data were analyzed using software (SAS version 9.2, SAS Institute, Cary, NC). For all tests, the type I error was set to 0.05 and all tests were two-sided.
Results
Between August 2008 and January 2011, 556 patients were included. After surgery, 26 women (4.7%) were excluded because invasive breast cancer was not pathologically confirmed; 58 (10.4%) patients dropped out because they withdrew consent and major protocol violation was observed for 6 (1.1%) patients. Therefore, 466 patients were followed. Table 1 shows the demographic and clinical characteristics of the patients.
Table 1.
Baseline patient demographics and clinical characteristics (n = 466)
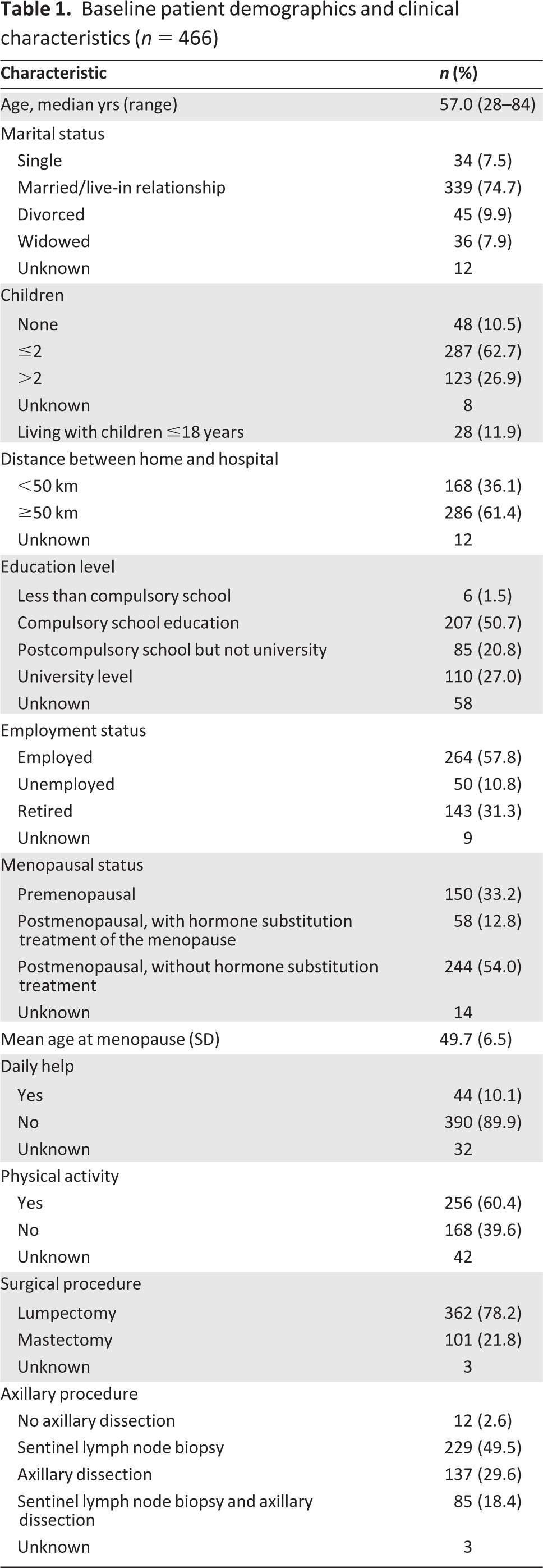
Table 1.
Continued
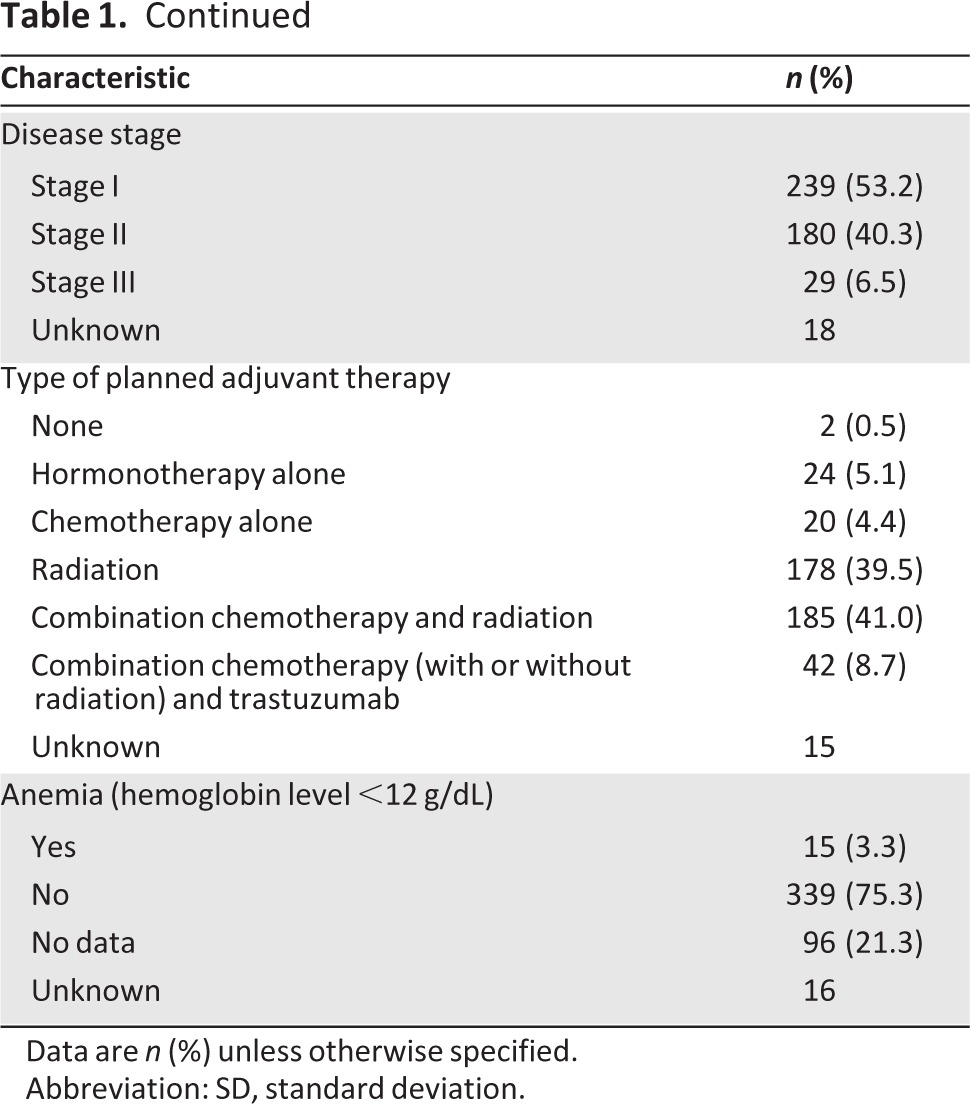
Data are n (%) unless otherwise specified.
Abbreviation: SD, standard deviation.
There were no significant differences in demographic and clinical characteristics between patients who completed the study and those who withdrew. Patients who dropped out had higher scores for the reduced motivation (p = .004) scale of MFI-20, lower scores for the emotional functioning scale of QLQ-C30 (p = .046), and higher scores for nausea (p = .026).
The average time between the two measures was less than 2 months (mean = 47 days, SD = 17 days). Intervals between surgery and first chemotherapy treatment and delays between surgery and beginning of radiotherapy were not significantly different (50 ± 19 days and 46 ± 14 days, respectively; p = .11). Patients treated by hormonotherapy alone or without adjuvant treatment (n = 26) had a significantly shorter delay between the two measures (38 ± 11 days, p = .002).
Missing Data
Before surgery, 98.5%, 98.3%, 98.9%, and 97.2% of the patients completed the MFI-20, QLQ-C30, LOT, and STAI Y-B questionnaires, respectively. After surgery, 88.2% of the patients completed the MFI-20 and QLQ-C30 questionnaires; 87.5% of patients completed the state anxiety questionnaire. No differences between patients who completed questionnaires and patients who did not were found. We observed a very low rate of missing items concerning the fatigue questionnaire (0.89% missing items before surgery and 0.79% after surgery).
Course of Fatigue and Quality of Life Before and After Surgery
Table 2 shows the mean values and standard deviation for the questionnaire scores before and after surgery and the mean change in scores. Scores for all subscales of the MFI-20 significantly increased after surgery. Moderate reductions (score difference superior to 10 points) in the MFI-20 activity and motivation dimensions were reported by 43% and 39% of the patients, respectively.
Table 2.
Questionnaire scores before and after surgery
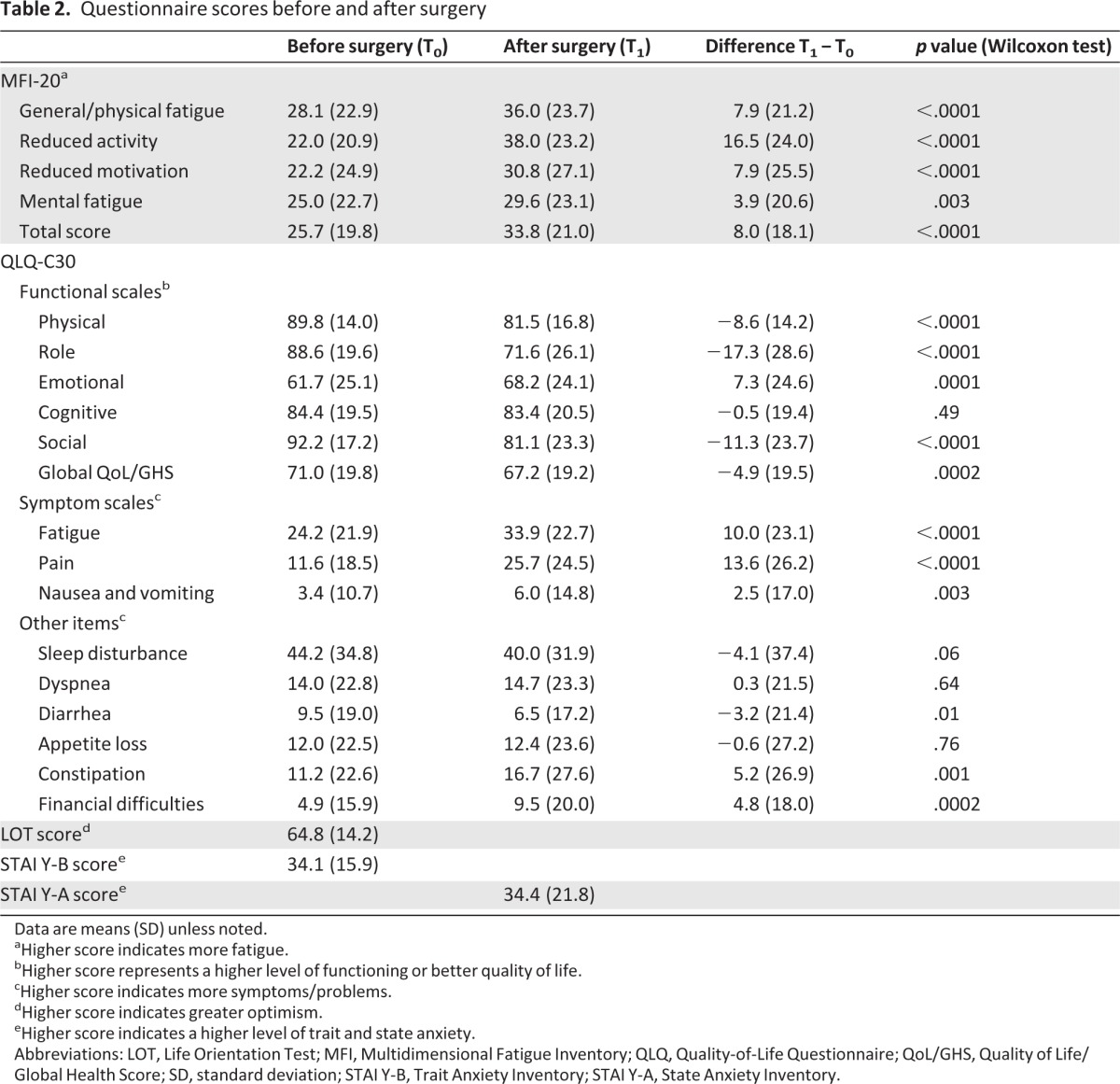
Data are means (SD) unless noted.
aHigher score indicates more fatigue.
bHigher score represents a higher level of functioning or better quality of life.
cHigher score indicates more symptoms/problems.
dHigher score indicates greater optimism.
eHigher score indicates a higher level of trait and state anxiety.
Abbreviations: LOT, Life Orientation Test; MFI, Multidimensional Fatigue Inventory; QLQ, Quality-of-Life Questionnaire; QoL/GHS, Quality of Life/Global Health Score; SD, standard deviation; STAI Y-B, Trait Anxiety Inventory; STAI Y-A, State Anxiety Inventory.
The physical, role, and social functioning scores of the QLQ-C30 significantly decreased after surgery (p < .0001). Patients reported a significantly poorer global health status/QoL score after surgery (p = .0002). The emotional functioning score improved after surgery (Δ T1-T0 = 7.3, p = .0001). A total of 49% of patients reported a moderate decrease in the role functioning dimension. The mean scores for fatigue (p < .0001), pain (p < .0001), nausea and vomiting (p = .003), constipation (p = .001), and financial difficulties (p = .0002) increased significantly after surgery. We also observed that 47% and 21% of patients presented with moderate increases in pain and insomnia, respectively.
Postoperatively, patients who had undergone mastectomy reported lower scores of role, physical, and social functioning on EORTC QLQ-C30 scales than those who had had a lumpectomy (mean score, role functioning: 65.3 [SD: 26.2] vs. 73.2 [SD: 25.9], p = .01; mean score, physical functioning: 77.6 [SD: 17.9] vs. 82.5 [SD: 16.4], p = .014; mean score, social functioning: 76.1 [SD: 25.4] vs. 82.4 [SD: 22.6], p = .02). Patients who underwent mastectomy also reported more nausea/vomiting (mean score: 9.5 [SD: 21.6] vs. 5.1 [SD: 12.2]; p = .01) and more appetite loss (mean score: 18.2 [SD: 30.1] vs. 10.8 [SD: 21.3]; p = .01) after surgery and were less optimistic (mean score: 61.4 [SD: 15.2] vs. 65.8 [SD: 13.8]; p = .01). For the other QLQ-C30 scales, MFI-20, trait anxiety, and state anxiety, no other significant differences of the scores were observed.
Factors Associated With Change in Fatigue
According to our hypothesis, QoL and QLQ-C30 and MFI-20 fatigue scale scores might produce harmful multicolinearity (VIF ≥ 4). Therefore, these variables were eliminated from the final analysis because colinearity can increase estimates of parameter variance. Significant correlations existed among the other independent variables but none were considered to be multicolinear.
Table 3 presents variables significantly associated with change in cancer-related fatigue after surgery in bivariate and multivariate analyses. Bivariate statistics showed a strong association between a decrease in all functional scores of QLQ-C30 and an increased MFI-20 total score between T0 and T1 (p < .0001). Lower physical (p = .01), role (p = .003), emotional (p < .0001), cognitive (p < .0001), and social (p = .0005) functioning scales and lower global health status/QoL scale (p = .002) of QLQ-C30 measured before surgery and lower preoperative MFI-20 total score were also associated with an increase in cancer-related fatigue after surgery. Worsening pain, sleep disturbance, appetite loss (all p < .0001), and nausea/vomiting (p = .0005) were strongly associated with a worsening in fatigue.
Table 3.
Factors associated with change in MFI-20 total score after surgery (bivariate and multivariate analyses, regression model; n = 466)
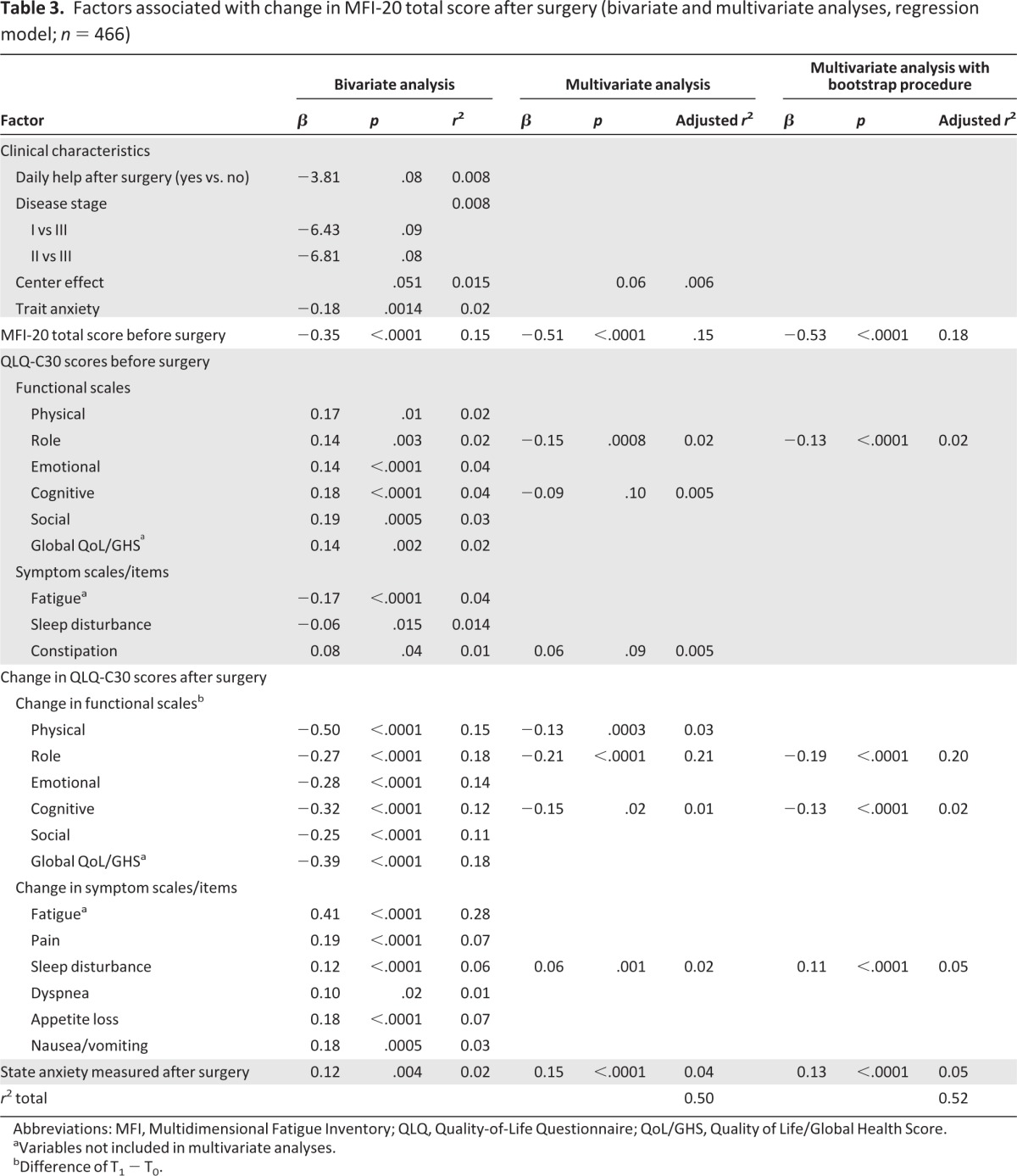
Abbreviations: MFI, Multidimensional Fatigue Inventory; QLQ, Quality-of-Life Questionnaire; QoL/GHS, Quality of Life/Global Health Score.
aVariables not included in multivariate analyses.
bDifference of T1 − T0.
Optimism was not associated with change in fatigue after surgery. Higher preoperative trait anxiety and postoperative state-anxiety were associated with an increase in cancer-related fatigue (p = .001 and p = .004, respectively). Daily help after surgery was not significantly associated with change in cancer-related fatigue (p = .08). Neither sociodemographic factors (age, marital status, having children at home, employment status, educational level) nor disease and treatment pattern (stage of disease, lymph node metastases, surgical procedure, type of planned adjuvant therapy) were significantly related to change in cancer-related fatigue. A center effect was nearly observed (p = .051). The comparison of demographical clinical characteristics between centers showed some variables were significantly different. Center effect and these variables were forced in the multivariate model.
Multiple regression analyses showed that worsening in cancer-related fatigue level before and after surgery was best predicted by higher cancer-related fatigue (p < .0001) and lower role functioning (p = .0008) before surgery; by deterioration in role, cognitive, and physical functioning (p < .0001, p = .02, and p = .0003, respectively) after surgery; by an increase in insomnia after surgery (p = .001); and by a higher state anxiety after surgery (p < .0001; Table 3). Subsequent analysis with variables selected through bootstrapping procedures revealed that physical functioning was not any more associated with change in cancer-related fatigue and confirmed that the best predictors of a worsening in cancer-related fatigue were higher cancer-related fatigue and lower role functioning before surgery, a deterioration in role and cognitive functioning, a higher sleep disturbance after surgery, and a higher state anxiety after surgery (all p < .0001; Table 3).
Discussion
As in previous studies [29, 30], participants in this study reported elevated levels of postoperative fatigue and their activity was strongly reduced. Patients rated their role functioning, social functioning, and QoL lower; their functional well-being also showed significant deterioration. Patients also reported significantly increased pain, constipation, and nausea/vomiting.
The strongest predictors for an increase of fatigue after surgery were lower role functioning before surgery, higher preoperative cancer-related fatigue, higher state anxiety after surgery, an increase in insomnia, and a decrease in QoL in most domains. It is important to note that presurgical fatigue and a worsening in role functioning accounted for 20% and 18%, respectively, of the variance in change in postsurgical fatigue.
According to our hypothesis, decreases in role, physical, and cognitive functioning would be predictors of an increase in cancer-related fatigue after surgery. Following the use of a sequential bootstrap technique that evaluated the precision of estimation, only changes in role and cognitive function demonstrated a strong association with cancer-related fatigue. Similar to Ancoli-Israel et al. [31], we found that a worsening in sleep disturbance was associated with a worsening in fatigue after surgery. Before treatments start, the baseline levels of sleep and circadian rhythms must be determined to limit fatigue during and after treatment.
Contrary to another study [32], we did not show that pain level was associated with worsening fatigue after surgery. Pain has a number of negative effects on mood, daily activities, sleep, cognitive functions, social life, and long-term QoL [11]. The number of dissected lymph nodes, type of surgery, and age may play an active role in the development of pain after breast cancer surgery, although the results are conflicting [33, 34].
Some studies showed that women who underwent mastectomies were more fatigued than women who underwent lumpectomies [35]. In addition, undergoing mastectomy was a significant predictor of cancer-related fatigue in a recent study [32]. This result can be explained by the possible psychological impact of mastectomy. In our study, type of surgery was not found to influence fatigue after surgery, but only 22% of our patients had mastectomies. Nevertheless, we observed that patients who had undergone mastectomies reported lower role, physical, and social functioning; had greater appetite loss; and were less optimistic than patients who had lumpectomies.
Our results suggest also that patients with breast cancer who presented with greater fatigue before surgery have a greater risk of experiencing postsurgical fatigue. Consequently, it might be beneficial to screen such patients prior to surgery to reduce their postsurgical cancer-related fatigue. It was previously suggested that emotional support is important at the time of diagnosis and after surgery [36]. Indeed, social and family support and communication have been found to be important factors, which may be partially responsible for better QoL and therefore less fatigue. In our study, social support and emotional functioning were correlated with fatigue; however, when these factors had to compete with others factors, the positive association did not hold. Another study also found that social support did not appear to play a role in fatigue measured 6 months after surgery [37].
Our study focused on two personality traits: optimism and trait anxiety. Optimism has been defined as a generalized tendency to have positive expectancies for the future [25, 38]. Some studies assessed the relationship between optimism and cancer-related fatigue in patients with breast cancer [16, 39]. They found that greater optimism was related to lower cancer-related fatigue in a longitudinal sample of patients with breast cancer who were interviewed postoperatively and over the next year [40]. Our results do not confirm the association between optimism before surgery and postsurgery cancer-related fatigue.
Literature indicates that patients with cancer have higher preoperative anxiety than patients without cancer [41]. The diagnosis of cancer induces stresses that are caused by the patient's perception of cancer and treatment. This state anxiety may fluctuate over time and can vary in intensity. Lehto and Cimprich investigated the relationship between anxiety and directed attention (the ability to focus and concentrate) in women awaiting breast cancer surgery [42]. They concluded that health care professionals should assess anxiety in women during the preoperative period and assist them in coping with the psychological and cognitive demands associated with this highly stressful period.
In contrast to the transitory nature of state anxiety, trait anxiety reflects the disposition to experience anxiety in threatening situations. Studies have reported that trait anxiety assessed prior to diagnosis was a significant predictor of depressive symptoms, fatigue [43], and QoL [44] in women with breast complaints 6 months after the diagnosis of a benign breast problem or surgery to treat breast cancer. Greater trait anxiety and state anxiety were associated with an increase in cancer-related fatigue after surgery in bivariate analysis; however, in multivariate analysis, only state anxiety (i.e., the level of momentary anxiety) was significantly associated with change in cancer-related fatigue. The personality traits of patients did not have a significant influence on change in fatigue immediately after surgery. The relationship between personality trait and fatigue needs to be more fully explored, particularly in longitudinal studies.
Literature shows that higher fatigue after surgery is associated with lower physical activity [18, 19]. Indeed, De Jong et al. examined the course of the activity level in patients with breast cancer who were receiving adjuvant chemotherapy; an increase in fatigue accompanied by a reduction in activity level was reported [45]. A reduced functional capacity means that patients with breast cancer expend greater effort relative to maximal ability to perform usual activities, thus leading to higher level of fatigue. Exercise training attenuates the loss and even increases functional capacity.
A number of studies on patients with breast cancer during and after their adjuvant treatment have investigated the effects of physical activity in reducing cancer-related fatigue, with no definitive conclusions regarding its effectiveness [46]. Nevertheless, exercise seems to be beneficial for patients with breast cancer, and the evidence suggesting that physical activity limits cancer-related fatigue is particularly strong [47]. To our knowledge, the current study is the first to seek physical activity prior to surgery as a predictor of change in cancer-related fatigue after surgery. However, in multivariate analysis, the association between physical activity and fatigue was not significant. Nevertheless, physical activity was analyzed as a dichotomous variable and no validated questionnaire was used. Further research about this association is necessary.
Some limitations of this study must be discussed. The first limitation of this study might be attrition bias. The sample of patients who dropped out is small (10%), but these patients were significantly less motivated than followed patients. Secondly, we did not include data on comorbidities, such as diabetes, and other chronic conditions that could affect fatigue.
Our results suggest that changes in cancer-related fatigue after surgery in patients with breast cancer are determined by physical and psychological distress rather than by the clinical characteristics of the cancer. This study indicates the cross-sectional and short-term longitudinal association of various variables and changes in fatigue after surgery, but it is difficult to infer causal relationship. Long-term follow-up studies should be conducted to explore the impact of demographics, medical history, and personality traits on fatigue at different stages of disease and treatment.
Conclusions
Waiting for surgery is a very stressful experience for patients with breast cancer. Preoperative experiences and coping with breast cancer have postoperative impacts. To decrease fatigue after surgery, it is necessary to account for the level of cancer-related fatigue, assess the presence of correlates, and provide guidelines when developing intervention strategies for supporting these patients. In addition, screening measures should be implemented at the time of diagnosis, before the beginning of breast cancer treatment, to identify psychologically vulnerable patients and offer them professional support. More recently, some authors suggested that introduction of palliative care services earlier in the course of the disease could have a meaningful positive effect on patients' QoL and moods [48, 49]. The early integration of palliative care in the disease trajectory of patients is explicitly recommended by the World Health Organization. Nevertheless, these hypotheses require further study.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This study was supported by the Lorraine Regional Council, Cancéropôle du Grand-Est, and Nancy University Hospital.
Author Contributions
Conception/Design: Christine Rotonda, Francis Guillemin, Franck Bonnetain, Thierry Conroy
Provision of study material or patients: Franck Bonnetain, Michel Velten, Thierry Conroy
Collection and/or assembly of data: Christine Rotonda
Data analysis and interpretation: Christine Rotonda, Francis Guillemin, Franck Bonnetain, Michel Velten, Thierry Conroy
Manuscript writing: Christine Rotonda, Francis Guillemin, Franck Bonnetain, Michel Velten, Thierry Conroy
Final approval of manuscript: Christine Rotonda, Francis Guillemin, Franck Bonnetain, Michel Velten, Thierry Conroy
Disclosures
Gabriel Hortobágyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe (C/A); Novartis (RF); Taivex (O); founder and member of the board of directors for Citizen's Oncology Foundation; Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Sanofi, Ortho-Biotech, Amgen, Bristol-Myers Squibb (C/A), (H).
Reviewer “A”: None
Reviewer “B”: None
References
- 1.Tyczynski JE, Plesko I, Aareleid T, et al. Breast cancer mortality patterns and time trends in 10 new EU member states: mortality declining in young women, but still increasing in the elderly. Int J Cancer. 2004;112:1056–1064. doi: 10.1002/ijc.20514. [DOI] [PubMed] [Google Scholar]
- 2.Roila F, Cortesi E. Quality of life as a primary end point in oncology. Ann Oncol. 2001;12(suppl 3):S3–S6. doi: 10.1093/annonc/12.suppl_3.s3. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.McCready D, Holloway C, Shelley W, et al. Surgical management of early stage invasive breast cancer: A practice guideline. Can J Surg. 2005;48:185–194. [PMC free article] [PubMed] [Google Scholar]
- 5.Arora NK, Gustafson DH, Hawkins RP, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma: A prospective study. Cancer. 2001;92:1288–1298. doi: 10.1002/1097-0142(20010901)92:5<1288::aid-cncr1450>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Dabakuyo TS, Fraisse J, Causeret S, et al. A multicenter cohort study to compare quality of life in breast cancer patients according to sentinel lymph node biopsy or axillary lymph node dissection. Ann Oncol. 2009;20:1352–1361. doi: 10.1093/annonc/mdp016. [DOI] [PubMed] [Google Scholar]
- 7.Han J, Grothuesmann D, Neises M, et al. Quality of life and satisfaction after breast cancer operation. Arch Gynecol Obstet. 2010;282:75–82. doi: 10.1007/s00404-009-1302-y. [DOI] [PubMed] [Google Scholar]
- 8.Nissen MJ, Swenson KK, Ritz LJ, et al. Quality of life after breast carcinoma surgery: A comparison of three surgical procedures. Cancer. 2001;91:1238–1246. [PubMed] [Google Scholar]
- 9.Pandey M. Early effect of surgery on quality of life in women with operable breast cancer. J Clin Oncol. 2006;36:468–472. doi: 10.1093/jjco/hyl065. Thomas breast cancer, Ramdas K et al. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt GK, Friedman LL. Physical and psychosocial outcomes of midlife and older women following surgery and adjuvant therapy for breast cancer. Oncol Nurs Forum. 1998;25:761–768. [PubMed] [Google Scholar]
- 11.Gartner R, Jensen MB, Nielsen J, et al. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 12.Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119:16–25. doi: 10.1016/j.pain.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Cella D, Lai JS, Chang CH, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 14.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 15.Stone P, Richards M, A'Hern R, et al. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol. 2000;11:561–567. doi: 10.1023/a:1008331230608. [DOI] [PubMed] [Google Scholar]
- 16.Von Ah DM, Kang DH, Carpenter JS. Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nurs. 2008;31:134–144. doi: 10.1097/01.NCC.0000305704.84164.54. [DOI] [PubMed] [Google Scholar]
- 17.De Jong N, Courtens AM, Abu-Saad HH, et al. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Cancer Nurs. 2002;25:283–297. doi: 10.1097/00002820-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- 19.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: A pilot study. Oncol Nurs Forum. 2000;27:1443–1448. [PubMed] [Google Scholar]
- 20.Jacobsen PB, Hann DM, Azzarello LM, et al. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 21.Rotonda C, Guillemin F, Bonnetain F, et al. Factors correlated with fatigue in breast cancer patients before, during and after adjuvant chemotherapy: The FATSEIN study. Contemp Clin Trials. 2011;32:244–249. doi: 10.1016/j.cct.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Gentile S, Delaroziere JC, Favre F, et al. Validation of the French multidimensional fatigue inventory (MFI 20) Eur J Cancer Care (Engl) 2003;12:58–64. doi: 10.1046/j.1365-2354.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 23.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 25.Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger CD. State-Trait Anxiety Inventory: A comprehensive bibliography. Mountain View, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- 27.Dancey J, Zee B, Osoba D, et al. Quality of life scores: An independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Qual Life Res. 1997;6:151–158. doi: 10.1023/a:1026442201191. [DOI] [PubMed] [Google Scholar]
- 28.Van SK, Curran D, Kramer J, et al. Multicollinearity in prognostic factor analyses using the EORTC QLQ-C30: Identification and impact on model selection. Stat Med. 2002;21:3865–3884. doi: 10.1002/sim.1358. [DOI] [PubMed] [Google Scholar]
- 29.Debess J, Riis JO, Pedersen L, et al. Cognitive function and quality of life after surgery for early breast cancer in North Jutland, Denmark. Acta Oncol. 2009;48:532–540. doi: 10.1080/02841860802600755. [DOI] [PubMed] [Google Scholar]
- 30.Rubin GJ, Hardy R, Hotopf M. A systematic review and meta-analysis of the incidence and severity of postoperative fatigue. J Psychosom Res. 2004;57:317–326. doi: 10.1016/S0022-3999(03)00615-9. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haghighat S, Akbari ME, Holakouei K, et al. Factors predicting fatigue in breast cancer patients. Support Care Cancer. 2003;11:533–538. doi: 10.1007/s00520-003-0473-5. [DOI] [PubMed] [Google Scholar]
- 33.Hack TF, Cohen L, Katz J, et al. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol. 1999;17:143–149. doi: 10.1200/JCO.1999.17.1.143. [DOI] [PubMed] [Google Scholar]
- 34.Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: A survey of 282 women. Pain. 1996;66:195–205. doi: 10.1016/0304-3959(96)03064-3. [DOI] [PubMed] [Google Scholar]
- 35.De Jong N, Candel MJ, Schouten HC, et al. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol. 2004;15:896–905. doi: 10.1093/annonc/mdh229. [DOI] [PubMed] [Google Scholar]
- 36.Arora NK, Finney Rutten LJ, et al. Perceived helpfulness and impact of social support provided by family, friends, and health care providers to women newly diagnosed with breast cancer. Psychooncology. 2007;16:474–486. doi: 10.1002/pon.1084. [DOI] [PubMed] [Google Scholar]
- 37.De Vries J, Van der Steeg AF, Roukema JA. Determinants of fatigue 6 and 12 months after surgery in women with early-stage breast cancer: A comparison with women with benign breast problems. J Psychosom Res. 2009;66:495–502. doi: 10.1016/j.jpsychores.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 39.Carver CS, Pozo C, Harris SD, et al. How coping mediates the effect of optimism on distress: A study of women with early stage breast cancer. J Pers Soc Psychol. 1993;65:375–390. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- 40.Carver CS, Lehman JM, Antoni MH. Dispositional pessimism predicts illness-related disruption of social and recreational activities among breast cancer patients. J Pers Soc Psychol. 2003;84:813–821. doi: 10.1037/0022-3514.84.4.813. [DOI] [PubMed] [Google Scholar]
- 41.McCleane GJ, Cooper R. The nature of pre-operative anxiety. Anaesthesia. 1990;45:153–155. doi: 10.1111/j.1365-2044.1990.tb14285.x. [DOI] [PubMed] [Google Scholar]
- 42.Lehto RH, Cimprich B. Anxiety and directed attention in women awaiting breast cancer surgery. Oncol Nurs Forum. 1999;26:767–772. [PubMed] [Google Scholar]
- 43.De Vries J, Van der Steeg AF, Roukema JA. Trait anxiety determines depressive symptoms and fatigue in women with an abnormality in the breast. Br J Health Psychol. 2009;14:143–157. doi: 10.1348/135910708X310200. [DOI] [PubMed] [Google Scholar]
- 44.Van der Steeg AF, De VJ, Van der Ent FW, et al. Personality predicts quality of life six months after the diagnosis and treatment of breast disease. Ann Surg Oncol. 2007;14:678–685. doi: 10.1245/s10434-006-9175-9. [DOI] [PubMed] [Google Scholar]
- 45.De Jong N, Kester AD, Schouten HC, et al. Course of fatigue between two cycles of adjuvant chemotherapy in breast cancer patients. Cancer Nurs. 2006;29:467–477. doi: 10.1097/00002820-200611000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Mock V, Frangakis C, Davidson NE, et al. Exercise manages fatigue during breast cancer treatment: A randomized controlled trial. Psychooncology. 2005;14:464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 47.Kirshbaum MN. A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2007;16:104–121. doi: 10.1111/j.1365-2702.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 48.Gaertner J, Wuerstlein R, Klein U, et al. Integrating palliative medicine into comprehensive breast cancer therapy: A pilot project. Breast Care. 2011;6:215–220. doi: 10.1159/000328162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]


