Abstract
Paracoccidioidomycosis (PCM) is a systemic mycosis caused by the thermodimorphic fungus Paracoccidioides brasiliensis. Leukotrienes and lipoxins are lipid mediators produced after 5-lipoxygenase (5-LO) activation that exhibit pro- and anti-inflammatory roles, respectively. Here, we have investigated the contribution of 5-LO enzymatic activity in PCM using an experimental model of P. brasiliensis infection. B6.129 wild-type (B6.129) and 5-LO-deficient (5-LO−/−) mice were intravenously inoculated with a virulent strain of P. brasiliensis (Pb18), and the survival rate of the infected mice was investigated on different days after yeast infection. 5-LO−/− mice exhibited an increased survival rate associated with a decreased number of CFU. The resistance of 5-LO−/− during PCM was associated with augmented nitric oxide (NO) production and the formation of compact granulomas. In addition, the absence of 5-LO was associated with a diminished number of CD4+ CD25+ regulatory T cells, higher levels of gamma interferon and interleukin-12, and increased T-bet (a T-box transcription factor that directs Th1 lineage commitment) mRNA levels in the lungs. Taken together, our results show for the first time that 5-LO enzymatic activity increases susceptibility to P. brasiliensis, suggesting that this pathway may be a potential target for therapeutic intervention during PCM.
INTRODUCTION
Paracoccidioidomycosis (PCM), an important chronic systemic mycosis endemic in rural areas of Latin America, is caused by the thermodimorphic fungus Paracoccidioides brasiliensis. PCM affects approximately 10 million people and results in a mortality rate of 1.4 per 1 million inhabitants (1, 2). Moreover, PCM is considered a critical social and economic problem because it occurs in individuals in the most productive stage of life and culminates in frequent anatomic and functional sequelae (3). T helper 1 (Th1)-type cytokines such as interleukin-12 (IL-12) and gamma interferon (IFN-γ) are implicated in resistance during P. brasiliensis infection, while the predominant production of Th2-type cytokines contributes to the disseminated form of the disease (4). The infection also leads to the differentiation of T CD4+ CD25+ FOXP3+ cells, which migrate to the lesion site to inhibit T cell proliferation and favor a latent form of the disease (5, 6).
Lipoxins and leukotrienes are lipid mediators formed by the 5-lipoxygenase (5-LO)-catalyzed oxidation of arachidonic acid (7, 8). The interaction of the 5-LO enzyme with a 5-LO-activating protein (FLAP) allows the conversion of arachidonic acid into the unstable leukotriene A4 (LTA4). The 5-LO biosynthetic pathways can be triggered after activation by a variety of stimuli, including fungal products (9). The two bioactive classes of leukotrienes, LTB4 and cysteinyl leukotrienes (CysLT), participate in protection during infectious diseases by promoting cellular activation, vasodilatation, and increased vascular permeability (10). Conversely, lipoxins are involved in the downmodulation of the inflammatory response, including the production of IL-12 and IFN-γ (11, 12).
Because little is known about the mechanisms underlying the immune response during PCM, we sought here to investigate the role of the 5-LO activity in a murine model of systemic P. brasiliensis infection using B6.129 wild-type and 5-LO-deficient mice (5-LO−/−). 5-LO−/− mice exhibited diminished fungal growth and increased survival rate compared to B6.129 mice. The protection observed in 5-LO−/− mice was associated with reduced expression of GITR and FOXP3 by CD4+ CD25+ cells and increased production of IFN-γ in the lungs. The blockage of the lipoxin receptor FPR2 in B6.129 mice led to a decreased number of fungal cells in the lungs, suggesting that FPR2 ligands, as lipoxins, could contribute to the inhibition of the host response in PCM. In conclusion, our data show for the first time that 5-LO enzymatic activity increases susceptibility during experimental P. brasiliensis infection in mice.
MATERIALS AND METHODS
Mice.
Male 6- to 8-week-old 5-LO-deficient mice (5-LO−/−) and strain-matched wild-type B6.129 mice, the most widely used genetic background for 5-LO genetically modified mice, were obtained from the Isogenic Breeding Unit–School of Medicine of Ribeirao Preto, University of Sao Paulo, Ribeirao Preto, Brazil. Experiments were conducted according to the ethical principles of animal research adopted by the Brazilian College of Animal Experimentation (COBEA) and approved by the Ethical Commission in Animal Research (protocol 086/2006).
Fungus, mouse infections, and mortality.
Yeast cells of a virulent P. brasiliensis strain (Pb18) were cultured for 10 to 12 days at 35 to 37°C in brain heart infusion-agar medium (Difco Laboratories) supplemented with 5% fetal calf serum and 5% culture filtrate from 2-week-old cultures of the slightly virulent isolate Pb265 as a source of growth-promoting factor (13, 14). For the preparation of inoculum, Pb18 cells were harvested and washed three times in phosphate-buffered saline (PBS; pH 7.2), and viability was determined as previously described (15). Fungal suspensions containing >80% viable cells were used. The mice were intravenously (i.v.) infected with 106 viable yeast cells. The survival of Pb18-infected B6.129 and 5-LO−/− mice (30 mice in each group) was verified daily for a period of 110 days.
In vivo treatment with lipoxin receptor antagonists.
The peptides BOC-2 (Boc-Phe-Leu-Phe-Leu-Phe; Phoenix Pharmaceuticals, Burlingame, CA) and WRW4 (Tocris Bioscience, Ellisville, MO) were used in vivo as nonselective and selective lipoxin receptor antagonists, respectively, as previously reported (16, 17). B6.129 mice were intraperitoneally treated with BOC-2 (10 μg/kg/day, 0.1 ml) every 48 h from days −1 to 29 postinfection (p.i.). Similarly infected control B6.129 mice were treated with vehicle (Vhc) alone (0.5% methanol). WRW4 treatment (1 mg/kg/day, 0.1 ml, given intraperitoneally) started on day −1 and was reinforced every 72 h until day 8 p.i. Control mice for WRW4 treatment received Vhc alone (dimethyl sulfoxide [DMSO] at 0.1%).
Assay for CFU.
The number of viable yeast cells in organs (lungs, liver, and spleen) from Pb18-infected mice was determined at 7, 15, 30, 60, and 90 days p.i. by counting CFU as previously described (6). Plates were incubated at 35 to 37°C for 7 days, and the amount of CFU per g of tissue was calculated.
Organ morphology and morphometry of pulmonary granulomas.
Whole lungs were excised, fixed with 10% formalin for 24 h, and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) for lesion analysis or impregnated with silver to demonstrate reticulum fibers using standard protocols. Images were captured with a digital camera (Olympus) adapted to a common optical microscope. Morphometric analyses were performed using panoramic images of lungs (magnification, ×10): the pulmonary inflammatory area was manually drawn and measured using ImageJ software (National Institutes of Health; http://rsbweb.nih.gov/ij/). The lesion index was then calculated (the sum of the inflammatory area × the lung area−1).
Immunohistochemistry.
Frozen lung sections (5 μm) were obtained from B6.129 and 5-LO−/− Pb18-infected mice, and an immunohistochemical reaction was performed as previously described (6). Slides were incubated with anti-mouse IgG as control or rabbit IgG anti-mouse 5-LO, NOS-2 or CD4 (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 100 times in PBS (5-LO and CD4) or PBS–0.01% saponin (NOS-2). We measured immunostained areas using ImageJ software. Briefly, after defining the range of brown color considered to be positive, the images were converted to 256 shades (8-bit) of gray. The grayscale image was converted to binary (black and white) to define the cutoff point. The threshold was adjusted, and the brown areas became black portions in the binary image. The percentage of stained area was automatically analyzed.
Measurement of cytokines and lipid mediators in lung supernatant.
Lungs were removed from mice on days 7, 15, 30, 60, and 90 p.i., weighed, and homogenized in 1 ml of protease inhibitor cocktail buffer (Complete; Roche, Mannheim, Germany) using a tissue homogenizer. After centrifugation (5,000 × g for 10 min), the supernatants were harvested and dispensed (0.05 ml) into a 96-well plate containing capture monoclonal antibodies. The levels of IFN-γ, IL-12p40, IL-12p70, IL-6, and IL-10 were measured by standard sandwich enzyme-linked immunosorbent assay (ELISA; BD Pharmingen, San Jose, CA). The optical densities were measured at 450 nm using a microplate ELISA reader (EMAX; Molecular Devices). To assay the amount of lipid mediators, the samples were centrifuged at 5,000 × g for 10 min, and the supernatant was purified with Sep-Pak C18 cartridges according to the manufacturer's instructions (Waters Corp., Milford, MA). Eicosanoid levels were measured using commercial kits obtained from Neogen Corp. (LXA4) and Cayman Chemical Co (LTB4 and CysLT).
Nitrite quantification in lung tissue.
The lungs were removed, weighed, homogenized, and centrifuged as described above. The assay was performed in a microplate by mixing 0.05 ml of lung homogenate with 0.05 ml of nitrate reductase solution to convert nitrate into nitrite. Next, 0.1 ml of Griess reagent was mixed (18), and the optical densities were measured at 540 nm. The nitrite concentration was determined with reference to a standard curve of 1 to 200 μM NaNO2.
Isolation of leukocytes from lung tissue and flow cytometry analysis.
Lungs were removed and placed in RPMI 1640 with Liberase CI enzymes (0.5 mg/ml; Boehringer Ingelheim Chemicals, Inc., Petersburg, VA). After 1 h at 37°C, the samples were crushed through a 50-μm-pore-size filter (Falcon products) and centrifuged (250 × g, 10 min). Next, leukocytes were collected, and the expression of CD11b, CD11c, CD3, CD8, CD4, CD25, CTLA-4, CD103, GITR, and FOXP3 was assessed by flow cytometry as previously reported (6). The data acquisition was performed on a fluorescence-activated cell sorting Canto II (BD Immunocytometry Systems) flow cytometer and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
cDNA synthesis and quantitative real-time PCR (qRT-PCR).
Total RNA was isolated from the lungs using an Illustra RNAspin minikit containing RNase-free DNase to avoid any genomic DNA contamination (GE Healthcare) as described by the manufacturer. The following primer pairs were used: 5-LO, 5′-CCA AAT GCC ACA AGG AT (forward) and 5′-CGC TTT TGA GTA GTT CAG AAC (reverse); Arginase-1, 5′-GTT CCC AGA TGT ACC AGG ATT C (forward) and 5′-CGA TGT CTT TGG CAG ATA TGC (reverse); NOS-2, 5′-CGA AAC GCT TCA CTT CCA A (forward) and 5′-TGA GCC TAT ATT GCT GTG GCT (reverse); T-bet, 5′-CCC CTG TCC AGT CAG TAA CTT (forward) and 5′-CTT CTC TGT TTG GCT GGC T (reverse); and Glyceraldehyde Phosphate Dehydrogenase (GAPDH), 5′-TGC AGT GGC AAA GTG GAG AT (forward) and 5′-CGT GAG TGG AGT CAT ACT GGA A (reverse). First-strand cDNA was synthesized from 2 μg of total RNA using a SuperScript III reverse transcriptase kit (Invitrogen) and oligo(dT) primers according to the manufacturer's instructions. Amplification assays were carried out in duplicate on a 7000 Sequence Detection Systems device (Applied Biosystems, Foster City, CA) in 15-μl volumes containing 5 μl of cDNA, 0.5 μl (1 to 2 μg) of each primer (reverse and forward), 6.5 μl of Platinum SYBR green qPCR master mix (Life Technologies, Carlsbad, CA), and 2.5 μl of Milli-Q H2O. After initial denaturation at 50°C for 2 min and 95°C for 2 min, amplifications were carried out for 40 cycles at 95°C for 15 s and 58°C for 30 s, and finally 72°C for 30 s. The expression of each mRNA was normalized to a housekeeping gene (GAPDH) by the threshold cycle (ΔΔCT) method.
Nitrite production in AM culture.
Bronchoalveolar lavage fluids were obtained from 5-LO−/− and B6.129 uninfected mice as previously described (19). After centrifugation (10 min, 350 × g, 4°C), the pellets were placed in RPMI 1640 (Invitrogen Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, 10 mM HEPES, 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate (all from Sigma-Aldrich). Cell count and viability were determined by trypan blue exclusion, and cells were plated (5 × 105 cells/well in a 96-well plate) and isolated by adherence after 4 h at 37°C in 5% CO2. After two washing steps with PBS, alveolar macrophages (AM) were cultivated for 18 h with fresh complete RPMI 1640 medium with 10% FBS. Then, the AM were cultured with or without P. brasiliensis yeasts cells at a 1:50 ratio of yeast to AM at 37°C in 5% CO2 in the presence of medium, LXA4 (100 μg/ml), or BOC-2 (50 μg/ml). After 48 h, supernatants were collected, and the production of nitric oxide was measured as described above.
Statistical analysis.
Data are expressed as means ± the standard errors of the mean (SEM). Statistical analysis was performed using analysis of variance to compare multiple groups, followed by the parametric Tukey-Kramer test for two-group comparison. The Gehan-Breslow-Wilcoxon method was used to compare survival curves. All analyses were performed with Prism software (version 5.0; GraphPad, San Diego, CA). A P value of <0.05 was considered statistically significant.
RESULTS
5-LO enzymatic activity is upregulated after P. brasiliensis infection.
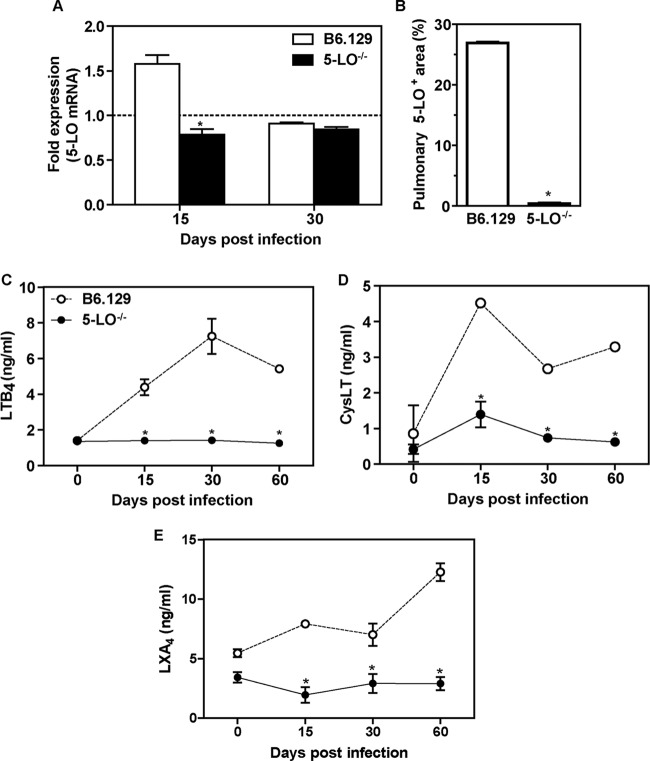
To evaluate whether P. brasiliensis drives the activation of 5-LO pathways, B6.129 and 5-LO−/− mice were infected with Pb18 yeast cells, and the transcript levels of 5-LO, as well as the production of leukotrienes and lipoxins, were determined in the lungs. 5-LO mRNA was increased in B6.129 mice at day 15 p.i. (1.575 ± 0.1) compared to the 5-LO−/− group (0.785 ± 0.062, P < 0.05). At day 30, both groups of mice exhibited similar levels of 5-LO transcripts (0.908 ± 0.013 versus 0.844 ± 0.029, P > 0.05) (Fig. 1A). To confirm these data, we investigated the effect of P. brasiliensis in the induction of 5-LO by immunohistochemistry in the lung from B6.129 mice at day 15 p.i. The immunostained area was determined by morphometric analysis, and, as shown in Fig. 1B, 27% of the total lung area was occupied by 5-LO+ cells in B6.129 mice, whereas <1% was found in 5-LO−/− lung (P < 0.05). We also observed that LTB4, CysLT, and LXA4 levels were increased in the lungs of B6.129 mice but not in 5-LO−/− mice (Fig. 1, C-E). These results demonstrate that 5-LO enzymatic activity is induced by P. brasiliensis in the lungs of infected mice.
Fig 1.
5-LO expression and leukotriene production in the lungs of P. brasiliensis-infected mice. (A) The transcript levels for 5-LO were measured by qRT-PCR in whole lung homogenate from B6.129 and 5-LO−/− mice at days 15 and 30 after (i.v.) infection with 106 P. brasiliensis (Pb18) yeast cells. Uninfected mice are represented by the dashed line. The results are expressed as the fold expression. (B) The percentages of the pulmonary area containing 5-LO+ cells are indicated. Lung sections from B6.129 and 5-LO−/− mice were stained for 5-LO at day 15 p.i. using immunohistochemistry. (C to E) LTB4 (C), CysLT (D), and LXA4 (E) production in whole lung homogenate. The data represent the means ± the SEM. *, P < 0.05 compared to B6.129 mice. Similar results were obtained in three independent experiments (n = 3 to 5 mice/group).
5-LO absence leads to an increased survival rate and better control of fungal growth.
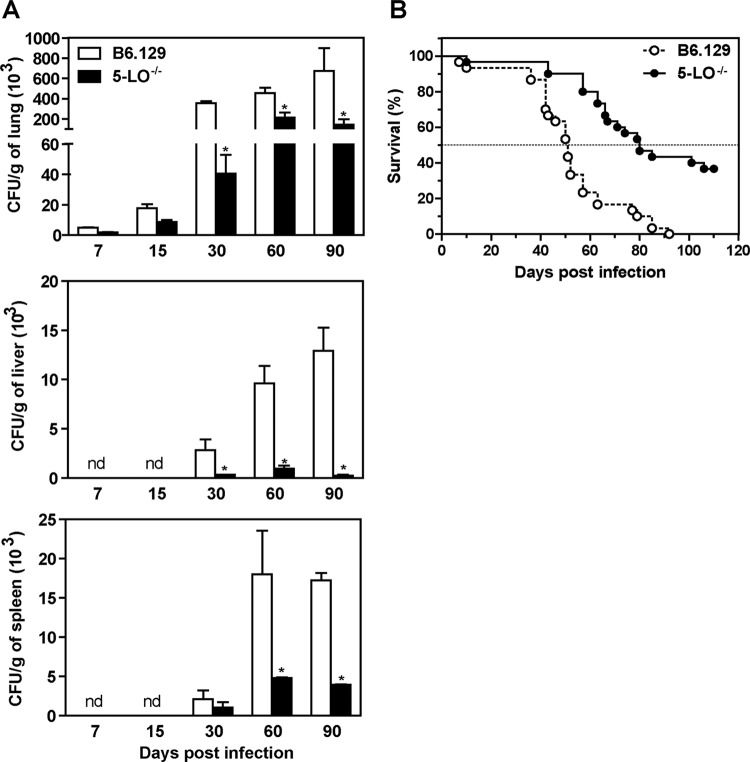
To directly assess whether 5-LO pathways are involved in susceptibility to PCM, B6.129 and 5-LO−/− mice were i.v. infected with 106 yeast cells, and the number of CFU was evaluated. At days 7 and 15 p.i., both B6.129 and 5-LO−/− mice exhibited a similar number of CFU in the lung, liver, and spleen (Fig. 2A). Interestingly, at days 30, 60, and 90 p.i., the number of CFU was significantly decreased in 5-LO−/− mice compared to the B6.129 group (Fig. 2A). These data indicate that the absence of 5-LO products might contribute to the inhibition of P. brasiliensis growing in pulmonary and also in extrapulmonary tissues. To determine the effect of 5-LO activation during PCM, the survival of B6.129 and 5-LO−/− infected mice was investigated for 110 days. As shown in Fig. 2B, mortality in both groups appeared around day 40 p.i. In addition, whereas a mortality rate of 50% was observed in B6.129 mice at day 50 p.i., this percentage was only achieved 80 days after fungal challenge in 5-LO−/− mice. We also observed that 32.1% of 5-LO−/− mice survived after 110 days of infection. Together, our data indicate that effective 5-LO enzymatic activity mediates susceptibility in our experimental model of P. brasiliensis infection.
Fig 2.
Increased survival and control of fungal growth in 5-LO−/− mice during experimental PCM. (A) B6.129 and 5-LO−/− mice were infected (i.v.) with 106 P. brasiliensis (Pb18) yeast cells, and the number of CFU was determined (n = 3 to 5 mice/group) in the lungs, liver, and spleen at days 7, 15, 30, 60, and 90 p.i. The data represent the means ± the SEM. *, P < 0.05 compared to B6.129 mice. nd, not detected. Similar results were obtained in three independent experiments. (B) The survival rate was assessed for a period of 110 days p.i. (n = 30 mice/group).
5-LO−/− mice exhibit compact granulomas in the lungs.
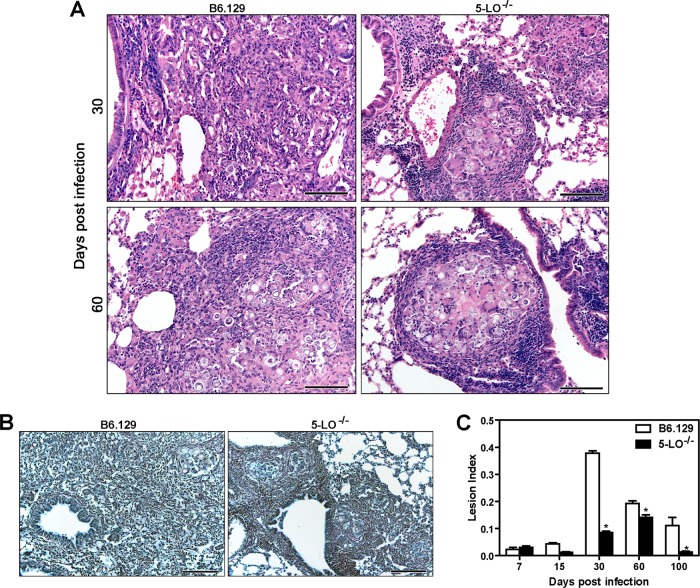
To address whether the protection observed in 5-LO−/− mice is associated with the formation of compact granulomas and reduced pulmonary injury, histological lung sections from B6.129 and 5-LO−/− mice were analyzed at days 7, 15, 30, 60, and 90 p.i. No differences were observed in the lungs from both groups until day 15 p.i. (data not shown). After days 30 and 60 p.i., 5-LO−/− mice exhibited foci of well-organized granulomatous lesions surrounded by a great number of lymphocytes. In contrast, B6.129 mice showed diffused granulomas at the same time points of infection (Fig. 3A). Moreover, the absence of 5-LO was associated with a normal pattern of reticulin fibers at day 30 p.i., while B6.129 mice showed an irregular distribution of these structures in the lung tissue (Fig. 3B). To determine the extent of the inflammatory lesions, lung sections stained with H&E were analyzed by morphometric analysis. As shown in Fig. 3C, the pulmonary injury was minor in B6.129 and 5-LO−/− mice in the early periods of infection (7 and 15 days) but significantly increased in the B6.129 group 30 days after infection. However, decreased injury (P < 0.05) was observed in 5-LO−/− mice at days 30 to 90 p.i. Taken together, these results indicate that 5-LO-products have a critical role in the exacerbation of pulmonary injury caused by P. brasiliensis.
Fig 3.
The absence of 5-LO contributes to the formation of compact granulomas during P. brasiliensis experimental infection. (A) Lung sections of B6.129 and 5-LO−/− mice after (i.v.) infection with 106 P. brasiliensis (Pb18) yeast cells at days 30 and 60 p.i. were stained with hematoxylin and eosin. (B) Silver impregnation (Gomori method) of lung tissue at day 30 p.i. Scale bar, 200 μm; optical magnification, ×20 (panels A and B). (C) The pulmonary lesion index was determined by the granuloma area × lung tissue−1. The data represent the means ± the SEM. *, P < 0.05 compared to B6.129 mice. Similar results were obtained in three independent experiments (n = 3 to 5 mice/group).
5-LO-dependent products modulate the influx of inflammatory cells to the lungs from Pb18-infected mice.
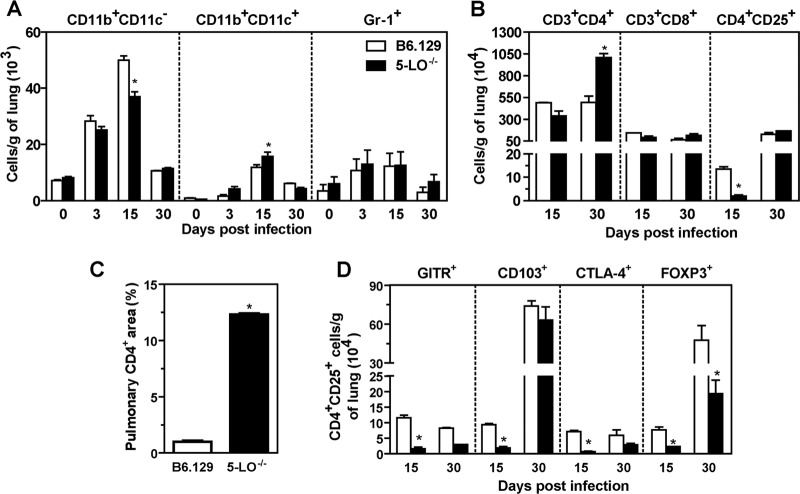
The migration and activation of inflammatory cells in the granulomas during PCM has been associated with a more efficient control of fungal growth and dissemination (20). Therefore, we next evaluated the role of 5-LO-products in cellular recruitment. Flow cytometry analysis of cells recovered from whole lung samples showed similar numbers of Gr-1+ cells in both B6.129 and 5-LO−/− mice (Fig. 4A). At day 15 p.i., we observed an increased number of CD11b+ CD11c+ cells in 5-LO−/− mice (15.761 ± 1.527) and a diminished frequency of CD11b+ CD11c− (36.997 ± 1.687) compared to the B6.129 group (11.882 ± 0.842 and 50 ± 1.466, respectively, P < 0.05) (Fig. 4A). To investigate whether T cell recruitment was affected in 5-LO−/− mice, whole lung cells were analyzed at days 15 and 30 p.i., which are time points at which we have observed an increased frequency of these cells during experimental PCM (6, 21). Although the number of CD8+ T cells was similar in both groups, the amount of CD4+ T cells was significantly increased in 5-LO−/− mice at day 30 p.i. (1,006.138 ± 45.879) compared to the B6.129 group (494.691 ± 70.667, P < 0.05) (Fig. 4B). Immunohistochemical analysis in pulmonary tissue showed that 1% of the total lung area was immunostained by anti-CD4 antibody in B6.129 mice at day 30 after fungal challenge compared to 12.3% (P < 0.05) in 5-LO−/− tissue (Fig. 4C). In addition, B6.129 mice exhibited an increased number (P < 0.05) of CD4+ CD25+ T cells at day 15 p.i. (Fig. 4B), indicating that 5-LO might positively modulate the recruitment of regulatory cells in this model. Because the molecule CD25 is commonly expressed by conventional CD4+ T cells (22), we analyzed other markers that are expressed in regulatory T (Treg) cells. As shown in Fig. 4D, significant levels of CD103, CTLA-4, GITR, and FOXP3 expression were observed in CD4+ CD25+ cells from B6.129 mice at day 15 p.i. Moreover, at day 30, the number of CD4+ CD25+ FOXP3+ cells was significantly increased in B6.129 mice compared to the 5-LO−/− group (Fig. 4D). Collectively, these results indicate that 5-LO products alter the composition of inflammatory cells in the infection foci.
Fig 4.
5-LO modulates the recruitment of inflammatory cells into the lungs during experimental PCM. B6.129 and 5-LO−/− mice were infected i.v. with 106 P. brasiliensis (Pb18) yeast cells. (A) The numbers of CD11b+ CD11c−, CD11b+ CD11c+, and Gr-1+ cells were determined by flow cytometry in the lungs at days 0, 3, 15 and 30 p.i. (B) The numbers of CD3+ CD4+, CD3+ CD8+, and CD4+ CD25+ cells were evaluated on days 15 and 30 after fungal infection. (C) CD4 expression was evaluated by immunohistochemistry on day 30 p.i., and the pulmonary area containing CD4+ cells was determined. (D) The numbers of CD4+ CD25+ cells expressing CD103, CTLA-4, GITR, and FOXP3. The data represent the means ± the SEM of 3 to 5 mice. *, P < 0.05 compared to B6.129 mice. Similar results were obtained in three independent experiments.
Increased NOS-2 expression in 5-LO−/− mice during experimental PCM.
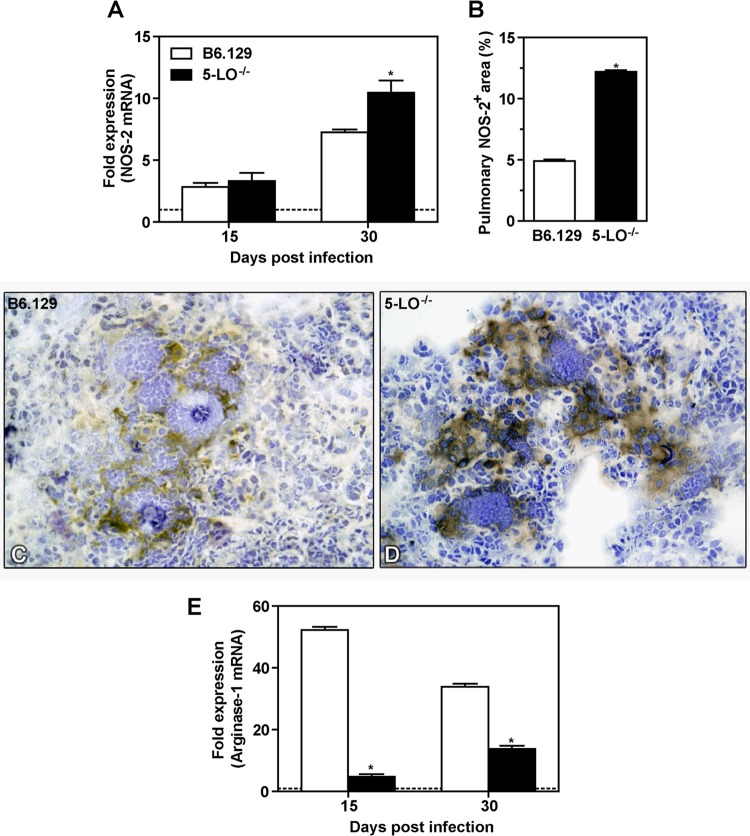
Nitric oxide (NO) produced by inflammatory cells plays a well-documented role in fungal clearance (23). Therefore, we hypothesized that the control of fungal growth observed in 5-LO−/− mice might be dependent on NO production. We evaluated transcript levels of nitric oxide synthase (NOS-2) by qRT-PCR in the lungs of B6.129 and 5-LO−/− mice at days 15 and 30 p.i. No differences were observed in NOS-2 mRNA levels in both B6.129 and 5-LO−/− groups at day 15 p.i. However, at day 30, 5-LO−/− mice exhibited significantly increased levels of NOS-2 mRNA compared to the B6.129 group (Fig. 5A). The percentage of NOS-2+ cells was increased 2.5-fold in 5-LO−/− mice compared to the B6.129 group (Fig. 5B). Figures 5C and D represent the expression of iNOS in B6.129 and 5-LO−/− mice, respectively. We also observed that, at day 30 p.i., NO levels were increased in the serum of 5-LO−/− mice compared to controls (data not shown). Since NOS-2 and arginase use a common substrate and can competitively metabolize l-arginine to NO and citrulline or urea and l-ornithine, respectively (24), we investigated whether arginase-1 transcripts were also affected in 5-LO−/− mice during experimental P. brasiliensis infection. Transcripts for arginase-1 were significantly decreased in 5-LO−/− lungs at days 15 and 30 p.i. compared to B6.129 (Fig. 5E). Taken together, our data show that 5-LO interferes with the NOS-2/arginase balance during experimental PCM, which might contribute to the lack of fungal clearance in B6.129 mice during this disease.
Fig 5.
Increased NOS-2 expression in the lungs from 5-LO−/− mice during P. brasiliensis infection. B6.129 and 5-LO−/− mice were infected (i.v.) with 106 P. brasiliensis (Pb18) yeast cells. (A) At days 15 and 30 p.i., NOS-2 mRNA levels were determined by qRT-PCR in whole lung homogenate. (B) The pulmonary area containing NOS-2+ was measured by morphometric analysis. (C and D) NOS-2 expression in lung sections from B6.129 (C) and 5-LO−/− (D) mice at day 30 p.i. was evaluated by immunohistochemistry (magnification, ×40). (E) Arginase-1 mRNA was determined by qRT-PCR in lung homogenate at days 15 and 30 p.i. Uninfected mice are represented by the dashed line. The data represent the means ± the SEM of 3 to 5 mice. *, P < 0.05 compared to B6.129 mice. Similar results were obtained in two independent experiments.
Absence of 5-LO increases the Th1 protective response during experimental PCM.
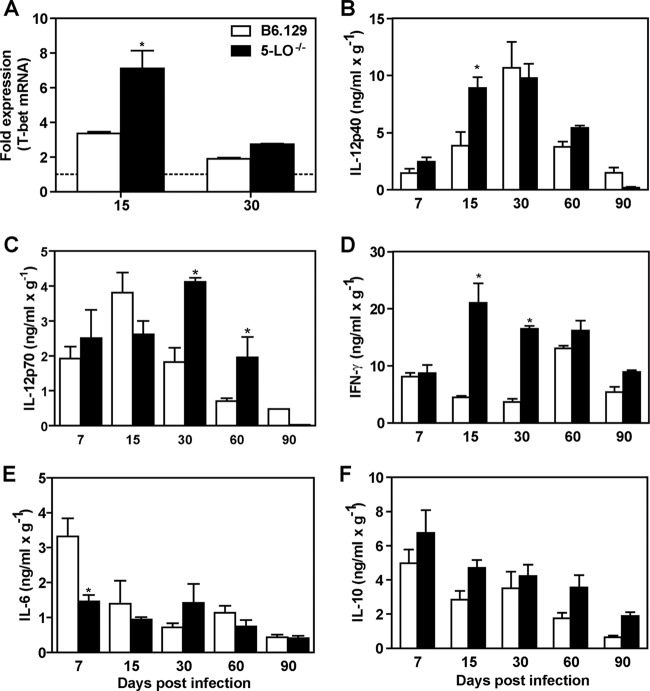
Th1-type cytokines are involved in both NO production and protection against fungal infection (25). To determine whether the resistance observed in 5-LO−/− mice is associated with Th1 cell activation, the transcript levels for T-bet, a T box transcription factor that directs Th1 lineage commitment, were evaluated in whole lungs of B6.129 and 5-LO−/− mice. As demonstrated in Fig. 6A, 5-LO−/− mice exhibited increased levels of T-bet mRNA at day 15 p.i. (7.104 ± 1.042) compared to B6.129 (3.363 ± 0.101, P < 0.05). However, no differences were observed between the groups at 30 days after fungal challenge. We also observed that IL-12p40 and IFN-γ transcripts were significantly increased in 5-LO−/− mice on day 15 of infection (data not shown). To confirm these data, IL-12 and IFN-γ levels were measured in lung homogenate from Pb18-infected mice at different time points. Our results showed that 5-LO−/− mice exhibited a significant increase in IL-12p40, IL-12p70, and IFN-γ production (Fig. 6, B-D). Moreover, IL-6 levels were significantly reduced in 5-LO−/− mice at day 7 p.i., but no differences were observed for other analyzed time points (Fig. 6E). IL-10 levels were similar in both groups at all analyzed time points (Fig. 6F). Together, these data suggest that the absence of 5-LO induces a prominent Th1 protective response in our model of P. brasiliensis experimental infection.
Fig 6.
5-LO products modulate cytokine production during experimental PCM. B6.129 and 5-LO−/− mice were infected (i.v.) with 106 P. brasiliensis (Pb18) yeast cells. (A) T-bet transcript levels were measured by qRT-PCR in whole lung homogenate at days 15 and 30 p.i. Uninfected mice are represented by the dashed line. (B to F) The levels of IL-12p40 (B), IL-12p70 (C), IFN-γ (D), IL-6 (E), and IL-10 (F) were measured by ELISA in lung homogenate from B6.129 and 5-LO−/− mice at different time points after the fungal challenge. The data represent the means ± the SEM of 3 to 5 mice. *, P < 0.05 compared to B6.129 mice. Similar results were obtained in three independent experiments.
FPR2 blockade partially controls fungal growth during P. brasiliensis infection.
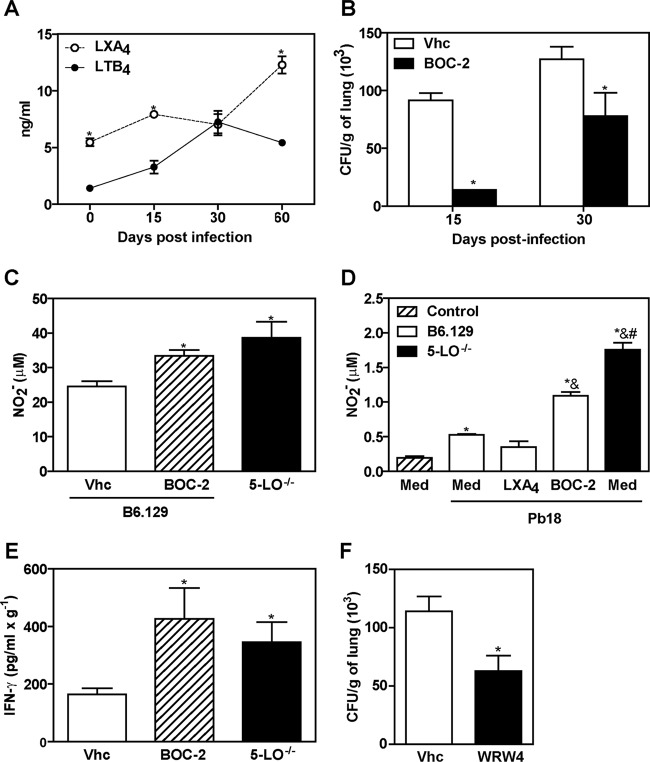
The imbalanced lipoxin and leukotriene production induced during 5-LO activation has been shown to play a critical role in the extension of the inflammatory response established against Mycobacterium tuberculosis (12, 26). To determine which 5-LO-product could be involved in susceptibility to P. brasiliensis, the levels of both LTB4 and LXA4 were measured in whole lung homogenates from infected B6.129 mice. As shown in Fig. 7A, the LXA4 levels were significantly increased at days 15 and 60 p.i. compared to LTB4 production at the same time points of infection. Because 5-LO-dependent LXA4 was preferentially produced in our model of fungal infection, we sought to verify whether the signaling through the lipoxin A4 receptor FPR2 is involved in susceptibility to P. brasiliensis. Initially, B6.129 mice were intraperitoneally treated with a nonselective antagonist of FPR2 receptor, Boc-Phe-Leu-Phe-Leu-Phe (BOC-2, 10 μg/kg/day), from day −1 until day 29 p.i. on every second day. On days 15 and 30 p.i., the number of CFU recovered from lungs was determined. Our results showed that BOC-2 treatment significantly decreased CFU counts compared to the untreated control group (Fig. 7B). In addition, NO levels were evaluated in whole lung homogenates at day 15 p.i. As shown in Fig. 7C, BOC-2 treatment resulted in increased NO production (24.58 ± 6.038) compared to untreated mice (33.39 ± 5.949, P < 0.05), which was also similar to the levels observed in 5-LO−/− mice (38.63 ± 18.81). To confirm these results, alveolar macrophages obtained from B6.129 and 5-LO−/− uninfected mice were cultured with medium alone or in the presence of P. brasiliensis at a 1:50 ratio of yeast per macrophage plus medium, LXA4 (100 μg/ml) or BOC-2 (50 μg/ml). NO levels were determined after 48 h. As shown in Fig. 7D, NO production was significantly increased when both B6.129 and 5-LO−/− alveolar macrophages were cultured with P. brasiliensis yeast cells. Moreover, the addition of LXA4 resulted in NO levels (0.348 ± 0.085) similar to B6.129 nonstimulated macrophages (0.1957 ± 0.023). Conversely, BOC-2 treatment significantly increased NO production (1.092 ± 0.09) compared to Pb18-stimulated B6.129 macrophages (0.5265 ± 0.028, P < 0.05) (Fig. 7D). Next, IFN-γ levels were measured in whole lung homogenates from B6.129 mice that were untreated or treated with BOC-2 at day 15 p.i. Our results showed increased IFN-γ production in BOC-2-treated mice compared to the vehicle-treated group (Fig. 7E). Since BOC-2 can also bind to the classical FPR receptor (27), we treated mice with WRW4, a specific FPR2 inhibitor (28). Corroborating our previous results, we found that disruption of the FPR2 signaling led to diminished fungal recovery in the lungs (P < 0.05) in comparison with B6.129 vehicle-treated (control) mice (Fig. 7F). Taken together, these data suggest that blockage of signaling through the lipoxin receptor FPR2 controls fungal growth during our model of P. brasiliensis infection.
Fig 7.
FPR2 blockade modulates the immune response during experimental PCM. B6.129 mice were infected (i.v.) with 106 P. brasiliensis (Pb18) yeast cells. (A) LTB4 and LXA4 levels were measured in lung homogenate at days 0, 15, 30, and 60 p.i. (B) The numbers of CFU were determined in the lung homogenates from P. brasiliensis-infected B6.129 mice intraperitoneally treated with vehicle (Vhc, 0.5% methanol) or BOC-2, a nonselective lipoxin receptor antagonist (10 μg/kg/day, 0.1 ml) from day −1 until 29 every 48 h. (C) Nitric oxide (NO) production was measured by the Griess method in the lung homogenates of 5-LO−/− and B6.129 mice treated with vehicle (Vhc) or BOC-2 at day 15 p.i. (D) NO production by alveolar macrophages (AM) obtained from uninfected 5-LO−/− and B6.129 mice. AM were cultured for 48 h with P. brasiliensis at a 1:50 ratio of yeast per macrophage plus medium, LXA4 (100 μg/ml), or BOC-2 (50 μg/ml). (E) IFN-γ levels were measured by ELISA in the lung homogenates at day 15 p.i. (F) The numbers of CFU were determined at day 30 post-P. brasiliensis infection in the lung homogenates from B6.129 mice intraperitoneally treated with vehicle (Vhc, 0.1% DMSO) or WRW4, a selective lipoxin receptor antagonist (1 mg/kg/day, 0.1 ml), from day −1 until 8 every 72 h. *, P < 0.05 compared to LTB4 production (A), B6.129 mice inoculated with Vhc solution (B, C, E, and F), or nonstimulated AM (D); &, P < 0.05 compared to B6.129 AM plus Pb18; #, P < 0.05 compared to B6.129 AM plus Pb18 and BOC-2. The data represent the means ± the SEM of 3 to 5 mice.
DISCUSSION
Leukotrienes and lipoxins are both involved in the modulation of immune responses during infectious diseases; while leukotrienes exhibit proinflammatory effects and increase antimicrobial defenses, lipoxins mediate anti-inflammatory responses and are associated with pathogen survival (29). In the present study, we investigated whether 5-LO-pathway products—leukotrienes and lipoxins—are involved in an experimental model of PCM induced by the i.v. inoculation of viable Pb18 yeast cells in mice. Although pulmonary PCM is usually acquired by the respiratory route, we have shown that lungs from intravenously infected mice are widely affected (21, 25, 30, 31), reproducing the characteristics of chronic pulmonary disease in humans and offering the advantages of reproducibility and standardization of the amount of fungal cells introduced into the host (32).
During PCM, the fungus P. brasiliensis is believed to grow inside immature granulomas and to further disseminate to different organs (20). After P. brasiliensis infection, the 5-LO−/− mice exhibited decreased CFU in the lungs, liver and spleen and an increased survival rate compared to the wild-type group (B6.129 mice). The protection observed in 5-LO−/− mice was associated with compact granuloma formation and IL-12, IFN-γ, and NOS-2 production. Thus, our results demonstrate for the first time that 5-LO-enzimatic activity contributes to the exacerbation of experimental PCM.
In addition, we found that the levels of 5-LO-pathway derived products —leukotrienes and lipoxins—were increased in B6.129-infected mice. However, the levels of LXA4 exceeded those of LTB4 in the lung homogenates of wild type-infected mice. Considering that the mortality rate and CFU counts in infected B6.129 mice were prominently increased compared to 5-LO−/− mice, these data indicate that 5-LO-derived products might have a critical role in the exacerbation and severity of PCM. Accordingly, one of the 5-LO-products (lipoxin) resulted in a diminished proinflammatory and Th1 response and increased susceptibility to experimental tuberculosis (12). The protective response observed in P. brasiliensis-infected 5-LO−/− mice indicates that the absence of both leukotrienes and lipoxins is favorable to more efficient fungal control. However, we did not administer stable LXA4 analogs to 5-LO-deficient mice to reverse the increased resistance of 5-LO−/− mice during P. brasiliensis infection, neither addressed the role of classical leukotrienes during experimental PCM. Thus, future research investigating the role of each 5-LO-derived lipid mediator in the course of P. brasiliensis infection would be enlightening.
We observed that infected 5-LO−/− mice developed compact granulomas that showed well-defined limits. In addition, these mice exhibited a decreased pulmonary lesion index characterized by diminished inflammatory infiltrates. A reduced inflammatory response was also reported in 5-LO−/− mice during infection with Trypanosoma cruzi (33), Mycobacterium tuberculosis (12), and Toxoplasma gondii (34). The granulomatous lesions are reservoirs of yeast cells that permit fungal survival in the host tissue, and the formation of compact granulomas is critical for the control of P. brasiliensis dissemination (20). We also observed an increased number of CD11b+ CD11c+ cells and significantly increased production of IL-12 and nitric oxide in 5-LO−/− mice at day 30 p.i. Given that 5-LO-dependent production of lipoxin promotes DC “paralysis,” a condition where these cells lose motility and the capacity to synthesize IL-12 (11), it is possible that the lipoxin levels observed in B6.129 mice are involved with paralysis during PCM. Accordingly, dendritic cells from 5-LO−/− mice infected with T. gondii did not exhibit paralysis (11), indicating that 5-LO activity is critical for this event to occur. Although we have not investigated the existence of paralysis in our model, the results observed in the 5-LO knockout mice infected with P. brasiliensis strongly indicate that this phenomenon might be implicated in the reduced production of IL-12 and NO, two important mediators involved in the resistance during experimental P. brasiliensis infection (25), in infected B6.129 mice.
In addition to the increased production of NO, we also observed that 5-LO−/− mice exhibited a significant decrease in arginase-1 transcripts compared to the B6.129 group. Given that NO and arginase-1 are expressed by classical (M1) or alternatively (M2) activated macrophages, respectively (35), these results suggest that 5-LO−/− mice might exhibit the predominant differentiation of M1 macrophages. Future research should determine the role of M1 and M2 cells and the prevalence of each subtype in 5-LO-deficient mice during P. brasiliensis infection. While M1 macrophages produce inflammatory cytokines and participate in the control of extracellular pathogens, M2 macrophages produce anti-inflammatory mediators and are involved with repair processes and termination of the immune response (36). During acute schistosomiasis, the blockage of arginase-1 was shown to inhibit M2 macrophage differentiation and resulted in the expansion of Treg cells (37), leading to the downmodulation of inflammatory responses. In fact, activated Treg cells can inhibit effector T cell proliferation and cytokine production (38).
We have previously observed that the severity of PCM in patients and mice is associated with the presence of FOXP3+ Treg lymphocytes in the host tissue (5, 6). These cells promote the persistence of P. brasiliensis in lesions and might contribute to further disseminated disease (5, 6). In our model, 5-LO−/− mice also exhibited a diminished number of Treg cells compared to B6.129 mice, indicating that the products of 5-LO activity appear to favor the expansion of regulatory T cells. Considering that IL-10 levels were similar in B6.129 and 5-LO−/− mice, it is unlikely that this cytokine is involved in the suppression of effector cells in this model as previously demonstrated (39).
Interestingly, B6.129 mice treated with selective or nonselective antagonists of the LXA4 receptor FPR2 (WRW4 and BOC-2, respectively) showed a significantly reduced fungal recovery in the lungs. Moreover, we observed that BOC-2 treatment restored NO and IFN-γ production. Taken together, our data suggest that FPR2 signaling could be a negative regulator of the immune response against fungal cells, and the activation of this pathway could contribute to susceptibility during P. brasiliensis experimental infection. Future studies can now be directed toward the investigation of the role of FPR2 receptors during PCM. In summary, the findings presented here show for the first time that 5-LO enzymatic activity increases susceptibility to P. brasiliensis and suggest that interruption of 5-LO signaling may represent a potential target for therapeutic intervention during paracoccidioidomycosis.
ACKNOWLEDGMENTS
We gratefully acknowledge the excellent technical support of Wander C. R. da Silva, Lúcia Pacheco, and Maria Elena Riul and the brilliant secretarial support of Ana Cristine S. Ferreira.
This study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). These funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 4 February 2013
REFERENCES
- 1. Restrepo A, McEwen JG, Castaneda E. 2001. The habitat of Paracoccidioides brasiliensis: how far from solving the riddle? Med. Mycol. 39:233–241 [DOI] [PubMed] [Google Scholar]
- 2. Bousquet A, Dussart C, Drouillard I, Charbel EC, Boiron P. 2007. Imported mycosis: a review of paracoccidioidomycosis. Med. Mal. Infect. 37(Suppl 3):S210–S214 [DOI] [PubMed] [Google Scholar]
- 3. Coutinho ZF, Silva D, Lazera M, Petri V, Oliveira RM, Sabroza C, Wanke B. 2002. Paracoccidioidomycosis mortality in Brazil (1980–1995). Cad Saude Publica 18:1441–1454 [DOI] [PubMed] [Google Scholar]
- 4. Ramos-e-Silva M, Saraiva LES. 2008. Paracoccidioidomycosis. Dermatol. Clin. 26:257–269 [DOI] [PubMed] [Google Scholar]
- 5. Cavassani KA, Campanelli AP, Moreira AP, Vancim JO, Vitali LH, Mamede RC, Martinez R, Silva JS. 2006. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J. Immunol. 177:5811–5818 [DOI] [PubMed] [Google Scholar]
- 6. Moreira AP, Cavassani KA, Tristao FSM, Campanelli AP, Martinez R, Rossi MA, Silva JS. 2008. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J. Immunol. 180:3049–3056 [DOI] [PubMed] [Google Scholar]
- 7. Zarini S, Gijon MA, Ransome AE, Murphy RC, Sala A. 2009. Transcellular biosynthesis of cysteinyl leukotrienes in vivo during mouse peritoneal inflammation. Proc. Natl. Acad. Sci. U. S. A. 106:8296–8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serhan CN. 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25:101–137 [DOI] [PubMed] [Google Scholar]
- 9. Peters-Golden M, Brock TG. 2003. 5-Lipoxygenase and FLAP. Prostaglandins Leukot. Essent. Fatty Acids 69:99–109 [DOI] [PubMed] [Google Scholar]
- 10. Lewis RA, Austen KF, Soberman RJ. 1990. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 323:645–655 [DOI] [PubMed] [Google Scholar]
- 11. Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. 2002. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat. Immunol. 3:76–82 [DOI] [PubMed] [Google Scholar]
- 12. Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. 2005. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J. Clin. Invest. 115:1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurita N, Sano A, Coelho KI, Takeo K, Nishimura K, Miyaji M. 1993. An improved culture medium for detecting live yeast phase cells of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 3:201–205 [DOI] [PubMed] [Google Scholar]
- 14. Singer-Vermes LM, Ciavaglia MC, Kashino SS, Burger E, Calich VL. 1992. The source of the growth-promoting factor(s) affects the plating efficiency of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 30:261–264 [DOI] [PubMed] [Google Scholar]
- 15. Calich VL, Purchio A, Paula CR. 1979. A new fluorescent viability test for fungi cells. Mycopathologia 66:175–177 [DOI] [PubMed] [Google Scholar]
- 16. Pamplona FA, Menezes-de-Lima O, Jr, Takahashi RN. 2010. Aspirin-triggered lipoxin induces CB1-dependent catalepsy in mice. Neurosci. Lett. 470:33–37 [DOI] [PubMed] [Google Scholar]
- 17. Stenfeldt AL, Karlsson J, Wenneras C, Bylund J, Fu H, Dahlgren C. 2007. Cyclosporin H, Boc-MLF, and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation 30:224–229 [DOI] [PubMed] [Google Scholar]
- 18. Green LC, Tannenbaum SR, Goldman P. 1981. Nitrate synthesis in the germfree and conventional rat. Science 212:56–58 [DOI] [PubMed] [Google Scholar]
- 19. Pina A, Bernardino S, Calich VL. 2008. Alveolar macrophages from susceptible mice are more competent than those of resistant mice to control initial Paracoccidioides brasiliensis infection. J. Leukoc. Biol. 83:1088–1099 [DOI] [PubMed] [Google Scholar]
- 20. Moreira AP, Campanelli AP, Cavassani KA, Souto JT, Ferreira BR, Martinez R, Rossi MA, Silva JS. 2006. Intercellular adhesion molecule-1 is required for the early formation of granulomas and participates in the resistance of mice to the infection with the fungus Paracoccidioides brasiliensis. Am. J. Pathol. 169:1270–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Souto JT, Aliberti JC, Campanelli AP, Livonesi MC, Maffei CM, Ferreira BR, Travassos LR, Martinez R, Rossi MA, Silva JS. 2003. Chemokine production and leukocyte recruitment to the lungs of Paracoccidioides brasiliensis-infected mice is modulated by interferon-gamma. Am. J. Pathol. 163:583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robb RJ, Munck A, Smith KA. 1981. T cell growth factor receptors: quantitation, specificity, and biological relevance. J. Exp. Med. 154:1455–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nascimento FR, Calich VL, Rodriguez D, Russo M. 2002. Dual role for nitric oxide in paracoccidioidomycosis: essential for resistance, but overproduction associated with susceptibility. J. Immunol. 168:4593–4600 [DOI] [PubMed] [Google Scholar]
- 24. Jansen A, Lewis S, Cattell V, Cook HT. 1992. Arginase is a major pathway of l-arginine metabolism in nephritic glomeruli. Kidney Int. 42:1107–1112 [DOI] [PubMed] [Google Scholar]
- 25. Livonesi MC, Souto JT, Campanelli AP, Maffei CM, Martinez R, Rossi MA, Silva JS. 2008. Deficiency of IL-12p40 subunit determines severe paracoccidioidomycosis in mice. Med. Mycol. 46:637–646 [DOI] [PubMed] [Google Scholar]
- 26. Peres CM, de Paula L, Medeiros AI, Sorgi CA, Soares EG, Carlos D, Peters-Golden M, Silva CL, Faccioli LH. 2007. Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis. Microbes Infect. 9:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gavins FN, Yona S, Kamal AM, Flower RJ, Perretti M. 2003. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood 101:4140–4147 [DOI] [PubMed] [Google Scholar]
- 28. Bae YS, Lee HY, Jo EJ, Kim JI, Kang HK, Ye RD, Kwak JI, Ryu SH. 2004. Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J. Immunol. 173:607–614 [DOI] [PubMed] [Google Scholar]
- 29. Aliberti J, Bafica A. 2005. Anti-inflammatory pathways as a host evasion mechanism for pathogens. Prostaglandins Leukot. Essent. Fatty Acids 73:283–288 [DOI] [PubMed] [Google Scholar]
- 30. Panagio LA, Tristao FSM, Moreira AP, Pereira MS, Cavassani KA, Milanezi CM, Rossi MA, Silva JS. 2008. Role of interleukin (IL)-18 in experimental paracoccidioidomycosis. Med. Mycol. 46:435–442 [DOI] [PubMed] [Google Scholar]
- 31. Cavassani KA, Tristao FSM, Oliveira LL, Rocha FA, Vancim JO, Moreira AP, Campanelli AP, Panagio LA, Milanezi CM, Martinez R, Rossi MA, Silva JS. 2011. Cell-free antigens from Paracoccidioides brasiliensis drive IL-4 production and increase the severity of paracoccidioidomycosis. PLoS One doi:10.1371/journal.pone.0021423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moscardi-Bacchi M, Franco M. 1985. Experimental paracoccidioidomycosis in the mouse. III. Histopathological and immunological findings after intravenous infection in the presence or absence of previous immunization. Rev. Soc. Bras. Med. Trop. 18:101–108 [Google Scholar]
- 33.Pavanelli WR, Gutierrez FR, Mariano FS, Prado CM, Ferreira BR, Teixeira MM, Canetti C, Rossi MA, Cunha FQ, Silva JS. 2010. 5-Lipoxygenase is a key determinant of acute myocardial inflammation and mortality during Trypanosoma cruzi infection. Microbes Infect. 12:587–597 [DOI] [PubMed] [Google Scholar]
- 34. Aliberti J, Serhan C, Sher A. 2002. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 196:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. 2001. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 167:6533–6544 [DOI] [PubMed] [Google Scholar]
- 36. Martinez FO, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front. Biosci. 13:453–461 [DOI] [PubMed] [Google Scholar]
- 37. Herbert DR, Orekov T, Roloson A, Ilies M, Perkins C, O'Brien W, Cederbaum S, Christianson DW, Zimmermann N, Rothenberg ME, Finkelman FD. 2010. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J. Immunol. 184:6438–6446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu G, Liu Z, Zheng C, Zheng SG. 2010. Antigen-nonspecific regulation centered on CD25+ Foxp3+ Treg cells. Cell Mol. Immunol. 7:414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]