Abstract
Cryptococcus neoformans var. grubii is the most frequent cause of AIDS-associated cryptococcosis worldwide, while Cryptococcus gattii usually infects immunocompetent people. To understand the mechanisms which cause differential susceptibility to these cryptococcal species in HIV infection, we established and characterized a model of cryptococcosis in CD4C/HIVMutA transgenic (Tg) mice expressing gene products of HIV-1 and developing an AIDS-like disease. Tg mice infected intranasally with C. neoformans var. grubii strain H99 or C23 consistently displayed reduced survival compared to non-Tg mice at three graded inocula, while shortened survival of Tg mice infected with C. gattii strain R265 or R272 was restricted to a single high inoculum. HIV-1 transgene expression selectively augmented systemic dissemination to the liver and spleen for strains H99 and C23 but not strains R265 and R272. Histopathologic examination of lungs of Tg mice revealed large numbers of widely scattered H99 cells, with a minimal inflammatory cell response, while in the non-Tg mice H99 was almost completely embedded within extensive mixed inflammatory cell infiltrates. In contrast to H99, R265 was dispersed throughout the lung parenchyma and failed to induce a strong inflammatory response in both Tg and non-Tg mice. HIV-1 transgene expression reduced pulmonary production of CCL2 and CCL5 after infection with H99 or R265, and production of these two chemokines was lower after infection with R265. These results indicate that an altered immune response in these Tg mice markedly enhances C. neoformans but not C. gattii infection. This model therefore provides a powerful new tool to further investigate the immunopathogenesis of cryptococcosis.
INTRODUCTION
Cryptococcal meningitis is one of the most important HIV-related opportunistic infections worldwide, especially in sub-Saharan Africa (1). Globally, approximately 957,900 cases occur each year, resulting in 624,700 deaths among persons living with HIV/AIDS (1). Although cryptococcosis can occur in apparently healthy hosts, most infections are observed in HIV-infected patients, who are particularly susceptible to this life-threatening fungal infection (1). Inhalation of basidiospores or yeast cells of Cryptococcus from the environment results in pulmonary infection and preferential dissemination to the central nervous system, causing meningoencephalitis. Cryptococcus neoformans var. grubii (serotype A) is by far the most frequent cause of AIDS-associated cryptococcosis worldwide, with fewer cases caused by Cryptococcus neoformans var. neoformans (serotype D), Cryptococcus gattii (serotypes B and C) (2–7), or, exceptionally, a C. neoformans var. grubii serotype A × C. gattii serotype B hybrid (8, 9). In contrast to C. neoformans var. grubii, C. gattii usually infects immunocompetent people (10) and is only occasionally found in patients with HIV/AIDS (2–6). In a survey from South Africa, however, although only 2.4% of all Cryptococcus isolates were confirmed to be C. gattii, 24 of these cases occurred in HIV-infected patients, and only a single case involved an HIV-uninfected person (6). Accordingly, although HIV/AIDS may potentially augment susceptibility to C. gattii infection in specific circumstances combining both environmental exposure in an area of endemicity and limited access to antiretroviral therapy, most of the enhanced burden of cryptococcal infection in HIV/AIDS is caused by the ubiquitous C. neoformans var. grubii (6).
A major endemic outbreak of C. gattii infection that began on Vancouver Island in 1999 led to 239 reported cases and at least 19 deaths by the end of 2008 (10–12; www.BCCDC.ca), and it has now spread to mainland British Columbia and the Pacific Northwest in the United States (10, 13–15). Consistent with the epidemiology of C. gattii infections in Australia and New Zealand (7, 16), these infections in the British Columbia outbreak occurred mainly in immunocompetent people, and only 6.2% of confirmed C. gattii-infected patients were infected with HIV (12).
The mechanisms underlying the differential ability of C. gattii and C. neoformans var. grubii to cause disease in healthy persons or patients with HIV/AIDS are largely unknown. As a first step toward understanding the ability of C. gattii to cause disease in immunocompetent hosts, a previous study revealed reduced levels of neutrophil infiltration and reduced inflammatory cytokine production in the lungs of C57BL/6 mice infected with C. gattii compared to those of mice infected with C. neoformans var. grubii (17). However, a comprehensive analysis of virulence and host immune cell responses to these Cryptococcus species would be facilitated greatly by the availability of a relevant animal model of cryptococcosis in HIV infection. We previously devised a novel model of mucosal candidiasis in CD4C/HIV transgenic (Tg) mice expressing gene products of HIV-1 in immune cells and developing an AIDS-like disease (18). These CD4C/HIV Tg mice are immunodeficient and exhibit severe atrophy and fibrosis of lymphoid organs and a preferential depletion of CD4+ T cells, with altered CD4+ T-cell proliferation in vitro, loss of CD4+ T-cell help, CD4+ T-cell and B-cell activation, and impaired dendritic cell (DC) function (19–23). In addition, diseases of the lung (lymphocytic interstitial pneumonitis), heart (myocytolysis and myocarditis), and kidney (tubulointerstitial nephritis, segmental glomerulosclerosis, and microcystic dilatation) develop in these Tg mice (19, 24). Mucosal Candida infection in these Tg mice closely mimics the clinical and pathological features of candidal infection in human HIV infection (18, 25) and has allowed us to perform controlled studies on the immunopathogenesis of mucosal candidiasis in HIV infection (26–28).
With the recognition that a cause-and-effect analysis of the immunopathogenesis of cryptococcosis and the virulence of Cryptococcus species could potentially be achieved with these Tg mice, the present study was undertaken to establish and characterize a novel model of cryptococcosis in these animals and to examine the infections caused by C. neoformans var. grubii and C. gattii, using survival assays, organ fungal burdens, histopathology, and assessments of the host immune response during a time course of infection.
MATERIALS AND METHODS
Strains.
C. neoformans var. grubii strains H99 and C23 and C. gattii strains R265 and R272 were used in this study. Clinical strains H99 and C23, both of molecular type VNI (29), were obtained from Joseph Heitman and Thomas Mitchell (Duke University Medical Center). R265 and R272 were both isolated in 2001 from the bronchial washings of immunocompetent patients infected during the outbreak on Vancouver Island and belong to the major VGIIa and less frequent VGIIb molecular types of C. gattii causing this outbreak, respectively (11).
Infection of Tg mice expressing HIV-1.
CD4C/HIVMutA Tg mice have been described elsewhere (19). CD4C/HIVMutA mutant DNA harbors mouse CD4 enhancer and human CD4 promoter elements to drive expression of the nef, env, and rev genes of HIV-1 in CD4+ CD8+ and CD4+ thymocytes, peripheral CD4+ T cells, macrophages, and DCs. The founder mouse F21388 was bred on the C3H background. Animals from this line express moderate levels of the transgene, with 50% survival at 3 months (19). Several HIV-1 genes (gag, pol, vif, vpr, tat, and vpu) are mutated in the CD4C/HIVMutA DNA, whereas nef, env, and rev are intact. Specific-pathogen-free male and female Tg mice and non-Tg littermates were housed in sterilized individual cages equipped with filter hoods, supplied with sterile water, and fed with sterile mouse chow. All animal experiments were approved by the animal care committee of the University of Montreal.
Cryptococcus strains were grown in yeast extract-peptone-dextrose (YPD) medium for 24 h at 30°C, washed twice with phosphate-buffered saline (PBS), counted in a hemacytometer, and resuspended in PBS at a density of 2.5 × 106 or 2.5 × 105 yeast cells/ml. Intranasal inoculation of the mice was performed as described previously (17). For the survival assay, animals reaching predetermined morbidity endpoints (>20% weight loss, immobile, no response when stimulated, or irregular/labored abdominal respiration) were designated premortem and euthanized with a lethal dose of ketamine and xylazine (18). For all other assays, mice were euthanized on the indicated days. Quantification of Cryptococcus in internal organs, histopathology, and determination of Cryptococcus cell body diameters and capsule thicknesses in mucicarmine-stained tissue sections were done using methods described elsewhere (17, 18, 30).
Flow cytometry analysis of lung immune cell populations.
Groups of five CD4C/HIVMutA Tg and non-Tg littermates (42 to 69 days old) were infected intranasally with 1.25 × 104 CFU of C. neoformans H99 or 1.25 × 105 CFU of C. gattii R265 and assessed at 7 and 14 days postinfection. Uninfected control mice received intranasal PBS alone. Independent experiments were conducted by pooling cells from all mice within each group. Mice were anesthetized with a mixture of ketamine and xylazine and then exsanguinated with 0.9% NaCl. Single-cell suspensions of lung tissue were prepared by mechanical disruption in a mortar containing 3 ml of PBS and incubation at 37°C for 1 h with 1% collagenase type IV (Sigma) in RPMI 1640 medium (Wisent Inc., St. Bruno, Canada) supplemented with 5% heat-inactivated fetal bovine serum (FBS; Wisent), 100 U/ml penicillin-streptomycin, and 50 μg/ml gentamicin. Cells were filtered through a sterile nylon mesh (pore size, 80 μm) to obtain a homogeneous suspension. Cells were surface stained with anti-mouse anti-CD45, anti-CD11b, anti-CD11c, and anti-F4/80 fluorescence-labeled monoclonal antibodies and their respective isotype controls (all from BioLegend, San Diego, CA) for quantitation of interstitial (CD45+ CD11b+ CD11c− F4/80+) and alveolar (CD45+ CD11b+ CD11c+ F4/80+) macrophages and dendritic cells (CD45+ CD11b+ CD11c+ F4/80−); with anti-CD45, anti-CD3, and anti-Gr-1 to quantitate Gr-1+ cells (CD45+ CD3− Gr-1+); and with anti-CD45, anti-CD4, and anti-CD8 to quantitate CD4+ (CD45+ CD4+ CD8−) and CD8+ (CD45+ CD4− CD8+) T-cell populations. Red blood cells were removed with FACS lysing solution (BD Biosciences), and the remaining total extracted cells were counted using a hemacytometer. Cell surface marker analysis was conducted on a FACSCalibur flow cytometer (BD Biosciences) equipped with CellQuest software. Data were acquired for 30,000 events by gating on CD45+ cells. Results for each immune cell population were calculated as both the percentage of CD45+ cells and the absolute number of cells extracted from the lungs of a single mouse.
Production of cytokines.
To assay the production of cytokines, lungs were harvested from CD4C/HIVMutA Tg mice and non-Tg littermates 7 or 14 days after intranasal infection with 1.25 × 104 CFU of C. neoformans H99 or 1.25 × 105 CFU of C. gattii R265. Uninfected control mice received intranasal PBS. Lungs were mechanically disrupted in a mortar containing 2 ml of PBS. Lung homogenates were centrifuged, and supernatants were stored at −80°C. Cytokines in supernatants were assayed using a BD Flex cytometric bead array set (BD Biosciences) according to the manufacturer's protocol on a FACSCalibur flow cytometer equipped with BD CellQuest software. Data analysis was performed using BD FCAP array software 3.0.
Statistical analysis.
Kaplan-Meier modeling and a log rank (Mantel-Cox) test were used to compare survival of C. neoformans var. grubii- and C. gattii-infected Tg and non-Tg mice. Organ burdens of Cryptococcus were compared using the Kruskal-Wallis test, and significant interactions were further analyzed by use of the Mann-Whitney test. Cryptococcus cell body diameters and capsule thicknesses, lung immune cell populations, and cytokine production were analyzed with SPSS, version 19, software (SPSS, Chicago, IL), using analysis of variance. Differences were considered significant if the P value was <0.05.
RESULTS
Enhanced susceptibility to cryptococcosis in Tg mice.
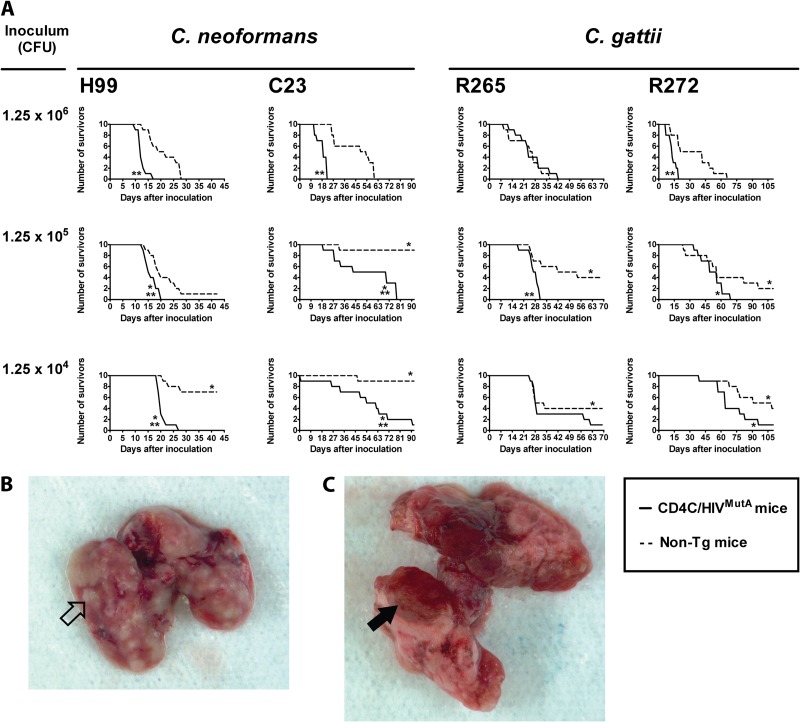
Tg and non-Tg mice were infected intranasally with three graded inocula of C. neoformans (strain H99 or C23) or C. gattii (strain R265 or R272) and then assessed for survival and organ burdens. Survival of both Tg and non-Tg mice was inversely correlated with the inoculum size of C. neoformans and C. gattii, with the single exception of Tg mice infected with strain R265 (Fig. 1A). Although C. neoformans strain C23 was less virulent than C. neoformans strain H99 in Tg and non-Tg mice at all three inocula (P < 0.03), Tg mice infected with these two C. neoformans strains consistently displayed reduced survival compared to non-Tg mice infected at the same three inocula. The enhanced susceptibility to cryptococcosis in the Tg mice was especially prominent in animals infected with the low inoculum of 1.25 × 104 CFU of C. neoformans H99, none of which survived, in comparison to the 70% survival of the non-Tg animals (Fig. 1A). Likewise, the mortality of Tg mice infected with C. neoformans C23 at this inoculum was 90%, compared to 10% for the non-Tg mice (Fig. 1A). In contrast to the C. neoformans infections, shortened survival of Tg mice infected with C. gattii strain R265 or R272 was restricted to a single higher inoculum (1.25 × 105 or 1.25 × 106, respectively) (Fig. 1A). Lungs harvested at necropsy from Tg and non-Tg mice infected with C. neoformans H99 or C. gattii R265 were macroscopically indistinguishable. All showed multiple hemorrhagic and abscess-like surface lesions (Fig. 1B and C). Taken together, the results of these survival studies clearly demonstrated that HIV-1 transgene expression markedly and consistently enhanced susceptibility to C. neoformans, independent of the inoculum, while this effect was discernible only at a single inoculum with C. gattii.
Fig 1.
(A) Survival of Tg and non-Tg mice infected with Cryptococcus neoformans (strain H99 or C23) or Cryptococcus gattii (strain R265 or R272). Ten mice were infected intranasally at each of the indicated inocula. Significant differences are indicated as follows: *, P < 0.01 versus mice infected with the same strain at an inoculum of 1.25 × 106 CFU; and **, P < 0.01 for Tg versus non-Tg mice infected with identical inocula of the same strain. (B and C) Lungs harvested at necropsy from Tg and non-Tg mice infected with C. neoformans H99 or C. gattii R265 all showed multiple hemorrhagic (filled arrowhead) and abscess-like (open arrowhead) surface lesions. Representative examples are shown for a non-Tg mouse infected with C. neoformans H99 (B) and a Tg mouse infected with C. gattii R265 (C).
Organ burdens of euthanized mice premortem, determined as CFU/g (Tables 1 and 2), demonstrated a close correlation with survival of Tg and non-Tg mice infected with C. neoformans or C. gattii. In non-Tg mice, organ burdens in the liver and spleen increased significantly with the inoculum size of the two C. neoformans strains (P ≤ 0.001), but not the two C. gattii strains (P > 0.05), but in Tg mice, inoculum size had no significant effect on organ burdens of either C. neoformans or C. gattii (P > 0.05). The two C. neoformans strains produced comparable burdens in the liver and spleen within the Tg and non-Tg groups of mice (P > 0.05), but both sets of burdens were greater than those produced by the two C. gattii strains (P < 0.03), which did not differ significantly from each other (P > 0.05). Interestingly, the reduced survival of Tg mice infected with C. neoformans compared to infected non-Tg animals was correlated with strikingly enhanced systemic dissemination to the liver and spleen of strains H99 and C23 at the two lowest inocula (1.25 × 104 and 1.25 × 105 CFU) (P < 0.03) (Table 1). In contrast, burdens of C. gattii strains R265 and R272 in these organs were comparable at all three inocula in Tg and non-Tg mice (P > 0.05) (Table 2), demonstrating that HIV-1 transgene expression selectively augments systemic dissemination to the liver and spleen for C. neoformans but not C. gattii. However, enhanced burdens in brains of Tg compared to non-Tg mice were observed at the two lowest inocula with C. neoformans strain C23 only (P ≤ 0.002), not strain H99 (P > 0.05) or the two C. gattii strains (P > 0.05) (Tables 1 and 2), showing that HIV-1 transgene-mediated augmentation of C. neoformans dissemination to the brain may be strain dependent.
Table 1.
Viable CFU in organs of CD4C/HIVMutA Tg mice inoculated intranasally with Cryptococcus neoformans
| Strain and variable | Valuea |
|||||
|---|---|---|---|---|---|---|
| Tg mice | Control non-Tg mice | |||||
| C. neoformans H99 | ||||||
| Inoculum (CFU) | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 |
| No. of mice inoculated | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean (range) age at inoculation (days) | 59 (49–63) | 54 (50–57) | 60 (50–64) | 49 (42–63) | 53 (45–67) | 61 (50–64) |
| Mean (range) age at assessment (days) | 72 (61–80)b | 70 (64–75)b | 80 (70–84)b | 70 (58–80)b | 76 (65–93)c | 96 (73–106)c |
| Variables for organs culture positive for C. neoformans | ||||||
| Brain | ||||||
| No. of mice | 7 | 9 | 9 | 10 | 8 | 5 |
| C. neoformans count (CFU/g) | 8.8 × 107 | 2.5 × 107 | 7.1 × 107 | 6.0 × 107 | 5.3 × 107 | 6.2 × 107 |
| Range of counts | 7.4 × 103-3.8 × 108 | 1.4 × 104-1.0 × 108 | 2.7 × 104-2.8 × 108 | 3.3 × 105-1.6 × 108 | 2.7 × 104-1.3 × 108 | 7.9 × 104-2.0 × 108 |
| Lungs | ||||||
| No. of mice | 9 | 10 | 9 | 10 | 9 | 6 |
| C. neoformans count (CFU/g) | 2.0 × 109 | 1.3 × 109 | 2.5 × 109 | 1.2 × 109 | 1.0 × 109 | 3.8 × 108 |
| Range of counts | 2.7 × 108-8.7 × 109 | 1.8 × 107-5.0 × 109 | 9.6 × 107-4.7 × 109 | 8.6 × 107-5.4 × 109 | 6.2 × 106-6.3 × 109 | 6.9 × 105-6.9 × 108 |
| Liver | ||||||
| No. of mice | 9 | 10 | 9 | 10 | 9 | 4 |
| C. neoformans count (CFU/g) | 4.6 × 105 | 1.3 × 106 | 3.5 × 105 | 2.9 × 105 | 8.8 × 104 | 7.3 × 104 |
| Range of counts | 2.5 × 104-2.5 × 106 | 4.2 × 104-7.6 × 106 | 1.3 × 105-6.5 × 105 | 2.9 × 104-8.4 × 105 | 1.1 × 104-3.2 × 105 | 1.9 × 104-2.1 × 105 |
| Spleen | ||||||
| No. of mice | 8 | 9 | 9 | 10 | 8 | 3 |
| C. neoformans count (CFU/g) | 3.6 × 106 | 1.5 × 106 | 1.5 × 106 | 4.0 × 105 | 2.2 × 105 | 6.0 × 104 |
| Range of counts | 1.1 × 105-2.2 × 107 | 2.3 × 105-2.8 × 106 | 3.6 × 105-4.0 × 106 | 5.4 × 104-1.1 × 106 | 1.8 × 104-6.5 × 105 | 9.1 × 103-1.2 × 105 |
| C. neoformans C23 | ||||||
| Inoculum (CFU) | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 |
| No. of inoculated mice | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean (range) age at inoculation (days) | 53 (50–56) | 53 (46–60) | 46 (43–52) | 45 (43–57) | 49 (49–50) | 52 (50–54) |
| Mean (range) age at assessment (days) | 71 (62–77)b | 105 (65–134)b | 100 (53–135)d | 90 (69–113)b | 135 (81–142)d | 140 (99–146)d |
| Variables for organs culture positive for C. neoformans | ||||||
| Brain | ||||||
| No. of mice | 8 | 7 | 6 | 6 | 0 | 0 |
| C. neoformans count (CFU/g) | 2.2 × 107 | 4.4 × 106 | 8.1 × 106 | 1.2 × 107 | NA | NA |
| Range of counts | 3.6 × 104-7.1 × 107 | 5.9 × 103-2.0 × 107 | 9.5 × 103-1.4 × 107 | 8.2 × 105-4.3 × 107 | NA | NA |
| Lungs | ||||||
| No. of mice | 10 | 7 | 4 | 8 | 1 | 0 |
| C. neoformans count (CFU/g) | 4.2 × 108 | 1.3 × 108 | 2.2 × 108 | 5.7 × 108 | 4.4 × 106 | NA |
| Range of counts | 1.2 × 108-9.4 × 108 | 7.7 × 105-3.2 × 108 | 2.1 × 106-3.9 × 108 | 1.7 × 107-1.5 × 109 | NA | NA |
| Liver | ||||||
| No. of mice | 10 | 7 | 4 | 7 | 1 | 0 |
| C. neoformans count (CFU/g) | 6.1 × 105 | 6.0 × 105 | 2.2 × 106 | 3.4 × 106 | 4.4 × 103 | NA |
| Range of counts | 3.9 × 104-2.5 × 106 | 1.5 × 103-2.1 × 106 | 1.4 × 104-4.3 × 106 | 9.6 × 103-8.6 × 106 | NA | NA |
| Spleen | ||||||
| No. of mice | 10 | 5 | 4 | 5 | 0 | 0 |
| C. neoformans count (CFU/g) | 2.8 × 106 | 2.8 × 106 | 3.1 × 107 | 4.1 × 106 | NA | NA |
| Range of counts | 1.8 × 105-1.7 × 107 | 2.0 × 105-7.7 × 106 | 1.0 × 105-7.4 × 107 | 1.6 × 105-8.5 × 106 | NA | NA |
Mice studied included Tg and control non-Tg offspring derived from the founder mouse F21388. NA, not applicable.
Assessment was done on the day of euthanization because of severe illness.
Assessment was done on the day of euthanization because of severe illness; survivors were euthanized 42 days after inoculation with C. neoformans.
Assessment was done on the day of euthanization because of severe illness; survivors were euthanized 92 days after inoculation with C. neoformans.
Table 2.
Viable CFU in organs of CD4C/HIVMutA Tg mice inoculated intranasally with Cryptococcus gattii
| Strain and variable | Valuea |
|||||
|---|---|---|---|---|---|---|
| Tg mice | Control non-Tg mice | |||||
| C. gattii R265 | ||||||
| Inoculum (CFU) | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 |
| No. of inoculated mice | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean (range) age at inoculation (days) | 57 (55–62) | 57 (54–61) | 53 (49–62) | 56 (52–62) | 59 (57–61) | 53 (49–62) |
| Mean (range) age at assessment (days) | 83 (70–100)b | 84 (72–91)b | 91 (74–131)c | 79 (61–92)b | 107 (83–130)c | 98 (74–131)c |
| Variables for organs culture positive for C. gattii | ||||||
| Brain | ||||||
| No. of mice | 6 | 6 | 0 | 4 | 3 | 0 |
| C. gattii count (CFU/g) | 5.7 × 104 | 1.4 × 105 | NA | 8.3 × 104 | 3.9 × 104 | NA |
| Range of counts | 1.4 × 104-2.2 × 105 | 6.1 × 103-7.1 × 105 | NA | 7.4 × 104-2.9 × 105 | 6.1 × 103-9.0 × 104 | NA |
| Lungs | ||||||
| No. of mice | 10 | 10 | 7 | 9 | 7 | 7 |
| C. gattii count (CFU/g) | 1.5 × 108 | 1.3 × 108 | 8.3 × 107 | 1.3 × 108 | 4.2 × 107 | 6.4 × 107 |
| Range of counts | 4.6 × 107-3.8 × 108 | 7.7 × 107-1.9 × 108 | 2.3 × 107-1.9 × 108 | 5.9 × 107-3.2 × 108 | 1.1 × 107-9.2 × 107 | 7.5 × 106-1.2 × 108 |
| Liver | ||||||
| No. of mice | 4 | 4 | 1 | 3 | 2 | 3 |
| C. gattii count (CFU/g) | 1.3 × 106 | 1.5 × 106 | 2.4 × 103 | 3.3 × 104 | 6.2 × 103 | 2.8 × 105 |
| Range of counts | 1.8 × 103-3.9 × 106 | 8.8 × 104-4.3 × 106 | NA | 1.4 × 104-6.8 × 104 | 2.0 × 103-1.0 × 104 | 3.6 × 104-7.4 × 105 |
| Spleen | ||||||
| No. of mice | 1 | 0 | 0 | 1 | 0 | 0 |
| C. gattii count (CFU/g) | 1.8 × 106 | NA | NA | 3.1 × 105 | NA | NA |
| Range of counts | NA | NA | NA | NA | NA | NA |
| C. gattii R272 | ||||||
| Inoculum (CFU) | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 | 1.25 × 106 | 1.25 × 105 | 1.25 × 104 |
| No. of inoculated mice | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean (range) age at inoculation (days) | 53 (47–59) | 64 (63–69) | 49 (44–53) | 56 (46–59) | 63 (60–64) | 59 (57–61) |
| Mean (range) age at assessment (days) | 66 (58–75)b | 115 (98–133)b | 120 (84–162)d | 89 (62–125)b | 129 (84–173)d | 149 (96–170)d |
| Variables for organs culture positive for C. gattii | ||||||
| Brain | ||||||
| No. of mice | 1 | 5 | 1 | 5 | 1 | 5 |
| C. gattii count (CFU/g) | 5.9 × 103 | 2.4 × 104 | 6.4 × 106 | 9.6 × 104 | NA | 5.9 × 107 |
| Range of counts | NA | 5.6 × 103-4.2 × 104 | NA | 1.8 × 104-2.4 × 105 | NA | 1.2 × 107-1.7 × 108 |
| Lungs | ||||||
| No. of mice | 8 | 9 | 6 | 8 | 6 | 8 |
| C. gattii count (CFU/g) | 7.2 × 107 | 5.9 × 107 | 3.8 × 107 | 1.0 × 108 | 4.9 × 107 | 1.5 × 107 |
| Range of counts | 5.0 × 107-1.1 × 108 | 2.1 × 107-1.5 × 108 | 1.7 × 107-6.2 × 107 | 2.0 × 108-3.5 × 108 | 8.3 × 106-1.7 × 108 | 3.6 × 106-3.4 × 107 |
| Liver | ||||||
| No. of mice | 1 | 3 | 3 | 2 | 1 | 3 |
| C. gattii count (CFU/g) | 1.9 × 104 | 1.2 × 105 | 7.7 × 104 | 2.7 × 104 | 8.7 × 105 | 1.3 × 104 |
| Range of counts | NA | 4.6 × 103-2.5 × 105 | 3.9 × 103-2.0 × 105 | 2.1 × 104-3.2 × 104 | NA | 1.2 × 103-2.1 × 104 |
| Spleen | ||||||
| No. of mice | 0 | 0 | 0 | 0 | 0 | 0 |
| C. gattii count (CFU/g) | NA | NA | NA | NA | NA | NA |
| Range of counts | NA | NA | NA | NA | NA | NA |
Mice studied included Tg and control non-Tg offspring derived from the founder mouse F21388. NA, not applicable.
Assessment was done on the day of euthanization because of severe illness.
Assessment was done on the day of euthanization because of severe illness; survivors were euthanized 69 days after inoculation with C. gattii.
Assessment was done on the day of euthanization because of severe illness; survivors were euthanized 110 days after inoculation with C. gattii.
Enhanced cryptococcal burdens and more frequent dissemination to the liver and spleen were also found in Tg compared to non-Tg mice euthanized at the fixed time of 14 days after infection with the lowest inoculum (1.25 × 104 CFU) of C. neoformans H99 (P < 0.05) (Table 3). Seven days after infection, however, no systemic dissemination had yet occurred, and pulmonary burdens were comparatively lower than those at day 14 (P < 0.02) and were not significantly different (P > 0.05) in Tg and non-Tg mice (Table 3). In contrast to the case with C. neoformans H99, however, lung burdens were comparable at days 7 and 14 (P > 0.05), the frequency of systemic dissemination remained low, and cryptococcal burdens in the liver and spleen were comparable in Tg and non-Tg mice 14 days after infection with an intermediate inoculum (1.25 × 105 CFU) of C. gattii strain R265 (P > 0.05) (Table 3).
Table 3.
Viable CFU in organs of CD4C/HIVMutA Tg mice inoculated intranasally with Cryptococcus spp.
| Strain (inoculum) and variable | Valuea |
|||
|---|---|---|---|---|
| Tg mice | Control non-Tg mice | |||
| Cryptococcus neoformans H99 (1.25 × 104 CFU) | ||||
| Days after inoculation | 7 | 14 | 7 | 14 |
| No. of inoculated mice | 6 | 6 | 6 | 6 |
| Variables for organs culture positive for C. neoformans | ||||
| Brain | ||||
| No. of mice | 0 | 4 | 0 | 2 |
| C. neoformans count (CFU/g) | NA | 2.5 × 106 | NA | 6.9 × 106 |
| Range of counts | NA | 4.4 × 104-8.5 × 106 | NA | 3.8 × 105-1.4 × 107 |
| Lungs | ||||
| No. of mice | 6 | 6 | 6 | 6 |
| C. neoformans count (CFU/g) | 1.1 × 108 | 6.1 × 108 | 6.7 × 107 | 2.0 × 108 |
| Range of counts | 3.3 × 107-3.9 × 108 | 4.6 × 107-1.1 × 109 | 3.6 × 107-1.0 × 108 | 1.0 × 108-3.1 × 108 |
| Liver | ||||
| No. of mice | 0 | 4 | 2 | 1 |
| C. neoformans count (CFU/g) | NA | 4.1 × 104 | 9.6 × 103 | 6.5 × 103 |
| Range of counts | NA | 2.3 × 104-5.8 × 104 | 7.9 × 103-1.1 × 104 | NA |
| Spleen | ||||
| No. of mice | 0 | 4 | 0 | 1 |
| C. neoformans count (CFU/g) | NA | 2.7 × 105 | NA | 1.8 × 104 |
| Range of counts | NA | 1.6 × 105-4.2 × 105 | NA | NA |
| Cryptococcus gattii R265 (1.25 × 105 CFU) | ||||
| Days after inoculation | 7 | 14 | 7 | 14 |
| No. of inoculated mice | 6 | 6 | 6 | 6 |
| Variables for organs culture positive for C. gattii | ||||
| Brain | ||||
| No. of mice | 0 | 1 | 4 | 0 |
| C. gattii count (CFU/g) | NA | 3.5 × 105 | 2.4 × 104 | NA |
| Range of counts | NA | NA | 1.3 × 104-5.9 × 104 | NA |
| Lungs | ||||
| No. of mice | 6 | 6 | 6 | 6 |
| C. gattii count (CFU/g) | 1.7 × 108 | 1.9 × 108 | 1.6 × 108 | 2.5 × 108 |
| Range of counts | 3.9 × 107-2.4 × 108 | 1.0 × 108-3.6 × 108 | 4.0 × 107-2.6 × 108 | 1.8 × 108-3.3 × 108 |
| Liver | ||||
| No. of mice | 0 | 2 | 1 | 1 |
| C. gattii count (CFU/g) | NA | 6.0 × 103 | 3.7 × 103 | 5.7 × 104 |
| Range of counts | NA | 4.2 × 103-7.8 × 103 | NA | NA |
| Spleen | ||||
| No. of mice | 0 | 0 | 0 | 0 |
| C. gattii count (CFU/g) | NA | NA | NA | NA |
| Range of counts | NA | NA | NA | NA |
Mice studied included Tg and control non-Tg offspring derived from the founder mouse F21388. NA, not applicable.
Defective inflammatory cell response to Cryptococcus in Tg mice.
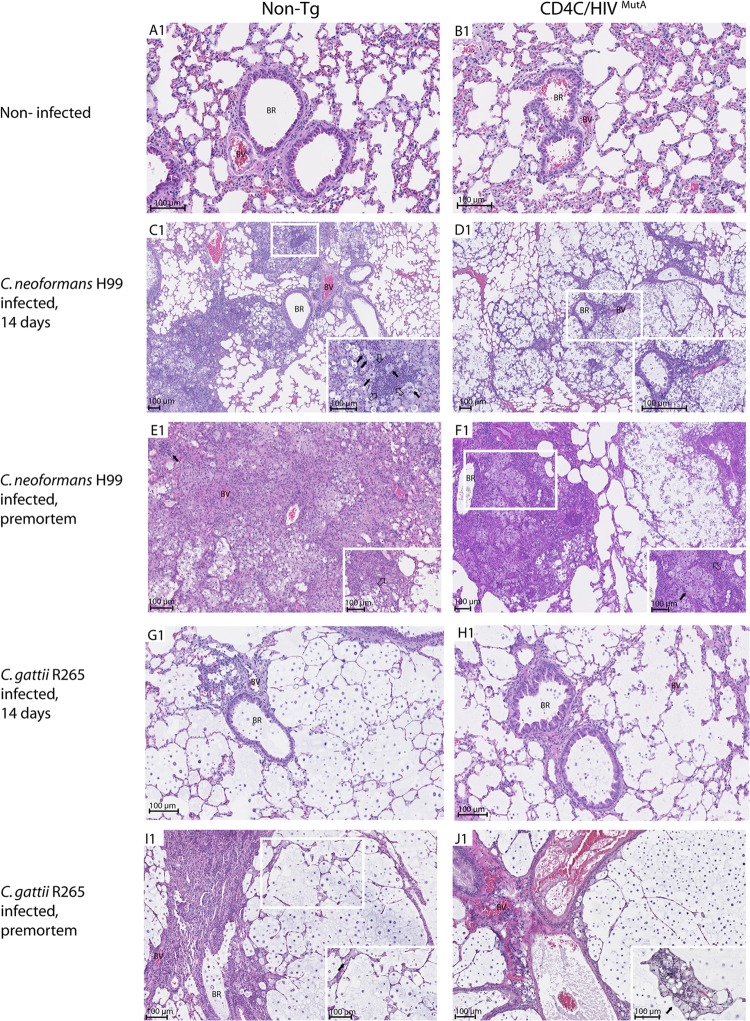
Histopathologic examination of lungs was conducted on days 7 and 14 after infection and premortem to identify the nature, location, and extent of the inflammatory cell response to C. neoformans strain H99 and C. gattii strain R265 (Fig. 2; see Fig. S1 to S3 in the supplemental material). The pulmonary inflammatory responses to C. neoformans were strikingly and consistently different in Tg and non-Tg mice. Seven days after infection of the Tg mice, numerous C. neoformans cells were located in the bronchioles and formed cysts or were individually dispersed throughout the lung parenchyma, with a minimal scattered mononuclear cell infiltrate (see Fig. S2L1 and S3L2). In contrast, the non-Tg mice displayed dense bronchovascular infiltrates containing mononuclear cells and polymorphonuclear leukocytes (PMNs) completely enclosing C. neoformans (see Fig. S2K1). Fourteen days after infection of the Tg mice, much larger numbers of C. neoformans cells were widely scattered in the lung tissue, with a minimal inflammatory cell response, and were rarely observed within discrete bronchovascular infiltrates containing mostly PMNs and a few mononuclear cells (Fig. 2D1; see Fig. S1D2). In striking contrast, in non-Tg mice, C. neoformans cells were almost entirely embedded within far more extensive mixed inflammatory infiltrates comprised of PMNs and macrophages and were seldom observed in the remaining lung parenchyma, which was devoid of inflammatory cells (Fig. 2C1). Finally, premortem non-Tg mice again displayed a widespread inflammatory response, with the added appearance at this late time point of necrotizing granulomas containing epithelioid cells and Langhans-type giant cells (Fig. 2E1). This was in contrast to the Tg mice, which displayed more limited inflammatory foci containing abundant macrophages and PMNs but no granulomas, as well as broad areas of lung parenchyma containing numerous C. neoformans cells but no inflammatory response (Fig. 2F1).
Fig 2.
Histopathology of lungs from Tg and non-Tg mice, either uninfected or assessed on day 14 or premortem after intranasal infection with 1.25 × 104 CFU of Cryptococcus neoformans H99 or 1.25 × 105 CFU of Cryptococcus gattii R265. Tissues were stained with hematoxylin phloxine saffron (HPS). Fourteen days after infection of non-Tg mice (C1), C. neoformans was present in great numbers and was almost entirely embedded within extensive mixed inflammatory infiltrates comprised of macrophages (filled arrowheads) and polymorphonuclear leukocytes (open arrowheads) (C1, enlarged inset) and only rarely observed in the remaining lung parenchyma, in marked contrast to the case with Tg mice, which displayed predominantly innumerable and widely scattered C. neoformans cells, with a minimal inflammatory cell response (D1) and only rarely enclosed within discrete bronchovascular infiltrates (enlarged inset). Premortem non-Tg mice displayed necrotizing granulomas containing epithelioid cells (filled arrowhead) and Langhans-type giant cells (open arrowhead) (E1), in contrast to Tg mice, which showed no granulomas but numerous macrophages (filled arrowhead) and polymorphonuclear leukocytes (open arrowhead) and wide areas of lung parenchyma containing numerous C. neoformans cells but no inflammatory response (F1). C. gattii was widely dispersed throughout the lung tissue and induced only a modest and localized inflammatory response comprised of macrophages (filled arrowheads) and polymorphonuclear leukocytes (open arrowhead) in Tg and non-Tg mice (G1 to J1). BR, bronchiole; BV, blood vessel. Images are representative of 2 (A1 and B1) or 6 (C1 to J1) mice per group, with consistent results.
In sharp contrast to the case for infection with C. neoformans, numerous C. gattii cells were widely dispersed throughout the lung tissue and induced only a sparse inflammatory response on days 7 and 14 after infection in both Tg and non-Tg mice (Fig. 2G1 and H1; see Fig. S1 to S3 in the supplemental material). A modest and circumscribed inflammatory response comprised of macrophages and PMNs appeared only in premortem animals and was independent of HIV-1 transgene expression (Fig. 2I1 and J1).
Interestingly, macrophages in lung tissue sections from Tg and non-Tg mice infected with C. neoformans or C. gattii often displayed the distinctive appearance of “hueco” cells filled with vesicles containing capsular polysaccharide (31, 32). These cells were observed beginning on day 14 after infection and became more abundant in mice assessed premortem.
Histopathologic examination of the brains of Tg and non-Tg mice on day 7 after infection with C. neoformans showed that the brains were entirely normal, in accordance with the absence of systemic dissemination to this organ at this early time point (Table 3). On day 14 after infection, however, histopathology revealed C. neoformans in the brain parenchyma of a single non-Tg mouse which displayed culture evidence of dissemination to this organ, but not in the other animals, which were either culture positive or negative (Table 3). Taken together with the organ burdens, these results indicated that the onset of dissemination to the brain for C. neoformans was detectable more than 7 days after infection in both Tg and non-Tg mice and did not occur earlier in the Tg mice, despite their enhanced frequency of systemic dissemination (Tables 1 and 2). Examination of the brains of Tg and non-Tg mice 7 and 14 days after infection with C. gattii did not show histopathologic evidence of the fungus, in accordance with lower burdens of C. gattii than of C. neoformans in this organ (Table 3).
Cell body diameters and capsule thicknesses of 100 randomly selected C. neoformans or C. gattii cells were determined in lung tissue sections from Tg and non-Tg mice 7 or 14 days after infection. For both C. neoformans and C. gattii, cell body diameters and capsule thicknesses increased significantly from day 7 to day 14 after infection of non-Tg mice (P < 0.001) but not Tg mice (P > 0.05), and both measurements were greater in non-Tg than in Tg mice on day 14 after infection with these two species (P < 0.001) (Table 4). However, cell body diameters and capsule thicknesses of C. neoformans H99 were markedly greater than those of C. gattii R265 both 7 and 14 days after infection of both Tg and non-Tg mice (P < 0.001), showing that the dimensions of these two species consistently differ in vivo, irrespective of time after infection or HIV-1 transgene expression (Table 4). Interestingly, using a cell body diameter threshold of 15 μm, 22 to 53% of C. neoformans H99 cells comprised giant (titan) cells (33), but these cells were seen less frequently (3 to 12% of cells) in tissue sections from mice infected with C. gattii 265.
Table 4.
Cell body diameters and capsule thicknesses of C. neoformans H99 and C. gattii R265 in mucicarmine-stained lung tissue sections 7 or 14 days after infection of CD4C/HIVMutA Tg or non-Tg mice
| Measurement and strain | Value after infectiona |
|||
|---|---|---|---|---|
| Tg mice |
Non-Tg mice |
|||
| 7 days | 14 days | 7 days | 14 days | |
| Cell body diameter (μm) | ||||
| C. neoformans H99 | 11.8 ± 4.2b | 11.4 ± 4.5b | 12.4 ± 3.6b | 15.4 ± 3.5b,c,d |
| C. gattii R265 | 10.3 ± 2.8 | 9.8 ± 2.7 | 9.5 ± 2.9 | 12.0 ± 2.8c,d |
| Capsule thickness (μm) | ||||
| C. neoformans H99 | 5.0 ± 1.9b | 5.6 ± 2.5b | 6.3 ± 1.8b | 8.2 ± 2.0b,c,d |
| C. gattii R265 | 4.9 ± 1.4 | 4.7 ± 2.3 | 3.5 ± 1.3 | 6.2 ± 2.0c,d |
Data are means ± standard deviations for 100 randomly selected cells.
P < 0.001 compared to C. gattii R265.
P < 0.001 compared to non-Tg mice at day 7.
P < 0.001 compared to Tg mice at day 14.
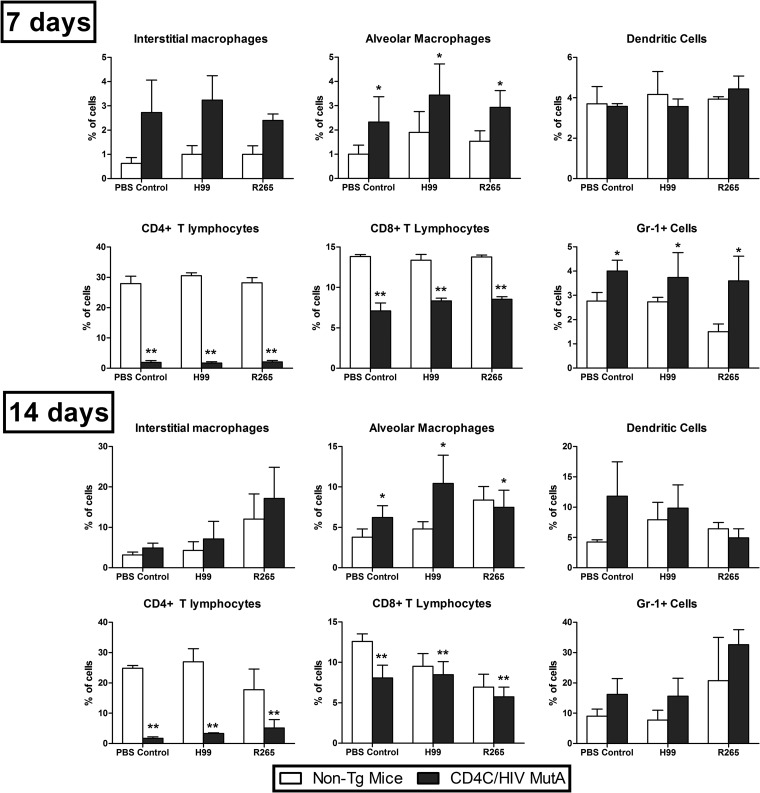
Altered lung immune cell populations in response to Cryptococcus in Tg mice.
To quantitatively assess the impact of HIV-1 transgene expression on lung immune cell populations, multiparametric flow cytometry analysis was conducted on CD4C/HIVMutA Tg mice and non-Tg littermates 7 and 14 days after infection or no infection with C. neoformans H99 or C. gattii R265. On both days, transgene expression independently caused striking reductions in the percentages of CD4+ and CD8+ T cells (P < 0.001) (Fig. 3). Furthermore, on day 14, total numbers of extracted pulmonary cells were significantly lower in Tg mice than in non-Tg mice (P = 0.002), correlating with the defective inflammatory cell response to Cryptococcus observed on histopathology. Independent of cryptococcal infection, percentages of alveolar macrophages were significantly increased (P < 0.05) in Tg compared to non-Tg mice on days 7 and 14 (Fig. 3). Similar findings were observed with Gr-1+ cells, but they reached statistical significance only on day 7 (Fig. 3). In addition, from day 7 to day 14, in both Tg and non-Tg mice, the percentages of dendritic cells, alveolar macrophages, and Gr-1+ cells were significantly increased in animals infected with either C. neoformans or C. gattii (P ≤ 0.02), while a similar increase in interstitial macrophages during the same interval was restricted to C. gattii (P < 0.001). We cannot formally exclude the possibility that in addition to PMNs, plasmacytoid dendritic cells and inflammatory monocytes, expressing Ly6C but not CD3, may have been recognized by the anti-Gr-1 antibody. Finally, absolute numbers of CD4+ and CD8+ cells, but not the other cell populations, were significantly diminished (P < 0.05) in the Tg compared to non-Tg mice on days 7 and 14 after infection or no infection with C. neoformans or C. gattii (data not shown).
Fig 3.
Flow cytometry analysis of lung immune cell populations in CD4C/HIVMutA Tg and non-Tg mice 7 and 14 days after infection or no infection with C. neoformans H99 or C. gattii R265. Data are presented as percentages of CD45+ cells and are the means ± standard errors of the means (SEM) of results from three or four independent experiments. Significant differences are indicated as follows: *, Tg > non-Tg mice (P < 0.05); and **, Tg < non-Tg mice (P < 0.001).
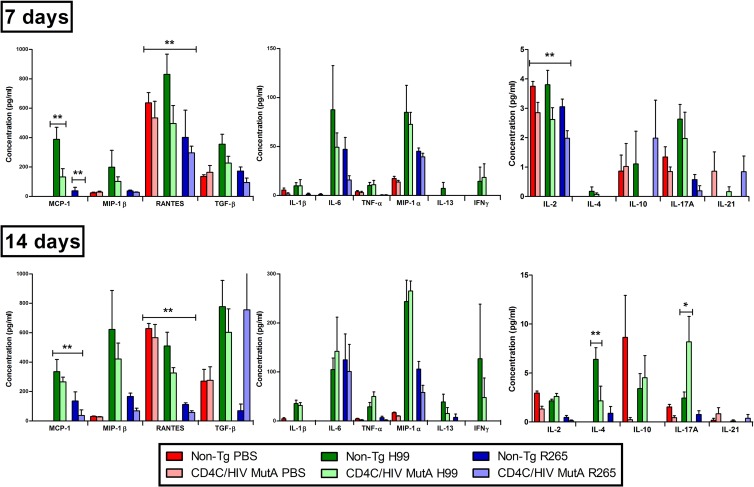
Altered production of pulmonary cytokines in response to Cryptococcus in Tg mice.
To determine if the reduced pulmonary inflammatory response to Cryptococcus observed in the Tg mice was associated with defective production of cytokines, Tg and non-Tg mice were assessed 7 or 14 days after infection or no infection with C. neoformans H99 or C. gattii R265. In comparison to the levels in non-Tg mice, HIV-1 transgene expression consistently reduced pulmonary production of the CC chemokines monocyte chemoattractant protein 1 (MCP-1; CCL2) and RANTES (CCL5) both 7 and 14 days after infection with C. neoformans or C. gattii (P ≤ 0.01) (Fig. 4), suggesting that defective production of these chemotactic cytokines may contribute to the defective inflammatory response to Cryptococcus in Tg mice. In addition, in comparison to the case with C. neoformans, production of these two chemokines was significantly lower after infection with C. gattii (P < 0.001), which may partially explain the markedly reduced pulmonary inflammatory response to C. gattii in comparison to that to C. neoformans in the non-Tg mice (Fig. 2). Indeed, a wide array of cytokines (interleukin-1β [IL-1β], tumor necrosis factor alpha [TNF-α], macrophage inflammatory protein 1β [MIP-1β], IL-13, transforming growth factor beta [TGF-β], and IL-4) increased significantly from day 7 to day 14 after infection with C. neoformans (P < 0.001) but not C. gattii (P > 0.05), and higher concentrations of TNF-α, MIP-1α, MIP-1β, IL-13, TGF-β, IL-2, and IL-4 were also found on day 14 after infection with C. neoformans compared to C. gattii (P < 0.001), independent of transgene expression. This differential production of cytokines after infection by the two species was especially prominent in the case of gamma interferon (IFN-γ), which was produced exclusively in response to infection with C. neoformans.
Fig 4.
Cytokine production in lungs of CD4C/HIVMutA Tg and non-Tg mice 7 and 14 days after infection or no infection with C. neoformans H99 or C. gattii R265. IL-12p70 (not shown) was undetectable in all mice. Data are the means ± SEM of results from six mice. Significant differences are indicated as follows: *, Tg > non-Tg mice (P < 0.05); and **, Tg < non-Tg mice (P < 0.05).
In sharp contrast, however, production of IL-6 and IL-10 increased significantly from day 7 to day 14 after infection with either of the two species (P < 0.05), suggesting that the lower pulmonary inflammatory response observed after infection with C. gattii than after infection with C. neoformans was not associated with a differing production of these two cytokines.
In addition to MCP-1 and RANTES, HIV-1 transgene expression resulted in decreased production of IL-2 on day 7 after infection (P ≤ 0.03), a defect previously associated with enhanced susceptibility to cryptococcosis at this early time point of infection (34). However, in comparison to non-Tg controls on day 14, Tg mice infected with C. neoformans unexpectedly had reduced production of IL-4 (P ≤ 0.001) and increased production of IL-17A (P ≤ 0.001), both of which are associated with a protective rather than nonprotective anticryptococcal host response (35–37).
DISCUSSION
The model which we established recapitulates the hallmark histopathological features of human pulmonary C. neoformans (38, 39) and C. gattii (40) infections, including a minimal inflammatory cell infiltrate in transgenic mice infected with C. neoformans that reproduces the pathological findings in AIDS patients (38). The present results also clearly establish, for the first time in an animal model, using controlled conditions with two strains each and three inocula of C. neoformans and C. gattii, that HIV-1 expression consistently augments susceptibility to C. neoformans but not that to C. gattii. This finding provides experimental evidence to support the results of epidemiological studies of cryptococcosis, which demonstrate that C. neoformans causes the overwhelming majority of infections in the setting of HIV infection, while C. gattii infections occur mostly in immunocompetent persons (12, 16). The lack of a significant transgene effect on mortality at the lowest inoculum of C. gattii (1.25 × 104 CFU), in contrast to an inoculum of 1.25 × 105 CFU, may have resulted from differing levels of the inflammatory response to C. gattii at these two inocula.
Assessments of organ burdens, lung histopathology, immune cell populations, and cytokine production were conducted at the fixed time points of 7 and 14 days after infection with 1.25 × 104 CFU of C. neoformans H99 or 1.25 × 105 CFU of C. gattii R265. These inocula were selected on the basis of the results of the survival study, which showed the greatest transgene effect on mortality at these inocula (Fig. 1), and therefore they were most likely to reveal differences in organ burdens at the fixed time points. Immune response parameters were assessed for the same inocula to allow a meaningful correlation with organ burden data.
In comparison to C. neoformans, infection of immunocompetent non-Tg C3H mice with C. gattii elicited a markedly reduced pulmonary inflammatory cell response, as reported previously for C57BL/6 and A/JCr mice infected with identical inocula of the two species (17, 41). It is therefore unlikely that the less robust pulmonary inflammatory cell response to C. gattii than that to C. neoformans which we found in the non-Tg mice was caused by the higher inoculum.
The lower pulmonary inflammatory cell response to C. gattii was closely correlated with diminished production of several cytokines and chemokines, including MCP-1, RANTES, MIP-1α, MIP-1β, IL-1β, IL-2, IL-4, IL-13, TNF-α, IFN-γ, and TGF-β. Among these, MCP-1, MIP-1α, TNF-α, and IFN-γ all play a role in leukocyte recruitment to the lungs in response to C. neoformans infection (42–52). Accordingly, reduced production of these four cytokines may explain, at least in part, the strikingly sparse inflammatory cell response to C. gattii compared to that to C. neoformans in the non-Tg C3H mice. Interestingly, we found greater capsule thicknesses of C. neoformans than C. gattii, and it has been reported that increasing capsule thicknesses of C. neoformans augment the magnitudes of IL-1β and TNF-α release by human PMNs (53). It would be relevant in future work to examine infection by C. neoformans 145A, which like C. gattii R265 induces a limited pulmonary inflammatory response (54), to determine if it behaves similarly to C. gattii in HIV-1-expressing Tg mice.
Despite these strikingly dissimilar host immune responses to C. neoformans and C. gattii, comparable lung burdens of both cryptococcal species were found on days 7 and 14 after infection and premortem. This seemingly paradoxical finding could possibly be explained by the antiphagocytic properties of the cryptococcal capsule (55) and the reduced phagocytosis of cryptococcal giant (titan) cells (33, 56–58), which would allow C. neoformans to proliferate at a rate comparable to that of C. gattii despite the enhanced inflammatory cell response. However, in a recent report (41), C. gattii R265 produced higher lung burdens than those of C. neoformans H99 after infection of C57BL/6 and BALB/c mice, suggesting that the protective pulmonary immune responses to Cryptococcus of these two mouse strains may differ qualitatively or quantitatively from those of non-Tg C3H mice. Nevertheless, in the non-Tg C3H mice, dissemination of C. neoformans to the liver and spleen at the time of euthanasia largely exceeded that of C. gattii, demonstrating a greater capacity of C. neoformans for systemic dissemination in the immunocompetent host (41). The greater capsule thickness of C. neoformans than that of C. gattii, providing protection against reactive oxygen and nitrogen species within phagocytes (55), may have facilitated dissemination by a “Trojan horse” mechanism (59). Despite this enhanced dissemination, however, the survival of non-Tg C3H mice infected with the C. neoformans and C. gattii strains did not differ significantly, suggesting that the variable virulence of strains within each species outweighs any potentially consistent difference in virulence between these two cryptococcal species. In fact, previous studies comparing the virulence of C. neoformans H99 and C. gattii R265 in C57BL/6 and BALB/c mice produced inconsistent results (17, 41), indicating that the virulence of C. neoformans and C. gattii is likely comparable in many, if not most, strains of immunocompetent mice. This interpretation is supported by the balanced upregulation in production of protective (IFN-γ) and nonprotective (IL-4 and IL-13) cytokines (36, 51, 52, 60, 61) in non-Tg C3H mice infected with C. neoformans compared to those infected with C. gattii. Taken together, the results of our survival studies demonstrate that HIV-1 transgene expression alters the course of cryptococcal infection to a far larger degree than any intrinsic differences in virulence, systemic dissemination, or host immune responses between C. neoformans and C. gattii.
Enhanced susceptibility to C. neoformans infection in the Tg mice was associated with a sharply reduced pulmonary inflammatory cell response and decreased production of the CC chemokines MCP-1 (CCL2) and RANTES (CCL5). The striking depletion of pulmonary CD4+ and CD8+ T cells in infected or uninfected Tg mice is congruent with the quantitative reductions of these cell populations in the oral mucosa, secondary lymphoid organs, and peripheral blood of these Tg mice (18, 23). The present results therefore suggest that the defective pulmonary CD4+ and CD8+ T-cell response to C. neoformans infection in Tg mice resulted from the primary depletion of these cell populations as a consequence of HIV-1 transgene expression, combined with a failure of their recruitment as a result of reduced production of the chemokines MCP-1 and RANTES, which attract activated T cells, monocytes, and dendritic cells. During pulmonary C. neoformans infection, upregulation of MCP-1 and MCP-3 (CCL7) production is required for CCR2-mediated recruitment of T cells, dendritic cells, and macrophages, formation of bronchovascular cell infiltrates, and development of protective Th1 immunity (42–48). Furthermore, SJL/J mice, which are resistant to C. neoformans infection, show enhanced MCP-1 mRNA expression compared to susceptible C57BL/6 mice (62). Potential cellular sources of MCP-1 in the lungs include epithelial cells, endothelial cells, fibroblasts, and macrophages (42). Of these specific cell populations, only macrophages express the HIV-1 transgene (19) and would thus be susceptible primarily to altered cytokine expression. In this regard, we have previously shown that F4/80+ macrophages recruited to the gastric submucosa and oral mucosa of HIV-1-expressing Tg mice in response to Candida albicans infection express the mannose receptor (CD206) almost uniformly, but MCP-1 only very infrequently (26), consistent with an alternatively activated (M2) phenotype known to be associated with susceptibility to cryptococcosis (36, 52). Furthermore, because it has been shown that experimental depletion of CD4+ and CD8+ T cells independently abrogates the appearance of a protective inflammatory response to pulmonary C. neoformans infection and augments systemic dissemination (63–65), it is likely that the depletion of these T-cell populations in the Tg mice contributed to the reduced pulmonary inflammatory cell response to C. neoformans and the augmented systemic dissemination to the liver and spleen. Despite the defective pulmonary inflammatory cell response to C. neoformans in the Tg mice, pulmonary fungal burdens were remarkably comparable to those in non-Tg mice, suggesting that reduced survival of the Tg mice was caused primarily by enhanced systemic dissemination rather than increased proliferation of C. neoformans in the lungs (66). Surprisingly, augmented susceptibility of the Tg mice to C. neoformans infection was associated with diminished pulmonary production of IL-4 and increased production of IL-17A, which result in an alteration of the Th1-Th2-Th17 balance associated with a protective rather than a nonprotective host response to C. neoformans (35–37, 55). The augmented dissemination of C. neoformans to the liver and spleen in Tg mice, also previously observed in IL-23p19−/− mice with impaired production of IL-17 (35), was therefore likely caused by perturbations other than a defective Th17 response.
Capsule thicknesses of C. neoformans and C. gattii in the lungs increased significantly during the course of infection of non-Tg mice (30) but not Tg mice. The mechanisms responsible for differences in capsule thickness in vivo are unknown (30) but could potentially include variations in iron, CO2, and nutrient concentrations in host tissues (30, 67). Interestingly, CD4C/HIVNef transgenic mice display increased circulating ferritin levels due to Nef-dependent release of ferritin from macrophages, and plasma ferritin levels are correlated with viral RNA in HIV-1-infected patients (68). C. neoformans can acquire iron bound to the major carrier transferrin by a reductive iron uptake pathway (69). Because growth of C. neoformans at high iron concentrations results in cells with thinner capsules (30) and lower expression of the CAP60 gene that is required for capsule production (70), increased availability of iron from the ferritin carrier may have contributed to the lack of capsule thickening during the course of cryptococcal infection in the Tg mice. However, despite the absence of capsule thickening during infection by both species, the capsule thickness of C. neoformans remained greater than that of C. gattii in the Tg mice and may have contributed to its enhanced systemic dissemination to the liver and spleen, which was also observed in the non-Tg mice.
The percentages of pulmonary dendritic cells, alveolar macrophages, and Gr-1+ cells increased from day 7 to day 14 after infection of Tg and non-Tg mice with C. neoformans, and absolute numbers of these cell populations extracted from the lungs were not significantly diminished in the Tg mice. Dendritic cells in CD4C/HIVMutA Tg mice have an immature phenotype, with low expression of major histocompatibility complex (MHC) class II and costimulatory molecules and a decreased capacity to present antigen in vitro (20, 27). In view of the defective production of MCP-1 in the Tg mice, dendritic cells could potentially have failed to accumulate in the lungs in response to C. neoformans infection because of defective CCR2-mediated recruitment and differentiation of monocytes (46). Preserved production of other CCR2 agonists, such as MCP-2 and MCP-3, may have compensated for the defective production of MCP-1. Because dendritic cells and alveolar macrophages play a critical role in the early innate protective host response against C. neoformans (71) and are associated with natural resistance to progressive infection (62), it is likely that functional defects of these cell populations also contributed to the increased susceptibility of the Tg mice to C. neoformans infection. Blood monocytes and alveolar macrophages from HIV-infected patients have impaired fungistatic activity against C. neoformans (72–76).
In summary, the present findings clearly demonstrate that HIV-1 transgene expression consistently augments susceptibility to C. neoformans but not C. gattii infection, and it reduces the pulmonary inflammatory cell response by both depletion of immune cells and diminished production of chemokines. In the absence of this protective host response in Tg mice, the greater capsule thickness of C. neoformans than that of C. gattii in vivo may become a primary determinant of the host-pathogen interaction and result in selectively enhanced virulence of C. neoformans, considering that both species qualitatively share all of the known major C. neoformans virulence traits (7, 77).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Canadian Institutes of Health Research (grant MOP-93597). Kassandre Leongson and Mathieu Goupil are recipients of a studentship award from the University of Montreal.
We thank Marie-Andrée Laniel for support in maintaining the Tg mouse colony, Christian Charbonneau for assistance with photomicrography, and Miguel Chagnon for statistical analysis.
Footnotes
Published ahead of print 22 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01339-12.
REFERENCES
- 1. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530 [DOI] [PubMed] [Google Scholar]
- 2. Bodasing N, Seaton RA, Shankland GS, Kennedy D. 2004. Cryptococcus neoformans var. gattii meningitis in an HIV-positive patient: first observation in the United Kingdom. J. Infect. 49:253–255 [DOI] [PubMed] [Google Scholar]
- 3. Chaturvedi S, Dyavaiah M, Larsen RA, Chaturvedi V. 2005. Cryptococcus gattii in AIDS patients, southern California. Emerg. Infect. Dis. 11:1686–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoang LM, Maguire JA, Doyle P, Fyfe M, Roscoe DL. 2004. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997–2002): epidemiology, microbiology and histopathology. J. Med. Microbiol. 53:935–940 [DOI] [PubMed] [Google Scholar]
- 5. Karstaedt AS, Crewe-Brown HH, Dromer F. 2002. Cryptococcal meningitis caused by Cryptococcus neoformans var. gattii, serotype C, in AIDS patients in Soweto, South Africa. Med. Mycol. 40:7–11 [DOI] [PubMed] [Google Scholar]
- 6. Morgan J, McCarthy KM, Gould S, Fan K, Arthington-Skaggs B, Iqbal N, Stamey K, Hajjeh RA, Brandt ME, Gauteng Cryptococcal Surveillance Initiative Group 2006. Cryptococcus gattii infection: characteristics and epidemiology of cases identified in a South African province with high HIV seroprevalence, 2002–2004. Clin. Infect. Dis. 43:1077–1080 [DOI] [PubMed] [Google Scholar]
- 7. Sorrell TC. 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39:155–168 [PubMed] [Google Scholar]
- 8. Bovers M, Hagen F, Kuramae EE, Hoogveld HL, Dromer F, St-Germain G, Boekhout T. 2008. AIDS patient death caused by novel Cryptococcus neoformans × C. gattii hybrid. Emerg. Infect. Dis. 14:1105–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. St-Germain G, Noel G, Chung KJ. 1988. Disseminated cryptococcosis due to Cryptococcus neoformans variety gattii in a Canadian patient with AIDS. Eur. J. Clin. Microbiol. Infect. Dis. 7:587–588 [DOI] [PubMed] [Google Scholar]
- 10. Galanis E, Hoang L, Kibsey P, Morshed M, Phillips P. 2009. Clinical presentation, diagnosis and management of Cryptococcus gattii cases: lessons learned from British Columbia. Can. J. Infect. Dis. Med. Microbiol. 20:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, Macdougall L, Boekhout T, Kwon-Chung KJ, Meyer W. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. U. S. A. 101:17258–17263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galanis E, Macdougall L. 2010. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg. Infect. Dis. 16:251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byrnes EJ, 3rd, Bildfell RJ, Frank SA, Mitchell TG, Marr KA, Heitman J. 2009. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J. Infect. Dis. 199:1081–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Datta K, Bartlett KH, Baer R, Byrnes E, Galanis E, Heitman J, Hoang L, Leslie MJ, MacDougall L, Magill SS, Morshed MG, Marr KA, Cryptococcus gattii Working Group of the Pacific Northwest 2009. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg. Infect. Dis. 15:1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fyfe M, MacDougall L, Romney M, Starr M, Pearce M, Mak S, Mithani S, Kibsey P. 2008. Cryptococcus gattii infections on Vancouver Island, British Columbia, Canada: emergence of a tropical fungus in a temperate environment. Can. Commun. Dis. Rep. 34:1–12 [PubMed] [Google Scholar]
- 16. Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, Marriott D, Pfeiffer T, Parr D, Byth K. 2000. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Clin. Infect. Dis. 31:499–508 [DOI] [PubMed] [Google Scholar]
- 17. Cheng PY, Sham A, Kronstad JW. 2009. Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect. Immun. 77:4284–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Repentigny L, Aumont F, Ripeau JS, Fiorillo M, Kay DG, Hanna Z, Jolicoeur P. 2002. Mucosal candidiasis in transgenic mice expressing human immunodeficiency virus type 1. J. Infect. Dis. 185:1103–1114 [DOI] [PubMed] [Google Scholar]
- 19. Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163–175 [DOI] [PubMed] [Google Scholar]
- 20. Poudrier J, Weng X, Kay DG, Hanna Z, Jolicoeur P. 2003. The AIDS-like disease of CD4C/human immunodeficiency virus transgenic mice is associated with accumulation of immature CD11bHi dendritic cells. J. Virol. 77:11733–11744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poudrier J, Weng X, Kay DG, Pare G, Calvo EL, Hanna Z, Kosco-Vilbois MH, Jolicoeur P. 2001. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-gamma and IL-6. Immunity 15:173–185 [DOI] [PubMed] [Google Scholar]
- 22. Priceputu E, Rodrigue I, Chrobak P, Poudrier J, Mak TW, Hanna Z, Hu C, Kay DG, Jolicoeur P. 2005. The Nef-mediated AIDS-like disease of CD4C/human immunodeficiency virus transgenic mice is associated with increased Fas/FasL expression on T cells and T-cell death but is not prevented in Fas-, FasL-, tumor necrosis factor receptor 1-, or interleukin-1beta-converting enzyme-deficient or Bcl2-expressing transgenic mice. J. Virol. 79:6377–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weng X, Priceputu E, Chrobak P, Poudrier J, Kay DG, Hanna Z, Mak TW, Jolicoeur P. 2004. CD4+ T cells from CD4C/HIVNef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro but are dispensable for the development of a severe AIDS-like organ disease. J. Virol. 78:5244–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kay DG, Yue P, Hanna Z, Jothy S, Tremblay E, Jolicoeur P. 2002. Cardiac disease in transgenic mice expressing human immunodeficiency virus-1 nef in cells of the immune system. Am. J. Pathol. 161:321–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Repentigny L, Lewandowski D, Jolicoeur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin. Microbiol. Rev. 17:729–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goupil M, Trudelle EB, Dugas V, Racicot-Bergeron C, Aumont F, Senechal S, Hanna Z, Jolicoeur P, de Repentigny L. 2009. Macrophage-mediated responses to Candida albicans in mice expressing the human immunodeficiency virus type 1 transgene. Infect. Immun. 77:4136–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewandowski D, Marquis M, Aumont F, Lussier-Morin AC, Raymond M, Senechal S, Hanna Z, Jolicoeur P, de Repentigny L. 2006. Altered CD4+ T cell phenotype and function determine the susceptibility to mucosal candidiasis in transgenic mice expressing HIV-1. J. Immunol. 177:479–491 [DOI] [PubMed] [Google Scholar]
- 28. Marquis M, Lewandowski D, Dugas V, Aumont F, Senechal S, Jolicoeur P, Hanna Z, de Repentigny L. 2006. CD8+ T cells but not polymorphonuclear leukocytes are required to limit chronic oral carriage of Candida albicans in transgenic mice expressing human immunodeficiency virus type 1. Infect. Immun. 74:2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Litvintseva AP, Mitchell TG. 2009. Most environmental isolates of Cryptococcus neoformans var. grubii (serotype A) are not lethal for mice. Infect. Immun. 77:3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rivera J, Feldmesser M, Cammer M, Casadevall A. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 66:5027–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feldmesser M, Kress Y, Novikoff P, Casadevall A. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feldmesser M, Tucker S, Casadevall A. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9:273–278 [DOI] [PubMed] [Google Scholar]
- 33. Feldmesser M, Kress Y, Casadevall A. 2001. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147:2355–2365 [DOI] [PubMed] [Google Scholar]
- 34. Hoag KA, Street NE, Huffnagle GB, Lipscomb MF. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13:487–495 [DOI] [PubMed] [Google Scholar]
- 35. Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, Straubinger RK, McClanahan T, Kastelein RA, Alber G. 2006. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J. Immunol. 176:1098–1106 [DOI] [PubMed] [Google Scholar]
- 36. Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, Schutze N, Straubinger RK, Blessing M, McKenzie AN, Brombacher F, Alber G. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 179:5367–5377 [DOI] [PubMed] [Google Scholar]
- 37. Voelz K, Lammas DA, May RC. 2009. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 77:3450–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gal AA, Koss MN, Hawkins J, Evans S, Einstein H. 1986. The pathology of pulmonary cryptococcal infections in the acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 110:502–507 [PubMed] [Google Scholar]
- 39. McDonnell JM, Hutchins GM. 1985. Pulmonary cryptococcosis. Hum. Pathol. 16:121–128 [DOI] [PubMed] [Google Scholar]
- 40. Torda A, Kumar RK, Jones PD. 2001. The pathology of human and murine pulmonary infection with Cryptococcus neoformans var. gattii. Pathology 33:475–478 [DOI] [PubMed] [Google Scholar]
- 41. Ngamskulrungroj P, Chang Y, Sionov E, Kwon-Chung KJ. 2012. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3:e00103–12 doi:10.1128/mBio.00103-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huffnagle GB, Strieter RM, Standiford TJ, McDonald RA, Burdick MD, Kunkel SL, Toews GB. 1995. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 155:4790–4797 [PubMed] [Google Scholar]
- 43. Traynor TR, Herring AC, Dorf ME, Kuziel WA, Toews GB, Huffnagle GB. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 168:4659–4666 [DOI] [PubMed] [Google Scholar]
- 44. Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB, Toews GB. 2011. Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am. J. Pathol. 178:198–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. 2008. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J. Immunol. 181:610–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. 2009. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J. Immunol. 183:8044–8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qiu Y, Zeltzer S, Zhang Y, Wang F, Chen GH, Dayrit J, Murdock BJ, Bhan U, Toews GB, Osterholzer JJ, Standiford TJ, Olszewski MA. 2012. Early induction of CCL7 downstream of TLR9 signaling promotes the development of robust immunity to cryptococcal infection. J. Immunol. 188:3940–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huffnagle GB, Traynor TR, McDonald RA, Olszewski MA, Lindell DM, Herring AC, Toews GB. 2000. Leukocyte recruitment during pulmonary Cryptococcus neoformans infection. Immunopharmacology 48:231–236 [DOI] [PubMed] [Google Scholar]
- 49. Huffnagle GB, Strieter RM, McNeil LK, McDonald RA, Burdick MD, Kunkel SL, Toews GB. 1997. Macrophage inflammatory protein-1alpha (MIP-1alpha) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J. Immunol. 159:318–327 [PubMed] [Google Scholar]
- 50. Huffnagle GB, Toews GB, Burdick MD, Boyd MB, McAllister KS, McDonald RA, Kunkel SL, Strieter RM. 1996. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529–4536 [PubMed] [Google Scholar]
- 51. Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie Q, Saito A. 1996. Contribution of interferon-gamma in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 13:123–130 [DOI] [PubMed] [Google Scholar]
- 52. Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346–6356 [DOI] [PubMed] [Google Scholar]
- 53. Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel TR. 1996. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 64:2897–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Curtis JL, Huffnagle GB, Chen GH, Warnock ML, Gyetko MR, McDonald RA, Scott PJ, Toews GB. 1994. Experimental murine pulmonary cryptococcosis. Differences in pulmonary inflammation and lymphocyte recruitment induced by two encapsulated strains of Cryptococcus neoformans. Lab. Invest. 71:113–126 [PubMed] [Google Scholar]
- 55. Voelz K, May RC. 2010. Cryptococcal interactions with the host immune system. Eukaryot. Cell 9:835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman J, Dromer F, Nielsen K. 2010. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 6:e1000953 doi:10.1371/journal.ppat.1000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. 2010. Fungal cell gigantism during mammalian infection. PLoS Pathog. 6:e1000945 doi:10.1371/journal.ppat.1000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okagaki LH, Nielsen K. 2012. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell 11:820–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Casadevall A. 2010. Cryptococci at the brain gate: break and enter or use a Trojan horse? J. Clin. Invest. 120:1389–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hernandez Y, Arora S, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 174:1027–1036 [DOI] [PubMed] [Google Scholar]
- 61. Kawakami K, Hossain Qureshi M, Zhang T, Koguchi Y, Xie Q, Kurimoto M, Saito A. 1999. Interleukin-4 weakens host resistance to pulmonary and disseminated cryptococcal infection caused by combined treatment with interferon-gamma-inducing cytokines. Cell. Immunol. 197:55–61 [DOI] [PubMed] [Google Scholar]
- 62. Guillot L, Carroll SF, Homer R, Qureshi ST. 2008. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect. Immun. 76:4745–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35–42 [DOI] [PubMed] [Google Scholar]
- 64. Huffnagle GB, Yates JL, Lipscomb MF. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 173:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huffnagle GB, Yates JL, Lipscomb MF. 1991. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect. Immun. 59:1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang JP, Lee CK, Akalin A, Finberg RW, Levitz SM. 2011. Contributions of the MyD88-dependent receptors IL-18R, IL-1R, and TLR9 to host defenses following pulmonary challenge with Cryptococcus neoformans. PLoS One 6:e26232 doi:10.1371/journal.pone.0026232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mogensen EG, Janbon G, Chaloupka J, Steegborn C, Fu MS, Moyrand F, Klengel T, Pearson DS, Geeves MA, Buck J, Levin LR, Muhlschlegel FA. 2006. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot. Cell 5:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Swingler S, Zhou J, Swingler C, Dauphin A, Greenough T, Jolicoeur P, Stevenson M. 2008. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe 4:63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jung WH, Sham A, Lian T, Singh A, Kosman DJ, Kronstad JW. 2008. Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog. 4:e45 doi:10.1371/journal.ppat.0040045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lian T, Simmer MI, D'Souza CA, Steen BR, Zuyderduyn SD, Jones SJ, Marra MA, Kronstad JW. 2005. Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 55:1452–1472 [DOI] [PubMed] [Google Scholar]
- 71. Osterholzer JJ, Milam JE, Chen GH, Toews GB, Huffnagle GB, Olszewski MA. 2009. Role of dendritic cells and alveolar macrophages in regulating early host defense against pulmonary infection with Cryptococcus neoformans. Infect. Immun. 77:3749–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reardon CC, Kim SJ, Wagner RP, Koziel H, Kornfeld H. 1996. Phagocytosis and growth inhibition of Cryptococcus neoformans by human alveolar macrophages: effects of HIV-1 infection. AIDS 10:613–618 [DOI] [PubMed] [Google Scholar]
- 73. Ieong MH, Reardon CC, Levitz SM, Kornfeld H. 2000. Human immunodeficiency virus type 1 infection of alveolar macrophages impairs their innate fungicidal activity. Am. J. Respir. Crit. Care Med. 162:966–970 [DOI] [PubMed] [Google Scholar]
- 74. Cameron ML, Granger DL, Matthews TJ, Weinberg JB. 1994. Human immunodeficiency virus (HIV)-infected human blood monocytes and peritoneal macrophages have reduced anticryptococcal activity whereas HIV-infected alveolar macrophages retain normal activity. J. Infect. Dis. 170:60–67 [DOI] [PubMed] [Google Scholar]
- 75. Harrison TS, Kornfeld H, Levitz SM. 1995. The effect of infection with human immunodeficiency virus on the anticryptococcal activity of lymphocytes and monocytes. J. Infect. Dis. 172:665–671 [DOI] [PubMed] [Google Scholar]
- 76. Harrison TS, Levitz SM. 1997. Mechanisms of impaired anticryptococcal activity of monocytes from donors infected with human immunodeficiency virus. J. Infect. Dis. 176:537–540 [DOI] [PubMed] [Google Scholar]
- 77. Kronstad JW, Attarian R, Cadieux B, Choi J, D'Souza CA, Griffiths EJ, Geddes JM, Hu G, Jung WH, Kretschmer M, Saikia S, Wang J. 2011. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 9:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.