To The Editor
We read with great interest the recent work by Gavrilovic et al. [1], reporting the occurrence of a geographic tongue (benign migratory glossitis) in four patients treated with the humanized anti-VEGF (vascular endothelial growth factor) monoclonal antibody bevacizumab (Avastin, Roche). Although geographic tongue is frequent, affecting up to 2.5% of the general population [2], we reckon, in line with the authors, that this was not a coincidence. Indeed, we have also observed several cases of female patients treated with this drug, who developed benign migratory glossitis after several therapeutic cycles (6 months on average) (Figs. 1, 2). Those cases were very likely attributable to bevacizumab, as these patients had no similar clinical history and reported the onset of oral discomfort and dysguesia during the course of treatment, which led to the discovery of oral lesions of geographic tongue. Moreover, the intensity of the lesions was sometimes correlated with bevacizumab cycles, showing a progressive fading during the 3 weeks following injection and rapid recurrence associated with new intravenous infusions (Fig. 2A, 2B). Finally, we have also observed similar symptoms, with the same characteristics and timing, occurring with some multitargeted kinase inhibitors acting on angiogenesis with a non-exclusive inhibitory activity on VEGF receptors (VEGFR 1–3). These patients were three white subjects treated with sunitinib (Sutent, Pfizer) (two patients) or sorafenib (Nexavar, Bayer) as monotherapy for metastatic renal cell carcinoma (Figs. 3, 4).
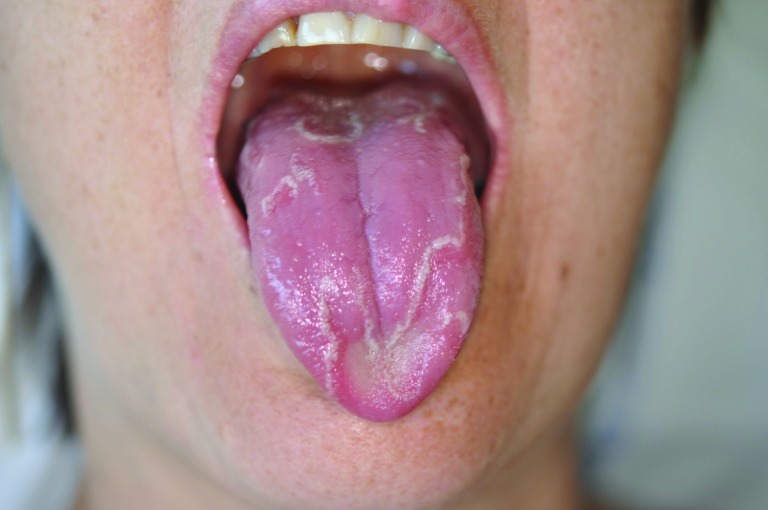
Figure 1.
Typical geographic tongue induced by bevacizumab.
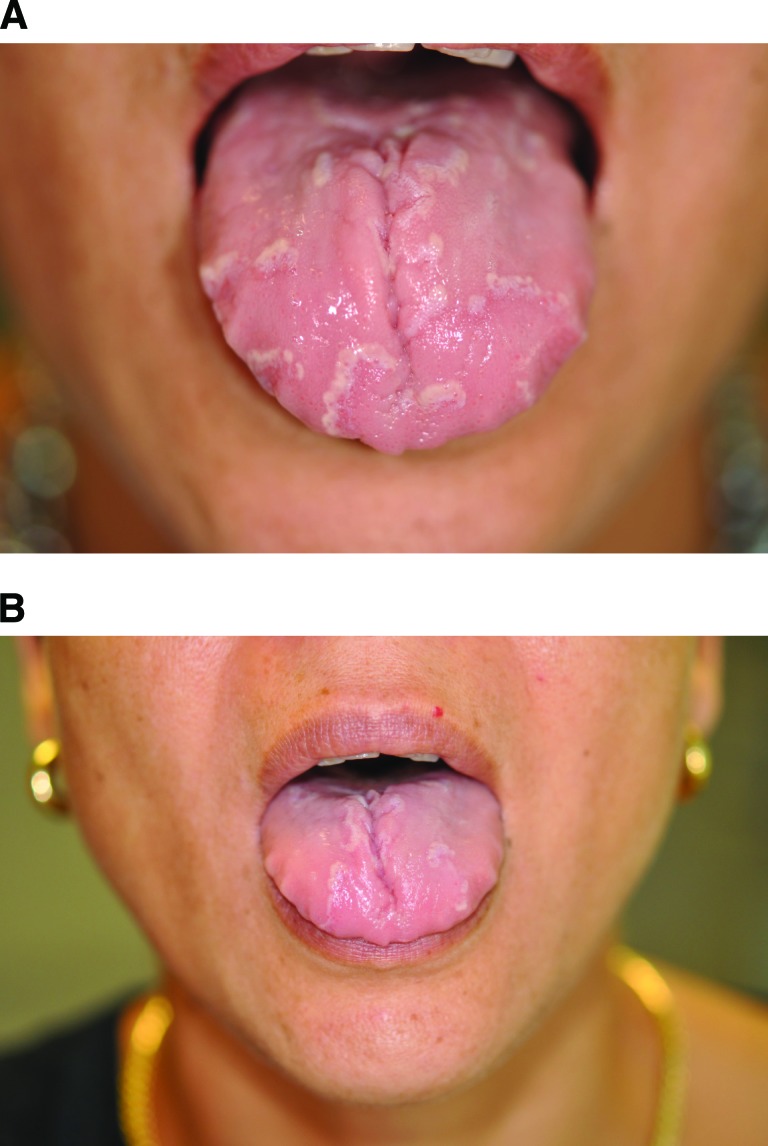
Figure 2.
A, B: Fading of the lesions 3 weeks after bevacizumab injection.
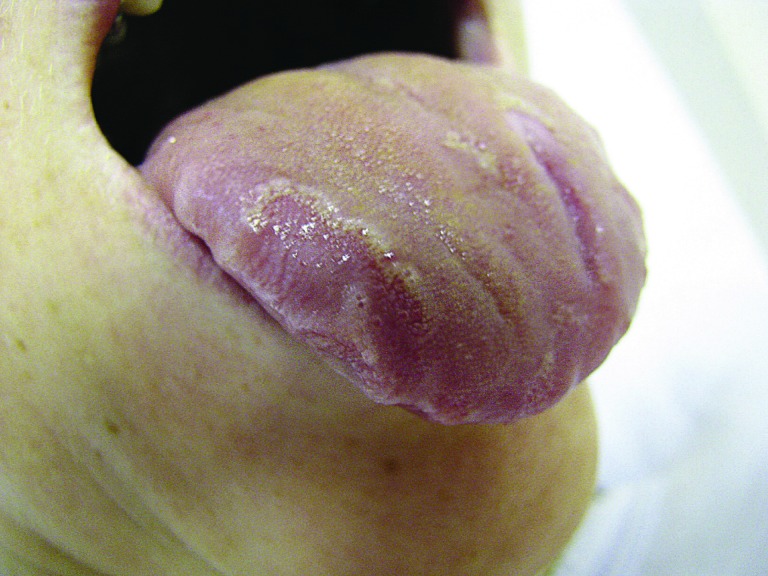
Figure 3.
(Sunitinib): Migratory well-demarcated areas with elevated yellowish hyperkeratotic borders.
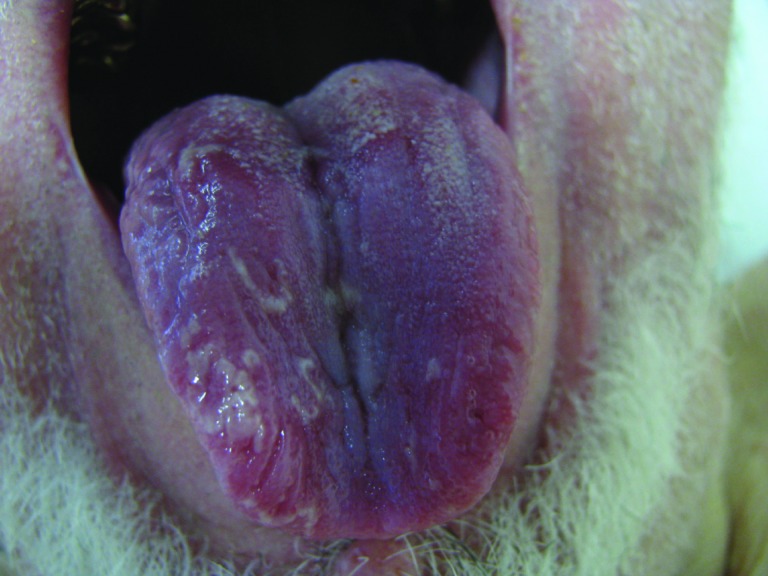
Figure 4.
(Sorafenib): Erythematous erosions, with loss of filiform papillae, surrounded by white circinate rims.
The pathophysiology of benign migratory glossitis is unknown [2]. However, given the established role of VEGF or VEGF receptors in the buccal mucosa homeostasis as well as in some oral diseases, such as squamous cell carcinomas [3, 4], we suggest, as the authors, that therapeutic inhibition of those specific targets by angiogenesis inhibitors could induce geographic tongue. Compared with mTOR inhibitors, the clinical presentation of oral adverse events induced by angiogenesis inhibitors is much less characterized [5, 6]. Stomatitis is however frequently reported in patient series and we cannot exclude that it could actually correspond in some cases to benign migratory glossitis.
Finally, it is worth to note that, as opposed to the classical and most often painless form [2], in almost all the cases reported here or by Gavrilovic et al. [1], benign migratory glossitis lesions were associated with dysguesia, especially triggered by some food.
It seems to us important that clinicians are aware of this potential adverse event, which remains benign, but whose morbidity in this setting can be noteworthy, leading to serious anxiety and discomfort for the patient. Benign migratory glossitis usually does not require any specific treatment, apart from reassurance about the benign nature of the lesions. Dose reduction or temporary discontinuation of these targeted treatments does not seem necessary.
Author Contributions
Conception/Design: Thomas Hubiche, Vincent Sibaud
Provision of study material or patients: Thomas Hubiche, Bruno Valenza, Christine Chevreau, Vincent Sibaud
Collection and/or assembly of data: Thomas Hubiche, Vincent Sibaud
Data analysis and interpretation: Thomas Hubiche, Jean-Christophe Fricain, Pascal Del Giudice, Vincent Sibaud
Manuscript writing: Thomas Hubiche, Vincent Sibaud
Final approval of manuscript: Thomas Hubiche, Bruno Valenza, Christine Chevreau, Jean-Christophe Fricain, Pascal Del Giudice, Vincent Sibaud
Disclosures
Vincent Sibaud: Pierre Fabre Laboratories (C/A). Jean-Christophe Fricain: Novartis (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Gavrilovic IT, Balagula Y, Rosen AC, et al. Characteristics of oral mucosal events related to bevacizumab treatment. The Oncologist. 2012;17:274–278. doi: 10.1634/theoncologist.2011-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assimakopoulos D, Patrikakos G, Fotika C, et al. Benign migratory glossitis or geographic tongue: an enigmatic oral lesion. Am J Med. 2002;113:751–755. doi: 10.1016/s0002-9343(02)01379-7. [DOI] [PubMed] [Google Scholar]
- 3.Maharaj ASR, Saint-Gebiez M, Maldonado AE, et al. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou G, Hasina R, Wroblewski K, et al. inhibition of vascular endothelial growth factor receptor and epidermal growth factor receptor is an effective chemopreventive strategy in the mouse 4-NQO model of oral carcinogenesis. Cancer Prev Res. 2010;3:1493–1502. doi: 10.1158/1940-6207.CAPR-10-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boers-Doets CB, Epstein JB, Raber-Durlacher JE, et al. Oral adverse events associated with tyrosine kinase and mammalian target of rapamycin inhibitors in renal cell carcinoma: a structured literature review. The Oncologist. 2012;17:135–144. doi: 10.1634/theoncologist.2011-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WJ, Lee JL, Chang SE, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol. 2009;161:1045–1051. doi: 10.1111/j.1365-2133.2009.09290.x. [DOI] [PubMed] [Google Scholar]