Abstract
The contraceptive depot medroxyprogesterone acetate (DMPA), with progestin as the single active compound, possesses selective glucocorticoid activity and can alter the expression of glucocorticoid receptor-regulated genes. We therefore propose that pharmacological doses of DMPA used for endocrine therapy could have significant immune modulatory effects and impact on susceptibility to, as well as clinical manifestation and outcome of, infectious diseases. We investigated the effect of contraceptive doses of DMPA in two different murine Mycobacterium tuberculosis models. Multiplex bead array analysis revealed that DMPA altered serum cytokine levels of tumor necrosis factor alpha (TNF-α), granulocyte colony-stimulating factor (G-CSF), and interleukin 10 (IL-10) in C57BL/6 mice and gamma interferon (IFN-γ) in BALB/c mice. DMPA also suppressed antigen-specific production of TNF-α, G-CSF, IL-10, and IL-6 and induced the production of IP-10 in C57BL/6 mice. In BALB/c mice, DMPA altered the antigen-specific secretion of IFN-γ, IL-17, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, and monocyte chemotactic protein 1 (MCP-1). Furthermore, we show that C57BL/6 mice treated with doses of DMPA, which result in serum concentrations similar to those observed in contraceptive users, have a significantly higher bacterial load in their lungs. Our data show for the first time that DMPA impacts tuberculosis (TB) disease severity in a mouse model and that the effects of this contraceptive are not confined to infections of the genital tract. This could have major implications for the contraceptive policies not only in developing countries like South Africa but also worldwide.
INTRODUCTION
Depot medroxyprogesterone acetate (DMPA), also known as Depo-Provera or Petogen, is the most commonly used contraceptive in developing countries, including South Africa (1). Some recent publications suggest that DMPA use is associated with a significantly higher risk of acquiring HIV (2–4), although other studies did not find this association (5, 6). Use of DMPA has previously also been linked to increased risk of acquisition of other sexually transmitted diseases, such as Trichomonas vaginalis, Neisseria gonorrhoeae, and Chlamydia trachomatis (7). DMPA is a contraceptive administered to women as an intramuscular injection once every 3 months. Some synthetic progestins bind with a high affinity not only to the progesterone receptor but also to the androgen receptor (8). Upon ligand binding, these receptors subsequently bind to a consensus sequence called the hormone response elements (HRE) in the promoter regions of their target genes (9, 10). DMPA, however, is pharmacologically unique to other synthetic progestins, including the 2-month injectable contraceptive norethisterone (NET), as it also binds with high affinity to the glucocorticoid receptor (11). DMPA has been shown by many, including us, to have partial GR agonist activity and has been shown to alter the expression of glucocorticoid receptor-regulated genes (12). However, DMPA is probably better referred to as a selective GR modulator, as it can act as an agonist as well as an antagonist depending on the cellular milieu (13).
Activation of the hypothalamus-pituitary-adrenal axis (HPA) has been implicated in the increased risk of developing tuberculosis (TB) (14–17). Furthermore, administration of synthetic glucocorticoids causes reactivation of TB in humans and animals (14, 15, 17). Due to its selective glucocorticoid properties, we hypothesize that DMPA would mimic the effects of cortisol during Mycobacterium tuberculosis infections. We have previously shown that DMPA mimics cortisol and not progesterone in human peripheral blood mononuclear cells (PBMCs) and suppresses Mycobacterium bovis bacillus Calmette-Guérin (BCG)-induced secretion of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 1α (IL-1α), IL-1β, IL-1ra, IL-2, IL-5, IL-6, IL-12p40, IL-13, IL-17, sCD40L, granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage inflammatory protein 1β (MIP-1β) (1). Cortisol and DMPA, but not progesterone, also upregulated the production of IP-10 and granulocyte colony-stimulating factor (G-CSF). Furthermore, PBMCs from healthy women using DMPA as a contraceptive produced significantly less IL-1α, IL-12p40, G-CSF, IL-10, and IL-13 when challenged with BCG compared to women not using contraceptives. Most interestingly, we showed that women using DMPA had significantly lower numbers of circulating monocytes. It is therefore possible that DMPA could alter susceptibility to TB and severity of active TB disease. This is of concern, as DMPA is currently the recommended contraceptive for patients with TB, as its efficacy is not diminished by liver enzyme induction due to TB drugs (18).
To assess whether this contraceptive can affect TB disease severity and immune responses to M. tuberculosis, we set up an MPA mouse model. The TB-resistant C57BL/6 and TB-susceptible BALB/c strains were included, as they also have different sensitivities to glucocorticoids (19). The aim, therefore, was to compare these two strains to our previously published human work and determine which strain is a suitable model to study the effect of DMPA in the context of TB. In this study, we highlight the similarities of the C57BL/6 mouse strain to that of our human data and we show that DMPA inhibits cytokine secretion in these mice similarly to that in women using DMPA as contraceptive. Most importantly DMPA increases the bacterial burden in the lungs of C57BL/6 mice, which warrants further investigation in contraceptive users.
MATERIALS AND METHODS
Ethics statement.
Ethical approval was obtained from the Animal Research Ethics Committee of the University of Cape Town (project number 009/054) and the Research Ethics Committee: Animal Care and Use of the University of Stellenbosch (project number 10NN_KLE01 and project number P09/10/014).
Mice.
Adult, 8- to 12-week-old female C57BL/6 and BALB/c mice were bred and housed under specific pathogen-free conditions at the animal facility of the University of Cape Town. Mice were allowed to acclimatize in the biosafety level 3 facility for 6 days before being used for any experimental procedures.
Hormone therapy.
Clinical-grade DMPA (Depo-Provera [150 mg/ml]; Pfizer, NY) was diluted to 20 mg/ml in sterile phosphate-buffered saline (PBS; Lonza, MA). Once a week, mice were injected intramuscularly into the right thigh with 1 mg of DMPA in 50 μl, and control mice were injected with the same volume of saline. Intramuscular injections of DMPA commenced 1 week before M. tuberculosis infection (week −1).
Mycobacterium tuberculosis infection.
M. tuberculosis H37Rv was obtained from the Trudeau Mycobacterial Culture Collection (Trudeau Institute, NY). M. tuberculosis was grown in Difco Middlebrook 7H9 broth (Becton, Dickinson [BD], San Diego, CA), supplemented with 0.05% Tween 80 (Sigma) and 10% Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment (BBL; BD) at 37°C until the mycobacteria reached mid-log phase. Mycobacterial viability and concentrations were determined by thawing an M. tuberculosis aliquot, disrupting the mycobacterial clumps by aspirating through a 29-gauge needle (Omnican; B. Braun, Melsungen, Germany) 30 times and plating 10-fold serial dilutions onto Difco Middlebrook 7H10 agar plates (BD) supplemented with 10% OADC (BD) and 0.5% glycerol (Sigma). A Glass-Col inhalation exposure system (model A4224; Glas-Col, Indiana) was used to deliver a low dose of approximately 30 CFU, as previously described (20), to the lungs of mice via aerosol inhalation 1 week after initiation of DMPA treatment (week 0). The infection dose was confirmed by enumeration of bacteria 1 day postinfection. Bacterial loads were assessed in the lungs and spleens of infected mice 3 and 8 weeks postinfection (10 mice/group) after euthanasia. An additional 16-week time point was, however, included in the BALB/c experiments, as this strain is known to be less sensitive to the effects of glucocorticoids (19). Lungs and spleens were weighed and homogenized in sterile PBS (Lonza) containing 0.04% Tween 20 (Sigma). Tenfold serial dilutions were plated in duplicate on Difco Middlebrook 7H11 agar (BD) plates supplemented with 0.5% glycerol (Sigma) and 10% OADC (BD). Plates were incubated for 18 to 21 days at 37°C, after which the colonies were enumerated and bacterial load per organ was determined.
Serum collection and ex vivo single-cell stimulation.
Mice were humanely euthanized by halothane exposure (5% in air) 3, 8, and 16 weeks postinfection and their organs aseptically removed. Blood was collected by cardiac puncture and serum stored at −70°C until cytokine analysis by Multiplex bead array assay. Single cells were prepared from the mediastinal lymph nodes (MLNs), collected from each group, by passing the organs through a 70-μm-pore-size nylon cell strainer (BD) in AIM-V medium (BD). After removal of erythrocytes, cells were cultured at a density of 2 × 105 cells/well in a total volume of 200 μl in 96-well cell culture plates (Greiner Bio-one, North Carolina) and restimulated ex vivo with a final concentration of 5 μg/ml of the M. tuberculosis antigens PPD (Statens Serum Institut, Copenhagen, Denmark) or ESAT-6 (Statens Serum Institut, Copenhagen, Denmark) for 4 days at 37°C and 5% CO2. The mitogen phytohemagglutinin (PHA; 5 μg/ml final concentration; Sigma) was included as a positive control. Supernatants were harvested and stored at −70°C until further analysis by Multiplex bead array analysis.
Histology.
To standardize the histological scoring, the left lung lobes were collected and fixed in 10% buffered formalin (Merck), processed, and embedded into paraffin wax. Tissues were sectioned (3 to 5 μm) and stained with hematoxylin and eosin and the lung pathology was semiquantitatively scored in a blinded manner from 0 to 4 according to the inflammatory score assigned to the perivascular infiltrates described in reference 21. A score of 0 indicated the absence of inflammation in the peribronchiolar, perivascular, and intra-alveolar regions of the lungs. Scores were assigned to the perivascular and peribronchiolar inflammation as follows: 1, minimal, few scattered areas of lymphocytic infiltrates which involve less than 15% of the blood vessel or bronchiole with no cuffing of lymphocytes; 2, mild, lymphocytic infiltration with formation of cuffs consisting of fewer than 5 layers of cells, with 15 to 25% involvement of blood vessel or bronchiole; 3, moderate, lymphocytic infiltration with cuff formation consisting of 5 to 10 layers of cells, with 25 to 75% involvement of blood vessel and bronchiole; and 4, severe, lymphocytic infiltration with formation of cuff consisting of more than 10 layers of cells, with 75% involvement of blood vessel and bronchiole. For the intra-alveolar regions, the scores were assigned by determining the percentage of area involved/inflamed in each scanned field of a specific lobe: 1, less than 15% of field area involved; 2, 15 to 25% involvement; 3, 25 to 75% of field involved; and 4, more than 75% of field involved in inflammation. Scores were grouped and averaged according to treatment group.
Multiplex bead array assay.
Cytokine analysis was done using mouse 7- and 21-plex Luminex assays (MPXMCYTO-70K-07, Merck-Millipore, Missouri) to simultaneously quantify the concentrations of IL-1α, IL-6, IL-10, IL-12p40, G-CSF, IFN-γ, and TNF-α in the mouse sera and IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, eotaxin, G-CSF, GM-CSF, IFN-γ, TNF-α, interferon-inducible protein 10 (IP-10), monocyte chemotactic protein 1 (MCP-1), MIP-1α, MIP-1β, and vascular endothelial growth factor (VEGF) in the MLN supernatants obtained from the ex vivo restimulations. The assay was done according to manufacturer's instructions, and the samples were evaluated in duplicate. Cytokine concentrations were measured on a Bio-Plex platform (Bio-Plex, Bio-Rad Laboratories). Two quality controls were included in each kit, and levels of all analytes were within the expected ranges. A standard curve ranging from 3.2 pg/ml to 10,000 pg/ml was used for all analytes. Bio-Plex Manager software (version 4.1.1) was used to analyze the data.
Determination of DMPA concentrations in mouse serum by LC-MS/MS.
Blood was collected from anesthetized mice by cardiac puncture, and the serum samples were stored at −80°C. The mouse sera were shipped to GBN Analytics in Mossel Bay, South Africa, where the concentrations of DMPA were measured using high-performance liquid chromatography (HPLC) with tandem mass spectrometry (LC-MS/MS) after a liquid-liquid extraction. Chromatographic separation was achieved on a Phenomenex Luna C18 (150- by 2.1-mm, 5-μm) analytical column using methanol and 5 mM ammonium formate (80:20 [vol/vol]) as the mobile phase under isocratic conditions. DMPA was detected with an Applied Biosystems API 4000 mass spectrometer using electrospray ionization in a positive and multiple reaction monitoring mode to monitor the precursor-to-product ion transition of medroxyprogesterone-17-acetate (m/z 387.2→327.3). Calibration and quality control standards were prepared by adding known amounts of DMPA in methanol to normal blank mouse serum to obtain the desired range. Standards were frozen at −80°C and subsequently used to assess the intra- and interbatch accuracy and precision of the detection method. Detection of DMPA in mouse serum was validated for the 0.165-to-84.2-ng/ml calibration range.
Statistics.
Data presented are of two independent experiments and were analyzed using the data analysis software GraphPad Prism 5 and Statistica 10, Statsoft (Ohio). P values of ≤0.05 were considered significantly different, and values of ≤0.09 and >0.05 were considered a trend. The letters a, b, and c were used to indicate statistical significance, with values with the same letter not being significantly different from each other.
RESULTS
Attaining murine serum concentrations of DMPA corresponding to human contraceptive levels.
The aim of the study reported here was to use a DMPA dosage that resulted in serum DMPA concentrations in mice similar to those found in human contraceptive users. In contraceptive users, the peak serum concentrations of DMPA have been shown to vary widely ranging from 1 ng/ml to 23 ± 6.90 ng/ml (18, 22–25). We therefore carried out a regimen study to identify a DMPA dose which would be comparable with the upper limit of peak serum concentrations observed in contraceptive users (22). The DMPA regimen study compared a weekly dose (Fig. 1, group A) to a single-dose (Fig. 1, group B) administration of 1 mg DMPA. Blood was drawn from both groups on day 1 and weeks 3, 8, and 16, and the DMPA serum concentrations were measured by LC-MS/MS. Electrospray ionization was used to produce ions, and DMPA showed a precursor ion at m/z 387.2. The DMPA serum concentration was 21.26 ng/ml 1 day after initiation of DMPA regimens (Fig. 1). The DMPA concentration thereafter increased in group A to 27.42 ng/ml after 3 weeks and remained high (31.6 ng/ml after 8 weeks and 29.42 ng/ml after 16 weeks) (Fig. 1) for the duration of the experiment. The serum concentration in group B, however, decreased to 6.05 ng/ml after 3 weeks and remained low (0.3 ng/ml after 8 weeks and 0.19 ng/ml after 16 weeks) (Fig. 1). We therefore decided to inject mice weekly with DMPA to achieve serum concentrations that are consistent with peak concentrations observed in humans. We believe this DMPA mouse model will allow us to gain insight into the immune modulatory effects of human contraceptive doses of DMPA.
Fig 1.
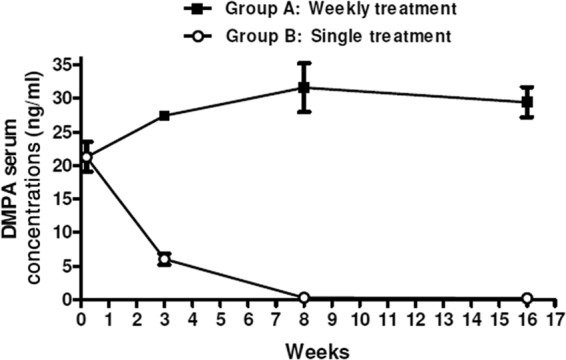
Mean serum concentration of DMPA in C57BL/6 mice after intramuscular administration. DMPA was administered to mice by means of two regimens. Group A received a weekly injection of 1 mg DMPA, whereas group B received a single injection with the same concentration of hormone. Serum of 5 mice in each group was collected at the following time points after initiation of DMPA treatment: day 1 and weeks 3, 8, and 16. DMPA serum concentrations were measured by high-performance liquid chromatography (HPLC) and tandem mass spectrometry (LC-MS/MS). Data are represented as means ± standard errors of the means (SEM).
Serum concentrations of DMPA resembling those observed in contraceptive users resulted in increased body weights compared to those of the appropriate control mice injected with saline. The body weight of each mouse was measured on a weekly basis as a determinant of their well-being. Mice from the C57BL/6 and BALB/c strains treated with DMPA (DMPA and DMPA-M. tuberculosis) gained significantly more weight than the saline (naïve and M. tuberculosis)-treated groups (Fig. 2A and B), a well-described side effect observed in women using DMPA as contraceptive (26). No significant differences were found between the DMPA and DMPA-M. tuberculosis groups and the naïve and M. tuberculosis groups, which is consistent with the low M. tuberculosis infection dose. An additional 16-week time point was included in the BALB/c experiments, as no differences in CFU were seen at the 3- and 8-week postinfection time points for this particular strain.
Fig 2.
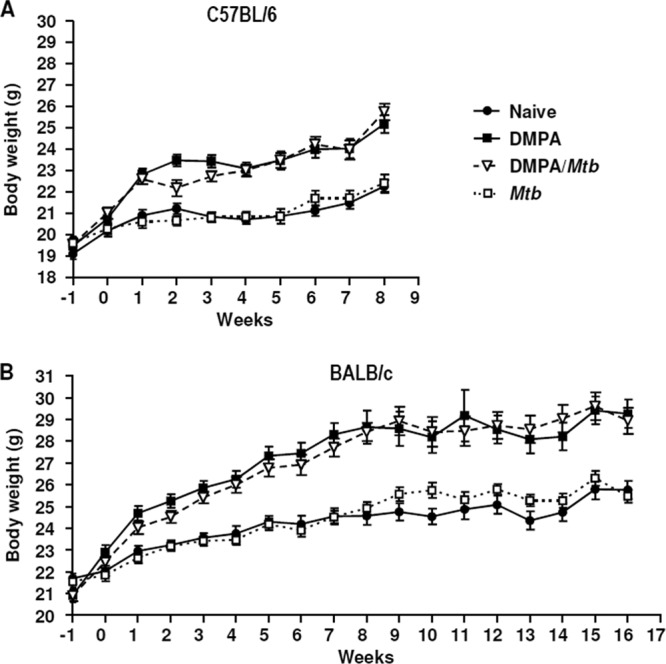
DMPA treatment results in enhanced body weight increases. Mice (n = 10 mice/group) from both the C57BL/6 (A) and BALB/c (B) strains received weekly intramuscular injections of 1 mg DMPA, and the control mice received an equal volume of saline (50 μl). DMPA injections commenced 1 week before (week −1) aerosol infection with M. tuberculosis H37Rv (week 0). Differences in body weight among the four groups were determined in two independent experiments by one-way analysis of variance (ANOVA) with the Bonferroni post hoc test at each of the time points, and the data are represented as means ± SEM. From 1 week postinfection, DMPA-treated mice (DMPA and DMPA-M. tuberculosis groups) gained significantly more weight than the untreated (naïve and M. tuberculosis) groups (0.001 < P < 0.05). No differences were found between naïve and M. tuberculosis-infected mice and between the two DMPA-treated groups. Each graph is a representative of the two independent experiments.
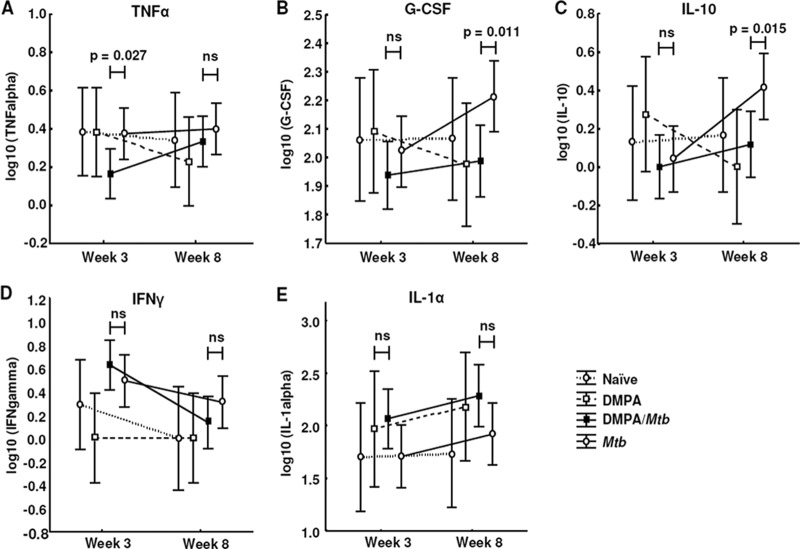
DMPA-treated mice infected with M. tuberculosis have lower serum levels of TNF-α, G-CSF, IL-10, and IFN-γ than untreated M. tuberculosis-infected mice.
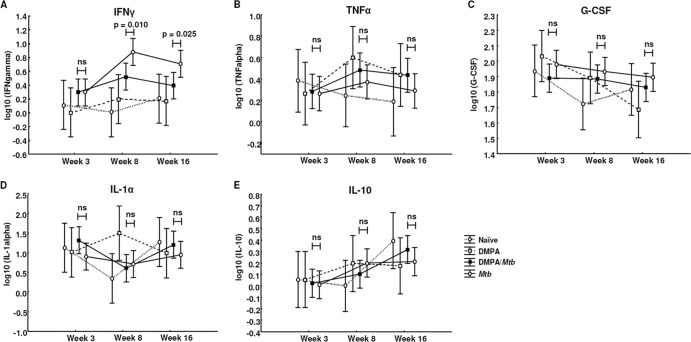
We previously showed that PBMCs of women using DMPA produce significantly less IL-1α, IL-12p40, IL-10, IL-13, and G-CSF in response to BCG, which coincided with lower levels of circulating monocytes (1). These results raised the question of whether DMPA users are more susceptible to TB and/or display more severe TB disease than noncontraceptive users. Addressing this question in humans proves very difficult, as a large number of study participants not only would have to be recruited but would also have to be followed up over a long period of time. The aim of this study was therefore to use a mouse model to better understand the overall immune regulatory properties of DMPA during M. tuberculosis infection. Serum concentrations of IL-1α, IL-6, IL-10, IL-12p40, G-CSF, IFN-γ, and TNF-α were measured by Luminex analysis in two independent experiments in both strains. C57BL/6 mice treated with DMPA and infected with M. tuberculosis had significantly lower circulating concentrations of TNF-α (P = 0.027; Fig. 3A) 3 weeks postinfection and of G-CSF (P = 0.011; Fig. 3B) and IL-10 (P = 0.015; Fig. 3C) 8 weeks postinfection than untreated, M. tuberculosis-infected mice. DMPA treatment had no effect on the serum concentrations of IFN-γ (Fig. 3D) and IL-1α (Fig. 3E) in C57BL/6 mice. DMPA therefore differentially altered cytokine production during the course of the infection. DMPA treatment affected IFN-γ concentrations in BALB/c mice infected with M. tuberculosis 8 weeks postinfection, with DMPA-treated mice having significantly lower circulating IFN-γ levels (P = 0.010; Fig. 4A) than untreated M. tuberculosis-infected mice. The treatment effect remained evident even 16 weeks postinfection (P = 0.025; Fig. 4A). No differences were observed in the serum concentrations of TNF-α, G-CSF, IL-1α, and IL-10 in BALB/c mice between the DMPA-treated and untreated M. tuberculosis groups (Fig. 4B to E). DMPA altered the concentrations of IL-10 and G-CSF in our previously published human study (1) as well as in the C57BL/6 DMPA mouse model. Although different antigens were used in these studies, both measure antimycobacterial responses, and the results suggest that the C57BL/6 strain is a good model to study the effect of contraceptive doses of DMPA on mycobacterial infections.
Fig 3.
Effects of DMPA treatment on serum cytokine concentrations in M. tuberculosis-infected C57BL/6 mice. Serum collected from C57BL/6 mice 3 and 8 weeks postinfection was subjected to cytokine measurement by Luminex analysis. DMPA-treated mice generally have lower levels of circulating cytokines than untreated mice. Pooled data from two independent experiments are shown. For each experiment, the serum of five mice in each of the infected groups (DMPA-M. tuberculosis and M. tuberculosis) and 3 of the naïve and DMPA-only groups were included for Luminex analysis. The log-transformed data were analyzed using a one-way ANOVA with a Fisher least significant difference (LSD) post hoc test and presented as least-squares means and 95% confidence intervals. A P value of <0.05 was regarded as significantly different (ns, not significant).
Fig 4.
Effects of DMPA on serum cytokine concentrations in M. tuberculosis-infected BALB/c mice. Serum collected from BALB/c mice 3, 8, and 16 weeks postinfection was subjected to cytokine measurement by Luminex analysis. DMPA treatment altered circulating concentrations of IFN-γ (A) but not TNF-α (B), G-CSF (C), IL-1α (D), and IL-10 (E) in these mice. Pooled data from two independent experiments are shown. For each experiment, the serum of five mice in each of the infected groups (DMPA-M. tuberculosis and M. tuberculosis) and 3 of the naïve and DMPA-only groups were included for Luminex analysis. The log-transformed data were analyzed using a one-way ANOVA with a Fisher LSD post hoc test and presented as least-squares means and 95% confidence intervals. A P value of <0.05 was regarded as significantly different (ns, not significant).
DMPA alters memory responses of mediastinal lymph node cells to M. tuberculosis-specific antigens.
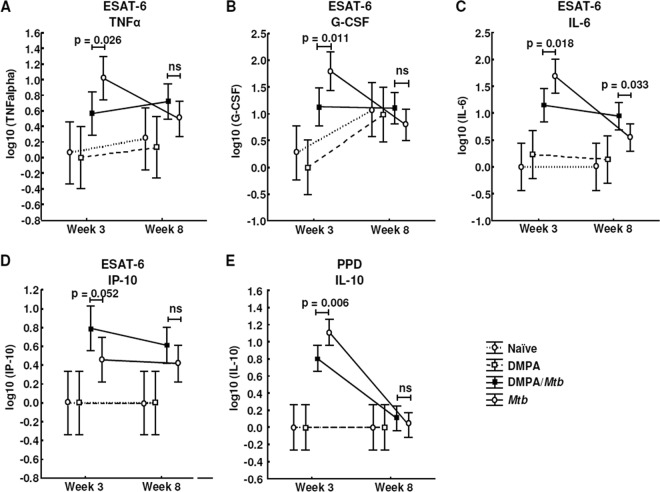
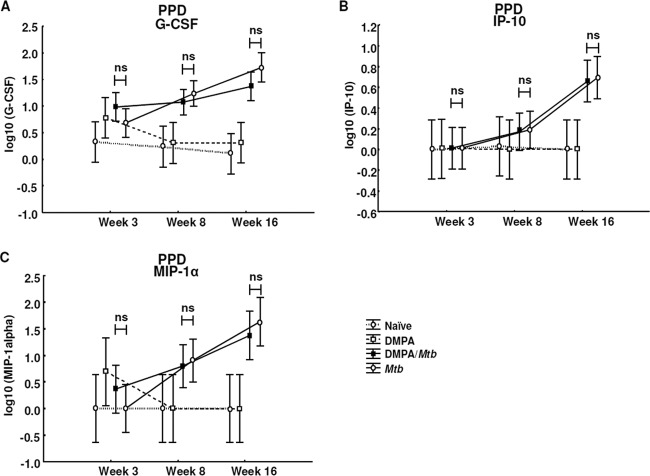
To further explore the effects of DMPA on the memory immune response of mice challenged with M. tuberculosis, the MLNs of each of the mice in the four groups were isolated and stimulated with the M. tuberculosis antigens purified protein derivative (PPD) and ESAT-6 for 4 days in the absence of hormone. The lymph node supernatants were also subjected to Luminex analysis, and the concentrations of IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, eotaxin, G-CSF, GM-CSF, IFN-γ, TNF-α, IP-10, MCP-1, MIP-1α, MIP-1β, and VEGF were measured. MLN cells isolated from DMPA-treated M. tuberculosis-infected C57BL/6 mice produced significantly less TNF-α (P = 0.026; Fig. 5A), G-CSF (P = 0.011; Fig. 5B), and IL-6 (P = 0.018; Fig. 5C) 3 weeks postinfection in response to ESAT-6 compared to untreated M. tuberculosis-infected mice. No differences were detected in the TNF-α and G-CSF levels in the culture supernatants 8 weeks postinfection. The effect of DMPA on IL-6 varied during the course of the infection, since the MLN cells of the DMPA-treated group produced more IL-6 (P = 0.033; Fig. 5C) 8 weeks postinfection. DMPA treatment, furthermore, resulted in increased production of IP-10 (P = 0.052; Fig. 5D) 3 weeks postinfection. Cells isolated from the MLNs of C57BL/6 mice also produced less IL-10 (P = 0.006; Fig. 5E) in response to PPD compared to the cells of untreated mice infected with M. tuberculosis. The effect on both these cytokines disappeared at 8 weeks. No differences were, however, observed in the antigen-induced responses of IFN-γ and MIP-1α (Fig. 6A and B) 3 and 8 weeks postinfection.
Fig 5.
DMPA treatment alters cytokine and chemokine secretion of PPD- and ESAT-6-stimulated MLN cells of C57BL/6 mice. C57BL/6 mice were injected intramuscularly with DMPA or saline weekly. Single cells were obtained from MLNs collected 3 (n = 5 mice/group) and 8 (n = 5 mice/group) weeks postinfection. Stimulations were done in triplicate in two independent experiments, and the cytokine levels were measured in the culture supernatant by Luminex analysis. DMPA treatment altered the ESAT-6 (A to D)- and PPD (E)-induced cytokine responses of the isolated MLN cells. Log-transformed data of two experiments were pooled and analyzed using a one-way ANOVA with a Fisher LSD post hoc test and presented as least-squares means and 95% confidence intervals. A P value of <0.05 was regarded as significantly different (ns, not significant).
Fig 6.
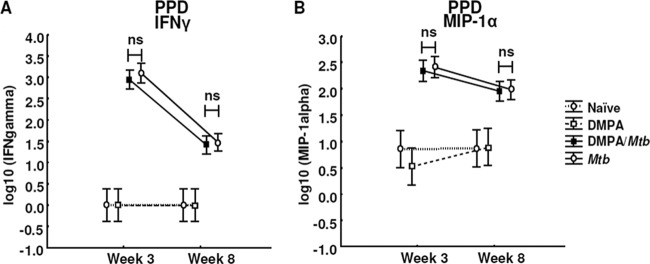
DMPA treatment does not alter the PPD-induced secretion of IFN-γ and MIP-1α by MLN cells of C57BL/6 mice. C57BL/6 mice were injected intramuscularly with DMPA or saline weekly. Single cells were obtained from MLNs collected 3 (n = 5 mice/group) and 8 (n = 5 mice/group) weeks postinfection. Stimulations were done in triplicate in two independent experiments, and the cytokine levels were measured in the culture supernatant by Luminex analysis. DMPA treatment did not alter the PPD-induced IFN-γ (A) and MIP-1α (B) responses of the isolated MLN cells. Log-transformed data from the two experiments were pooled and analyzed using a one-way ANOVA with a Fisher LSD post hoc test and presented as least-squares means and 95% confidence intervals. A P value of <0.05 was regarded as significantly different (ns, not significant).
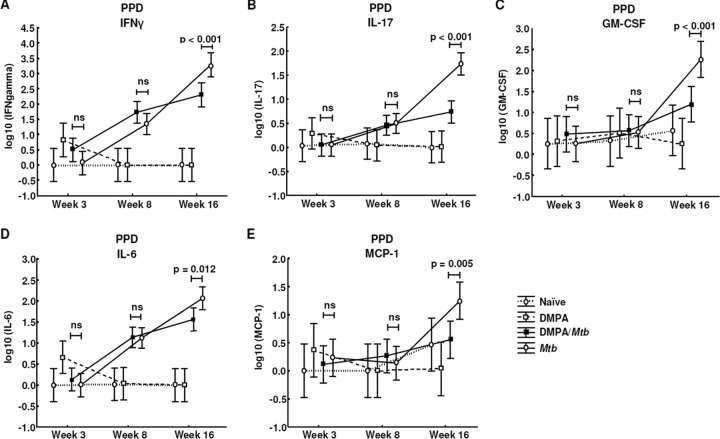
MLN cells of DMPA-treated BALB/c mice produced significantly less IFN-γ (P < 0.001; Fig. 7A), IL-17 (P < 0.001; Fig. 7B), GM-CSF (P < 0.001; Fig. 7C), IL-6 (P = 0.012; Fig. 7D), and MCP-1 (P = 0.005; Fig. 7E) in response to PPD 16 weeks postinfection. For this particular strain, no differences were found in the antigen-induced response of G-CSF, IP-10, and MIP-1α (Fig. 8A to C). From the results, it is clear that DMPA has an overall suppressive effect on cytokine production with the exception of IP-10 and IL-6 in the C57BL/6 strain. We have previously shown that DMPA upregulated the production of IP-10 in vitro when human PBMCs were stimulated with BCG for 3 and 6 days (1). PBMCs of human MPA users also produce lower levels of IL-10 and G-CSF upon BCG stimulation. The cytokine profile of the C57BL/6 strain therefore corresponds to the results previously reported in our ex vivo human experiments, although different mycobacterial antigens were used (1).
Fig 7.
DMPA treatment alters cytokine and chemokine secretion of PPD-stimulated MLN cells of BALB/c mice. BALB/c mice were injected intramuscularly with DMPA or saline weekly. Single cells were obtained from MLNs collected 3, 8, and 16 weeks postinfection of 5 mice/group. Stimulations were done in triplicate in two independent experiments, and cytokine levels were measured in the culture supernatants by Luminex analysis. DMPA treatment altered the PPD-induced cytokine responses of IFN-γ (A), IL-17 (B), GM-CSF (C), IL-6 (D), and MCP-1 (E) 16 weeks postinfection. Log-transformed data of two experiments were pooled and analyzed using a one-way ANOVA with a Fisher LSD post hoc test and presented as least-squares means and 95% confidence intervals. A P value of <0.05 was regarded as significantly different (ns, not significant).
Fig 8.
DMPA treatment does not alter the PPD-induced secretion of G-CSF, IP-10, and MIP-1α by MLN cells of BALB/c mice. BALB/c mice were injected intramuscularly with DMPA or saline weekly. Single cells were obtained from MLNs collected 3, 8, and 16 weeks postinfection from 5 mice/group. Stimulations were done in triplicate in two independent experiments, and cytokine levels were measured in the culture supernatants by Luminex analysis. DMPA treatment did not alter the PPD-induced G-CSF (A), IP-10 (B), and MIP-1α (C) responses of the isolated MLN cells. Log-transformed data from the two experiments were pooled and analyzed using a one-way ANOVA with a Fisher LSD post hoc test and presented as least-squares means and 95% confidence intervals. A P value of <0.05 was regarded as significantly different (ns, not significant).
DMPA inhibits mycobacterial clearance in the lungs of C57BL/6 mice.
After showing that DMPA treatment resulted in significant changes in the serum cytokine levels and altered the ability of MLN cells to produce cytokines in response to PPD and ESAT-6, we investigated whether DMPA influences the ability of mice to control M. tuberculosis replication. Ten mice in each group were killed at the time points indicated, and their lungs and spleens were aseptically removed and weighed. Organ weights were analyzed as a marker of inflammation. No differences were observed in the lung weights of infected C57BL/6 mice at any of the time points (data not shown); however, in the BALB/c strain, the lungs of infected mice treated with DMPA weighed significantly less than the lungs of untreated mice infected with M. tuberculosis 8 weeks postinfection (P < 0.05; data not shown). This effect, however, appeared to be transient, as no difference in organ weight was observed 16 weeks postinfection (data not shown). Similar to the lung weights, the spleens of the infected mice weighed more than the spleens of the uninfected (naïve and DMPA treatment only) mice, but no difference was observed in the spleen weights of DMPA-treated C57BL/6 and BALB/c mice compared to untreated mice (data not shown). Histopathological scoring was further done to determine the extent of perivascular, peribronchiolar, and intra-alveolar lymphocytic infiltration, as glucocorticoids are known to differentially alter the accumulation of inflammatory cells in the airways (27). DMPA treatment resulted in significantly decreased inflammation in the peribronchiolar regions 16 weeks postinfection (P = 0.037; Fig. 9A) and significantly increased inflammation in the intra-alveolar regions 8 weeks postinfection (P = 0.0002; Fig. 9B) in BALB/c mice. No difference in inflammatory score was observed in the perivascular region of BALB/c mice (data not shown) or in any of the 3 parameters in the C57BL/6 mouse strain (data not shown).
Fig 9.
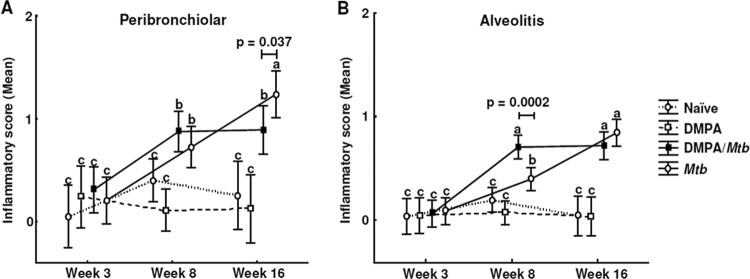
DMPA alters lymphocytic infiltration in lungs of M. tuberculosis-infected BALB/c mice. Mice were injected weekly with MPA or saline and infected with a low aerosol dose of H37Rv 1 week after initiation of DMPA injections. Histopathological scoring was done on the left lung lobes in a blinded fashion in two independent experiments, and tissue was scored from 0 to 4 for the extent of inflammatory cell infiltration. Perivascular, peribronchiolar, and alveolar infiltrates were scored separately. Zero to 1 represented no or little inflammation, whereas 4 signified severe inflammation. Data are expressed as mean inflammatory scores with 95% confidence intervals (CI), and the differences were determined with one-way ANOVA. Data shown are the pooled results from two the experiments, with each having 5 mice per group. The letters a, b, and c indicate statistical significance, where values with the same letter are not significantly different from each other. A P value of <0.05 was regarded as significantly different.
Glucocorticoids are known to induce reactivation of TB in mice (15), and since DMPA possesses selective glucocorticoid activity, we wanted to determine the effect of this contraceptive on the bacterial burden of M. tuberculosis-infected mice. Enumeration of the lung CFU of the C57BL/6 mice revealed that the DMPA-treated mice (DMPA-M. tuberculosis group) had significantly more bacteria in their lungs at 3 weeks (P = 0.021) and 8 weeks (P = 0.0002; Fig. 10A) postinfection than untreated mice (M. tuberculosis group). Dambuza et al. demonstrated that clearance of M. tuberculosis is dependent on TNF-α and that TNF−/− mice displayed high bacterial loads and succumb to the infection (28). The increase in lung CFU in this particular strain in DMPA-treated mice is therefore possibly attributable to the decreased TNF-α serum concentrations observed 3 weeks postinfection. No differences in CFU were found at any of the 3 time points for BALB/c mice (Fig. 10B). This again highlights the fact that these two strains have different sensitivities to glucocorticoid-induced immune regulation. Increases in the spleen weights between the infected and uninfected animals suggest that the infection spread to this organ; however, spleen weights were similar in DMPA-treated and untreated mice (data not shown). DMPA treatment did not affect the bacterial load in the spleens of either mouse strain.
Fig 10.
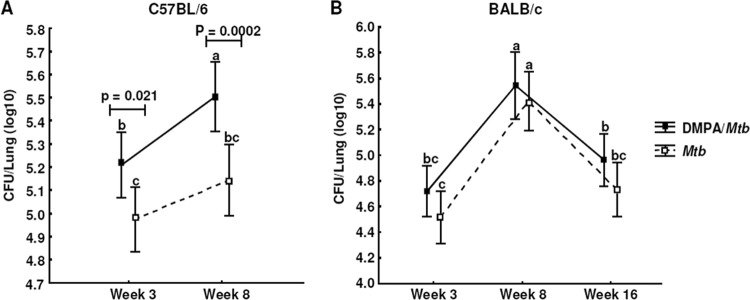
DMPA alters the bacterial burden in the lungs of M. tuberculosis-infected C57BL/6 mice. C57BL/6 (A) and BALB/c (B) mice were infected with ∼30 CFU by aerosol inhalation 1 week after commencement of intramuscular DMPA injections into the right thigh. Mice were treated with 50 μl containing 1 mg DMPA weekly, whereas control mice were injected with the same volume of saline. Bacterial load (CFU) was determined in the lungs 3, 8, and 16 weeks postinfection in two independent experiments, of which each had 10 mice/group. Data shown are the pooled results of the two experiments and expressed as means with 95% CI, and the differences were determined with one-way ANOVA. The letters a, b, and c indicate statistical significance, where values with the same letter are not significantly different from each other. A P value of <0.05 was regarded as significantly different.
DISCUSSION
Several effects and possible mechanisms of DMPA on infections of the genital tract have been documented (29–31). It was shown that the use of DMPA as a contraceptive causes a significant increase in the shedding of HIV-1 DNA in humans (31) and the administration of contraceptive doses of DMPA to rhesus macaques causes thinning of the vaginal epithelium (30). In a herpes simplex virus 2 (HSV-2) mouse model, DMPA causes a 10-fold increase in susceptibility to this sexually transmitted disease (29). DMPA also inhibits the effector function of CD8 T cells, including IFN-γ production, and induces reactivation of HSV-1 in mice (32, 33). A significant association was also found between the use of DMPA and the acquisition of sexually transmitted diseases other than HIV (7). Whether DMPA use increases the risk of acquiring HIV has been a matter of dispute, but recent reports by Morrison et al. and Heffron et al. supported this concept (2, 3). The effects of DMPA on infections outside the genital tract have not been investigated until recently, when we reported for the first time that DMPA acts similarly to cortisol and influences BCG-induced cytokine responses in PBMCs of noncontraceptive users and that PBMCs of DMPA users produce less IL-1α, IL-12p40, IL-10, IL-13, and G-CSF (1). Importantly, we also showed that DMPA users have significantly less circulating monocytes (1), a phenomenon also observed for low-dose glucocorticoid therapy (34).
Our goal was to determine the effects of DMPA in a model system which would have clinical relevance. Various DMPA regimens have been described in animal models in an attempt to achieve DMPA serum concentrations similar to either endogenous progesterone levels or DMPA levels observed in humans (35–37). One such study established an intramuscular DMPA regimen for mice which would achieve similar DMPA plasma concentrations observed in women who use this hormone as breast cancer therapy (37). Our pharmacokinetic study in mice identified an intramuscular DMPA regimen with serum concentrations comparable with the highest peak serum concentrations observed in women using it as injectable contraceptive. We are aware that the serum concentrations in women vary widely and do not remain at this level throughout the 3-month period until follow-up injection. Therefore, the timing after DMPA administration might be critical in inhibiting immune responses to M. tuberculosis in humans.
In the C57BL/6 model, DMPA-treated mice had lower serum levels of TNF-α 3 weeks and lower levels of G-CSF and IL-10 8 weeks after infection. Furthermore, cells isolated from the MLNs of DMPA-treated C57BL/6 mice also produced less TNF-α, G-CSF, and IL-6 and more IP-10 in response to ESAT-6 and less IL-10 in response to PPD. The production of IFN-γ and MIP-1α was not affected by DMPA in this model. BALB/c mice, on the other hand, had lower serum IFN-γ levels 8 and 16 weeks postinfection. Due to limited changes observed in the BALB/c strain at the earlier time points, a 16-week time point was included based on the findings of Hernandez-Pando et al. (38). Changes in the PPD-induced cytokine responses of the MLN cells were detected at this late time point, with MLN cells of DMPA-treated BALB/c mice producing less IFN-γ, IL-17, GM-CSF, IL-6, and MCP-1. In contrast to the C57BL/6 strain, the production of G-CSF and TNF-α was not affected by DMPA in BALB/c mice.
The clinical importance of TNF-α in responses against M. tuberculosis has been highlighted in patients receiving TNF-α-blocking therapies for other inflammatory diseases, as many of these patients experience relapse of TB (39). TNF-α, together with IFN-γ, mediates the activation of macrophages, which is required to control the growth of M. tuberculosis, and it was shown that TNF−/− mice have significantly more CFU in their lungs than wild-type mice and succumb during early stages of disease (28). Mice deficient in IL-10 generally have an enhanced ability to control M. tuberculosis; however, it was shown that these mice succumb to the disease due to severe immunopathology (40). Mice deficient in IL-6 are able to initiate the formation of granulomas but fail to maintain them and have a decreased ability to produce proinflammatory mediators during the early stages of the infection (41). IL-17 contributes to immune responses to M. tuberculosis in TB patients as well as latently infected individuals and possibly plays a role in granuloma formation and protective immunity (42, 43). G-CSF and IP-10 have also been implicated in inflammatory cytokine responses during active TB (44). By altering the production of these cytokines involved in the complex immune responses against M. tuberculosis, it is possible that MPA might affect disease progression and severity.
Contraceptive doses of DMPA also influenced the recruitment and accumulation of inflammatory cells to and at the site of disease. Glucocorticoid therapies are known to deplete monocyte populations in humans (34) and limit the recruitment of eosinophils and T lymphocytes but not neutrophils in the airways of mice (27). DMPA treatment furthermore resulted in an increased bacterial burden in C57BL/6 mice infected with M. tuberculosis compared to that of untreated mice. The increase in the bacterial load is likely due to the selective glucocorticoid properties of DMPA. Glucocorticoid treatment is known to induce a Th2 response in individuals receiving anti-inflammatory therapies (45). In a model of latent tuberculosis infection, glucocorticoid administration resulted in immunosuppression and the subsequent reactivation of the disease (14). The increase in bacterial load could also be explained by the reduction in serum TNF-α concentration and the inability of the MLNs to produce this particular cytokine, as this cytokine is known to play an important role in the confinement of mycobacteria (46).
Interstrain variations have been described for mice with regard to inflammatory and immunological functions (19, 47). Macrophages from different mouse strains also exhibit different sensitivities to the glucocorticoid dexamethasone (19). Dexamethasone treatment, for example, differentially inhibits the lipopolysaccharide-induced IL-1β and TNF-α production of bone morrow-derived macrophages of C57BL/6 and BALB/c mice (19). C57BL/6 mice were more sensitive to dexamethasone-mediated inhibition of cytokine production compared to BALB/c mice. The delay in treatment response in BALB/c mice is therefore possibly attributable to the fact that this strain is less sensitive to glucocorticoid-mediated effects (19). In keeping with the cytokine data, DMPA affected CFU counts in the lungs of C57BL/6 but not BALB/c mice.
In our human study, we found that DMPA treatment inhibited the BCG-induced production of a number of cytokines, including TNF-α, IL-6, IFN-γ, IL-17, and GM-CSF, and increased the BCG-induced production of IP-10 in PBMCs in vitro (1). However, BCG-stimulated PBMCs of DMPA users produced significantly less IL-10 and G-CSF in response to BCG than PBMCs from noncontraceptive users. Due to the similarities between the human study and the C57BL/6 model despite different mycobacterial antigenic stimulation in these studies, this strain serves as a good model to investigate the immune modulatory properties and mechanisms of action of DMPA in the context of TB.
These results show for the first time that DMPA impacts TB disease severity in a murine model and that the effects of this contraceptive are not confined to infections of the genital tract. We believe these findings could have major implications for the contraceptive policies not only in developing countries like South Africa but worldwide.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of South Africa grant FA2007022300004 (K.R.); The South African Tuberculosis and AIDS Training (SATBAT) program, funded by the National Institute of Health/Fogarty International Center grant 1U2RTW007370/3 (K.R.); SATBAT grant 1U2RTW007370/3 (L.K.); the Harry Crossley Foundation; and the KwaZulu-Natal Research Institute of TB-HIV (K-RITH) (L.K.).
We thank M. J. Smit for performing LC-MS/MS procedures and the staff of the UCT animal unit for assisting in animal care.
Footnotes
Published ahead of print 4 February 2013
REFERENCES
- 1. Kleynhans L, Du Plessis N, Black GF, Loxton AG, Kidd M, van Helden PD, Walzl G, Ronacher K. 2011. Medroxyprogesterone acetate alters Mycobacterium bovis BCG-induced cytokine production in peripheral blood mononuclear cells of contraceptive users. PLoS One 6:e24639 doi:10.1371/journal.pone.0024639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM. 2012. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect. Dis. 12:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, Byamugisha J, Padian N, Celentano DD, Salata RA. 2010. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS 24:1778–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leclerc PM, Dubois-Colas N, Garenne M. 2008. Hormonal contraception and HIV prevalence in four African countries. Contraception 77:371–376 [DOI] [PubMed] [Google Scholar]
- 5. Morrison CS, Skoler-Karpoff S, Kwok C, Chen PL, van de Wijgert J, Gehret-Plagianos M, Patel S, Ahmed K, Ramjee G, Friedland B, Lahteenmaki P. 2012. Hormonal contraception and the risk of HIV acquisition among women in South Africa. AIDS 26:497–504 [DOI] [PubMed] [Google Scholar]
- 6. Myer L, Denny L, Wright TC, Kuhn L. 2007. Prospective study of hormonal contraception and women's risk of HIV infection in South Africa. Int. J. Epidemiol. 36:166–174 [DOI] [PubMed] [Google Scholar]
- 7. Overton ET, Shacham E, Singhatiraj E, Nurutdinova D. 2008. Incidence of sexually transmitted infections among HIV-infected women using depot medroxyprogesterone acetate contraception. Contraception 78:125–130 [DOI] [PubMed] [Google Scholar]
- 8. Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. 1999. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol. Cell. Endocrinol. 154:11–20 [DOI] [PubMed] [Google Scholar]
- 9. Strahle U, Klock G, Schutz G. 1987. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc. Natl. Acad. Sci. U. S. A. 84:7871–7875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Z, Corden JL, Brown TR. 1997. Identification and characterization of a novel androgen response element composed of a direct repeat. J. Biol. Chem. 272:8227–8235 [DOI] [PubMed] [Google Scholar]
- 11. Kontula K, Paavonen T, Luukkainen T, Andersson LC. 1983. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem. Pharmacol. 32:1511–1518 [DOI] [PubMed] [Google Scholar]
- 12. Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP. 2005. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol. Cell. Endocrinol. 242:23–32 [DOI] [PubMed] [Google Scholar]
- 13. Zhao Q, Pang J, Favata MF, Trzaskos JM. 2003. Receptor density dictates the behavior of a subset of steroid ligands in glucocorticoid receptor-mediated transrepression. Int. Immunopharmacol. 3:1803–1817 [DOI] [PubMed] [Google Scholar]
- 14. Manabe YC, Kesavan AK, Lopez-Molina J, Hatem CL, Brooks M, Fujiwara R, Hochstein K, Pitt ML, Tufariello J, Chan J, McMurray DN, Bishai WR, Dannenberg AM, Jr, Mendez S. 2008. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb.) 88:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scanga CA, Mohan VP, Joseph H, Yu K, Chan J, Flynn JL. 1999. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect. Immun. 67:4531–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howard AD, Zwilling BS. 1999. Reactivation of tuberculosis is associated with a shift from type 1 to type 2 cytokines. Clin. Exp. Immunol. 115:428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jick SS, Lieberman ES, Rahman MU, Choi HK. 2006. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 55:19–26 [DOI] [PubMed] [Google Scholar]
- 18. Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, Mishell DR. 1977. Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J. Clin. Endocrinol. Metab. 44:32–38 [DOI] [PubMed] [Google Scholar]
- 19. Harizi H, Mormede P, Corcuff JB. 2008. Inter-strain differences in glucocorticoid and mineralocorticoid effects on macrophage and lymphocyte functions in mice. J. Neuroimmunol. 204:38–42 [DOI] [PubMed] [Google Scholar]
- 20. Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, O'Garra A. 2010. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 40:2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tilley SL, Jaradat M, Stapleton C, Dixon D, Hua X, Erikson CJ, McCaskill JG, Chason KD, Liao G, Jania L, Koller BH, Jetten AM. 2007. Retinoid-related orphan receptor gamma controls immunoglobulin production and Th1/Th2 cytokine balance in the adaptive immune response to allergen. J. Immunol. 178:3208–3218 [DOI] [PubMed] [Google Scholar]
- 22. Fang S, Sun D, Jiang H, Luo H. 2004. Concentration changes if medroxyprogesterone acetate in serum and milk in lactating women who used depo geston. J. Reprod. Contraception 15:157–162 [Google Scholar]
- 23. Smit J, Botha J, McFadyen L, Beksinska M. 2004. Serum medroxyprogesterone acetate levels in new and repeat users of depot medroxyprogesterone acetate at the end of the dosing interval. Contraception 69:3–7 [DOI] [PubMed] [Google Scholar]
- 24. Pfizer 2012. Depo-Provera U.S. physicians prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=522 Pfizer, New York, NY [Google Scholar]
- 25. Jeppsson S, Gershagen S, Johansson ED, Rannevik G. 1982. Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent. Acta Endocrinol. (Copenh.) 99:339–343 [DOI] [PubMed] [Google Scholar]
- 26. Beksinska ME, Smit JA, Kleinschmidt I, Milford C, Farley TM. 2010. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception 81:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ito K, Herbert C, Siegle JS, Vuppusetty C, Hansbro N, Thomas PS, Foster PS, Barnes PJ, Kumar RK. 2008. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am. J. Respir. Cell Mol. Biol. 39:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dambuza I, Keeton R, Allie N, Hsu NJ, Randall P, Sebesho B, Fick L, Quesniaux VJ, Jacobs M. 2011. Reactivation of M. tuberculosis infection in trans-membrane tumour necrosis factor mice. PLoS One 6:e25121 doi:10.1371/journal.pone.0025121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 77:4558–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084–1089 [DOI] [PubMed] [Google Scholar]
- 31. Wang CC, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K, Chohan B, Lavreys L, Ndinya-Achola J, Kreiss JK. 2004. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS 18:205–209 [DOI] [PubMed] [Google Scholar]
- 32. Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL. 2008. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J. Immunol. 181:969–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vicetti Miguel RD, Hendricks RL, Aguirre AJ, Melan MA, Harvey SA, Terry-Allison T, St Leger AJ, Thomson AW, Cherpes TL. 2012. Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection. J. Immunol. 189:3449–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heimbeck I, Hofer TP, Eder C, Wright AK, Frankenberger M, Marei A, Boghdadi G, Scherberich J, Ziegler-Heitbrock L. 2010. Standardized single-platform assay for human monocyte subpopulations: lower CD14+CD16++ monocytes in females. Cytometry A 77:823–830 [DOI] [PubMed] [Google Scholar]
- 35. Hughes GC, Martin D, Zhang K, Hudkins KL, Alpers CE, Clark EA, Elkon KB. 2009. Decrease in glomerulonephritis and Th1-associated autoantibody production after progesterone treatment in NZB/NZW mice. Arthritis Rheum. 60:1775–1784 [DOI] [PubMed] [Google Scholar]
- 36. McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. 2002. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke 33:1685–1691 [DOI] [PubMed] [Google Scholar]
- 37. Palmieri D, Halverson DO, Ouatas T, Horak CE, Salerno M, Johnson J, Figg WD, Hollingshead M, Hursting S, Berrigan D, Steinberg SM, Merino MJ, Steeg PS. 2005. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J. Natl. Cancer Inst. 97:632–642 [DOI] [PubMed] [Google Scholar]
- 38. Hernandez-Pando R, de la Luz SM, Orozco H, Arriaga K, Pavon L, Al-Nakhli SA, Rook GA. 1998. The effects of androstenediol and dehydroepiandrosterone on the course and cytokine profile of tuberculosis in BALB/c mice. Immunology 95:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098–1104 [DOI] [PubMed] [Google Scholar]
- 40. Higgins DM, Sanchez-Campillo J, Rosas-Taraco AG, Lee EJ, Orme IM, Gonzalez-Juarrero M. 2009. Lack of IL-10 alters inflammatory and immune responses during pulmonary Mycobacterium tuberculosis infection. Tuberculosis (Edinb.) 89:149–157 [DOI] [PubMed] [Google Scholar]
- 41. Welsh KJ, Abbott AN, Hwang SA, Indrigo J, Armitige LY, Blackburn MR, Hunter RL, Jr, Actor JK. 2008. A role for tumour necrosis factor-alpha, complement C5 and interleukin-6 in the initiation and development of the mycobacterial cord factor trehalose 6,6′-dimycolate induced granulomatous response. Microbiology 154:1813–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Curtis MM, Way SS. 2009. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 180:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Djoba Siawaya JF, Chegou NN, van den Heuvel MM, Diacon AH, Beyers N, van Helden P, Walzl G. 2009. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine 47:132–136 [DOI] [PubMed] [Google Scholar]
- 45. Dandona P, Aljada A, Garg R, Mohanty P. 1999. Increase in plasma interleukin-10 following hydrocortisone injection. J. Clin. Endocrinol. Metab. 84:1141–1144 [DOI] [PubMed] [Google Scholar]
- 46. Allie N, Alexopoulou L, Quesniaux VJ, Fick L, Kranidioti K, Kollias G, Ryffel B, Jacobs M. 2008. Protective role of membrane tumour necrosis factor in the host's resistance to mycobacterial infection. Immunology 125:522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tumes DJ, Cormie J, Calvert MG, Stewart K, Nassenstein C, Braun A, Foster PS, Dent LA. 2007. Strain-dependent resistance to allergen-induced lung pathophysiology in mice correlates with rate of apoptosis of lung-derived eosinophils. J. Leukoc. Biol. 81:1362–1373 [DOI] [PubMed] [Google Scholar]