Summary
Background
Bisphosphonates reduce the risk of skeletal events in patients with malignant bone disease, and zoledronic acid has shown potential anticancer effects in preclinical and clinical studies. We aimed to establish whether bisphosphonates can affect clinical outcomes in patients with multiple myeloma.
Methods
Patients of age 18 years or older with newly diagnosed multiple myeloma were enrolled from 120 centres in the UK. Computer-generated randomisation sequence was used to allocate patients equally, via an automated telephone service, to receive 4 mg zoledronic acid as an infusion every 3–4 weeks or 1600 mg oral clodronic acid daily. Patients also received intensive or non-intensive induction chemotherapy. No investigators, staff, or patients were masked to treatment allocation, and bisphosphonate and maintenance therapy continued at least until disease progression. The primary endpoints were overall survival, progression-free survival, and overall response rate. We assessed between-group differences with Cox proportional hazards models for progression-free survival and overall survival, and with logistic regression models for overall response rate. Analysis was by intention to treat. This trial is registered, number ISRCTN68454111.
Findings
1970 patients were enrolled between May, 2003, and November, 2007, of whom 1960 were eligible for intention-to-treat analysis: 981 in the zoledronic acid group (555 on intensive chemotherapy, 426 on non-intensive chemotherapy); and 979 on clodronic acid (556 on intensive chemotherapy, 423 on non-intensive chemotherapy). The treatment cutoff was Oct 5, 2009, with patients receiving bisphosphonates for a median of 350 days (IQR 137–632) before disease progression, with a median of 3·7 years' follow-up (IQR 2·9–4·7). Zoledronic acid reduced mortality by 16% (95% CI 4–26) versus clodronic acid (hazard ratio [HR] 0·84, 95% CI 0·74–0·96; p=0·0118), and extended median overall survival by 5·5 months (50·0 months, IQR 21·0 to not reached vs 44·5 months, IQR 16·5 to not reached; p=0·04). Zoledronic acid also significantly improved progression-free survival by 12% (95% CI 2–20) versus clodronic acid (HR 0·88, 95% CI 0·80–0·98; p=0·0179), and increased median progression-free survival by 2·0 months (19·5 months, IQR 9·0–38·0 vs 17·5 months, IQR 8·5–34·0; p=0·07). Rates of complete, very good partial, or partial response did not differ significantly between the zoledronic acid and clodronic acid groups for patients receiving intensive induction chemotherapy (432 patients [78%] vs 422 [76%]; p=0·43) or non-intensive induction chemotherapy (215 [50%] vs 195 [46%]; p=0·18). Both bisphosphonates were generally well tolerated, with similar occurrence of acute renal failure and treatment-emergent serious adverse events, but zoledronic acid was associated with higher rates of confirmed osteonecrosis of the jaw (35 [4%]) than was clodronic acid (3 [<1%]).
Interpretation
Consistent with the potential anticancer activity of zoledronic acid, overall survival improved independently of prevention of skeletal-related events, showing that zoledronic acid has treatment benefits beyond bone health. These findings support immediate treatment with zoledronic acid in patients with newly diagnosed multiple myeloma, not only for prevention of skeletal-related events, but also for potential antimyeloma benefits.
Funding
Medical Research Council (London, UK), with unrestricted educational grants from Novartis, Schering Health Care, Chugai, Pharmion, Celgene, and Ortho Biotech.
Introduction
Myeloma is a plasma-cell malignant disease that can result in osteolytic lesions, excess immunoglobulin secretion, impaired renal function, and myelosuppression.1 Myeloma plasma cells live in the bone marrow microenvironment1,2 and can secrete factors that stimulate osteoclast-mediated osteolysis,2 placing patients at risk for skeletal-related events such as fractures and bone pain requiring palliative radiotherapy.3
Bisphosphonates were developed mainly to impair malignant osteolysis, thereby breaking the cycle of bone destruction and cancer growth that can result in skeletal-related events.4 By blocking growth-factor release from the bone matrix, bisphosphonates can indirectly impede myeloma growth.5 Preclinical evidence suggests that bisphosphonates—especially nitrogen-containing bisphosphonates such as zoledronic acid—might have inherent anticancer activities.4,6 Moreover, zoledronic acid significantly extended survival in two mouse models of multiple myeloma.7,8 The antimyeloma effect was independent of the effect of zoledronic acid on bone, but dependent on inhibition of protein prenylation,8 a mechanism of action not shared by non-nitrogen-containing bisphosphonates such as clodronic acid (clodronate).4
Although differences in overall survival with bisphosphonates were not significant in the full populations of large randomised controlled trials in multiple myeloma,9–12 bisphosphonates seemed to improve overall survival in subsets of patients in phase 3 studies.9–11 For example, in the UK Medical Research Council (MRC) trial in patients with bone lesions from multiple myeloma (n=535), overall survival was similar between clodronic acid and placebo in the full population, but clodronic acid significantly improved overall survival versus placebo in the subset of patients who had not had fractures before study entry (n=153; p=0·006).11 Furthermore, in large-scale randomised trials in patients undergoing adjuvant endocrine therapy for early breast cancer, zoledronic acid significantly extended disease-free survival (n=1803 and n=1065),13,14 and clodronic acid extended overall survival in patients with metastatic prostate cancer (n=278).15 Moreover, zoledronic acid showed anticancer synergy with some chemotherapy agents in preclinical assays.6 Therefore, bisphosphonates might affect the disease course of multiple myeloma.
Despite strong consensus that antimyeloma therapies should be given to symptomatic patients with multiple myeloma,16,17 no optimal regimen has emerged. Therefore, we designed the MRC Myeloma IX trial, an innovative study with two randomisation steps to allow comparison of both first-line and maintenance treatments for adult patients with newly diagnosed multiple myeloma. The first randomisation step compared oral clodronic acid and an intravenous bisphosphonate with respect to overall survival, progression-free survival, response rates, and skeletal-related events. Of the two intravenous bisphosphonates approved for prevention of skeletal-related events in multiple myeloma, pamidronic acid (pamidronate) and zoledronic acid, we selected zoledronic acid because it has higher antiresorbtive activity and shorter infusion time than does pamidronic acid.4,12
Methods
Patients
In the MRC Myeloma IX trial, adult patients of age 18 years or older who had newly diagnosed and histologically confirmed symptomatic multiple myeloma were enrolled from 120 centres in the UK. Patients were excluded if they had previous or concurrent active malignancies, had acute renal failure (serum creatinine >500 μmol/L and unresponsive to 72 h of rehydration, urine output <400 mL/day, or need for dialysis), had previously received treatment for myeloma (apart from local radiotherapy, bisphosphonates, or low-dose corticosteroids), or were pregnant or lactating. A strict risk-management programme was adopted, and all patients who were women of childbearing potential, or were men engaging in heterosexual activity with a woman of childbearing potential, had to use contraception.
The trial was approved by the North West Multi-centre Research Ethics Committee, and by local review committees at all participating centres. All patients provided written informed consent.
Randomisation and masking
The Clinical Trials Research Unit of the University of Leeds, Leeds, UK, used a computer-generated randomisation sequence to assign treatment groups via an automated telephone service. The trial had a two-by-two factorial design, with two randomisation steps and equal allocation to two groups at each randomisation step. The first randomisation step compared first-line treatments, and the second compared maintenance treatments. The first randomisation used a minimisation algorithm stratified by centre, haemoglobin (<115 vs ≥115 g/L for men, <95 vs ≥95 g/L for women), serum calcium (<2·6 vs ≥2·6 mmol/L; corrected for serum albumin), serum creatinine (<140 vs ≥140 μmol/L), and platelet count (<150×109/L or ≥150×109/L). The maintenance randomisation used a minimisation algorithm stratified by centre and treatment group allocated at first randomisation. In this open-label trial, no investigators, staff at participating centres, or patients were masked to treatment allocation apart from individuals analysing treatment response from laboratory results.
Procedures
Before randomisation, patients were allocated to receive induction chemotherapy via one of two pathways, intensive and non-intensive. Pathway selection had no rigid age cutoff and was based on performance status, clinician judgment, and patient preference. The intensive pathway consisted of four–six 21-day cycles of either CVAD (500 mg oral cyclophosphamide per week, 0·4 mg vincristine daily combined with 9 mg/m2 doxorubicin daily as a 4-day continuous infusion, and 40 mg dexamethasone daily on days 1–4 and 12–15), or oral CTD (500 mg cyclophosphamide per week, 100 mg thalidomide daily and increasing to 200 mg daily as tolerated, and 40 mg dexamethasone daily on days 1–4 and 12–15). After completion of induction therapy, patients underwent peripheral blood stem-cell mobilisation and harvest, high-dose melphalan treatment (200 mg/m2), and autologous stem-cell transplantation. The non-intensive pathway consisted of six–nine 28-day cycles of either oral MP (7 mg/m2 melphalan and 40 mg prednisolone, both on days 1–4), or attenuated oral CTD (CTDa; 500 mg cyclophosphamide per week, 50 mg thalidomide daily initially and increasing to 200 mg daily as tolerated, and 20 mg dexamethasone daily on days 1–4 and 15–18); treatment was continued until best response.
In the first randomisation, patients in the intensive pathway were allocated to receive either clodronic acid or zoledronic acid plus induction chemotherapy with either CVAD or CTD, and patients in the non-intensive pathway were allocated to receive either clodronic acid or zoledronic acid plus induction chemotherapy with either MP or CTDa. Clodronic acid was given at a dose of 1600 mg per day orally, and zoledronic acid at a dose of 4 mg as a 15-min infusion every 3–4 weeks during induction chemotherapy and every 4 weeks thereafter. The dose of zoledronic acid was adjusted for patients with impaired renal function at baseline, and was delayed in patients with raised creatinine concentrations during the study, as per the prescribing information. After first-line therapy (about 100 days after autologous stem-cell transplantation in the intensive pathway), the second randomisation allocated eligible patients (no disease progression, and opted to continue study treatment according to the protocol) to receive either maintenance therapy with 50 mg thalidomide daily and increasing to 100 mg daily if tolerated, or no further treatment. Bisphosphonates and maintenance therapy were given continuously at least until disease progression.
We followed the recommendations of Weitzman and colleagues18 to reduce the risk of osteonecrosis of the jaw and to identify and manage such cases.18 All suspected cases of osteonecrosis of the jaw were referred to a dental professional for diagnosis and management, and reports were centrally reviewed by an investigator.
The primary endpoints were overall survival, progression-free survival, and overall response rate. Secondary endpoints included skeletal-related events and toxic effects. Overall survival and progression-free survival were calculated from first randomisation to death or to progression or death, respectively. Patients with missing follow-up data were censored at the last date they were known to be alive (overall survival), or alive without disease progression.
In both the intensive and non-intensive pathways, disease was assessed after each cycle of induction chemotherapy (before high-dose melphalan and autologous stem-cell transplantation in the intensive pathway), and every 3 months thereafter, with an assessment at 100 days after transplantation in the intensive pathway. Treatment response was monitored centrally at the University of Birmingham, Birmingham, UK, from serum and urine protein and free light-chain studies, or, if results of such studies were missing, by review of local laboratory results in which results were verified by an independent response-assessment panel. Assessors at the central laboratory were masked with respect to treatment; assessors of local laboratory results had access to treatment allocation information, but such information was not taken into consideration in assessment of response. Responses and disease progression were defined according to international response criteria.19 Briefly, complete response was defined as negative immunofixation (100% M-protein reduction), and very good partial response was defined as at least 90% M-protein reduction with positive immunofixation.19 Safety was assessed by continuous monitoring of adverse events. Serum creatinine was monitored monthly during induction chemotherapy and, thereafter, monthly for zoledronic acid and every 3 months for clodronic acid. Serious adverse events were defined as treatment emergent if they were judged by the treating physician to be potentially related to study drugs. Data for skeletal-related events, defined as vertebral fractures, other fractures, spinal cord compression, need for radiation or surgery to bone lesions, and new osteolytic bone lesions, were recorded until disease progression.
Statistical analysis
The sample size was calculated on the basis of the comparisons in chemotherapy regimens in the factorial design. In the intensive pathway, we aimed to recruit 1080 patients (540 per group) to test the hypothesis that CTD was not inferior to CVAD, with a hazard ratio (HR) of 1·2 and 80% power at a 5% significance level. In the non-intensive pathway, we aimed to recruit 850 patients (425 per group) to assess whether CTDa was superior to standard chemotherapy with MP, with 80% power at a 5% significance level. We calculated that the sample size for the two pathways combined had sufficient power (>80%) to detect a reduction of 10% in the proportion of patients with skeletal-related events in patients on zoledronic acid compared with those on clodronic acid.
Analyses were based on the treatments that patients were randomised to receive and the intention-to-treat population, defined as all randomised patients with histologically confirmed multiple myeloma who provided written informed consent. Between-group comparisons for overall survival and progression-free survival were done with Cox regression models stratified by pathway and adjusted for minimisation factors (including treatment centre) and type of induction chemotherapy. Between-group comparisons in overall response rate were assessed with logistic-regression models. We generated a Cox model for skeletal-related events, which included the minimisation factors, chemotherapy, and history of skeletal-related events at baseline (stratified by pathway); this model was used to calculate p values. A post-hoc analysis compared overall survival between patients randomised to receive zoledronic acid versus clodronic acid, with adjustment for time-dependent differences in rates of skeletal-related events.
Cox models were assessed for statistical violations. Adjustment for treatment centre in the Cox models of overall survival and progression-free survival was the only factor identified: a small treatment centre (n=12) reported unfavourable mortality results for patients treated with clodronic acid with a HR of 3·46 versus zoledronic acid. Additional exploratory analyses were done to validate the results when stratification by treatment centre was not included (based on the model violation), and to examine the relative effects of zoledronic acid versus clodronic acid during the first few months on the study drug in response to rapid separation of the overall survival curves. Resultant exploratory analyses focused on the first 4 months and included Kaplan-Meier assessments of overall survival and progression-free survival and assessments of early deaths (within the first 120 days on study drug) that were related to treatment or multiple myeloma.
The Kaplan-Meier analyses for overall survival were stratified by intensive versus non-intensive induction chemotherapy, with p values calculated by the log-rank test. HRs for overall and progression-free survival were developed with Cox proportional hazards models, stratified by pathway and adjusted for minimisation factors (treatment centre, haemoglobin, corrected serum calcium, serum creatinine, platelet count, and type of chemotherapy); p values were based on these models. For overall response rate, p values were calculated by logistic regression adjusted for minimisation factors (excluding treatment centre for exploratory post-hoc analyses because few patients were enrolled at some treatment centres) and chemotherapy. For between-group comparisons of adverse events, p values were calculated by Fisher's exact test.
Statistical analyses were done with SAS (version 9.2) and Digital Visual Fortran software (version 6.0A). All tests were two-sided and at the 5% significance level, without adjustment for multiplicity.
This trial is registered, number ISRCTN68454111.
Role of the funding source
None of the funding organisations was involved in the study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. All authors had full access to trial data, and the chief investigators had the final responsibility for the decision to submit for publication.
Results
Between May, 2003, and November, 2007, 1970 patients were recruited, of whom 1960 were eligible for analysis by intention to treat (figure 1). Demographic and disease characteristics were well balanced between the zoledronic acid and clodronic acid groups at baseline (table 1). Patients in the intensive pathway were generally younger and had more aggressive bone disease than did those in the non-intensive pathway. Overall, most patients (about 97%, n=1898) were white, and 71% (n=1401) had myeloma bone disease.
Figure 1.
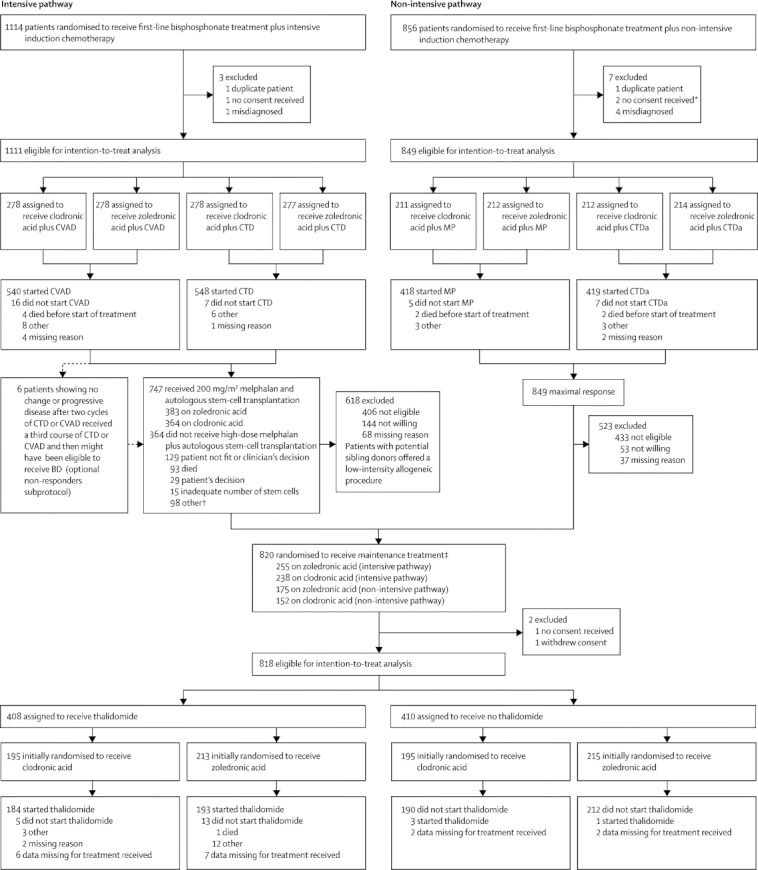
Trial profile
*Patients were included in the safety population. †Two patients on zoledronic acid received a conditioning regimen other than high-dose melphalan and underwent subsequent autologous stem-cell transplantation. ‡One patient in the non-intensive pathway who was excluded because no consent was received was then included in the randomisation to receive maintenance treatment; some of the 23 patients in the intensive pathway who did not start CVAD or CTD did receive high-dose melphalan plus autologous stem-cell transplantation, but none was included in the randomisation to receive maintenance treatment; none of the 12 patients in the non-intensive pathway who did not start MP or CTDa was included in the randomisation to receive maintenance treatment. CVAD=cyclophosphamide, vincristine, doxorubicin, and dexamethasone. CTD=cyclophosphamide, thalidomide, and dexamethasone. MP=melphalan and prednisolone. CTDa=attenuated CTD. BD=bortezomib and dexamethasone.
Table 1.
Demographic and disease characteristics at baseline (intention-to-treat population)
|
Intensive pathway |
Non-intensive pathway |
||||
|---|---|---|---|---|---|
| Zoledronic acid (n=555) | Clodronic acid (n=556) | Zoledronic acid (n=426) | Clodronic acid (n=423) | ||
| Age (years; median [range, IQR]) | 59 (31–74, 53–63) | 59 (33–78, 53–63) | 73 (59–89, 70–77) | 73 (57–88, 70–77) | |
| Sex | |||||
| Female | 201 (36%) | 218 (39%) | 191 (45%) | 185 (44%) | |
| Male | 354 (64%) | 338 (61%) | 235 (55%) | 238 (56%) | |
| ISS disease stage | |||||
| I | 129 (23%) | 146 (26%) | 63 (15%) | 47 (11%) | |
| II | 198 (36%) | 182 (33%) | 139 (33%) | 173 (41%) | |
| III | 174 (31%) | 169 (30%) | 173 (41%) | 160 (38%) | |
| Data unavailable | 54 (10%) | 59 (11%) | 51 (12%) | 43 (10%) | |
| β2-microglobulin (mg/L) | 4 (3–7) | 4 (3–7) | 5 (3–8) | 5 (4–8) | |
| Data unavailable | 43 (8%) | 52 (9%) | 47 (11%) | 37 (9%) | |
| Myeloma subtype | |||||
| IgG | 332 (60%) | 328 (59%) | 253 (59%) | 252 (60%) | |
| IgM | 2 (<1%) | 3 (1%) | 1 (<1%) | 2 (<1%) | |
| IgA | 125 (23%) | 116 (21%) | 99 (23%) | 102 (24%) | |
| IgD | 12 (2%) | 13 (2%) | 9 (2%) | 4 (1%) | |
| Light chain or non-expressing | 78 (14%) | 89 (16%) | 58 (14%) | 57 (13%) | |
| Data unavailable | 6 (1%) | 7 (1%) | 6 (1%) | 6 (1%) | |
| Bone disease | |||||
| Yes | 404 (73%) | 411 (74%) | 291 (68%) | 295 (70%) | |
| No | 149 (27%) | 138 (25%) | 130 (31%) | 123 (29%) | |
| Data unavailable | 2 (<1%) | 7 (1%) | 5 (1%) | 5 (1%) | |
| Bone pain | |||||
| Yes | 428 (77%) | 415 (75%) | 275 (65%) | 287 (68%) | |
| No | 120 (22%) | 132 (24%) | 147 (35%) | 131 (31%) | |
| Data unavailable | 7 (1%) | 9 (2%) | 4 (1%) | 5 (1%) | |
| Calcium after hydration (mmol/L) | 2·4 (2·2–2·5) | 2·4 (2·3–2·5) | 2·4 (2·2–2·5) | 2·4 (2·3–2·5) | |
| Data unavailable* | 37 (7%) | 51 (9%) | 37 (9%) | 37 (9%) | |
| Creatinine after hydration (μmol/L) | 100 (84–128) | 96 (79–122) | 101 (82–128) | 105 (88–137) | |
| Data unavailable* | 38 (7%) | 48 (9%) | 32 (8%) | 34 (8%) | |
| Haemoglobin (g/L) | 110 (90–120) | 110 (90–120) | 100 (90–120) | 110 (100–120) | |
| Data unavailable* | 19 (3%) | 20 (4%) | 20 (5%) | 15 (4%) | |
| Platelets (×109/L) | 237 (186–308) | 246 (194–310) | 226 (177–292) | 230 (177–292) | |
| Data unavailable* | 19 (3%) | 20 (4%) | 20 (5%) | 15 (4%) | |
Data are median (IQR) or number of patients (%), unless otherwise indicated. ISS=international staging system.
For all variables included in the minimisation algorithm, the stratification category (eg, serum calcium <2·6 vs ≥2·6 mmol/L) was recorded for all patients, even if the exact value was not documented.
By the cutoff date of Oct 5, 2009, most patients remained on treatment until disease progression or the end of the study period (n=746 for zoledronic acid, n=794 for clodronic acid). Median follow-up was 3·7 years (IQR 2·9–4·7), with patients receiving bisphosphonates for a median of 350 days (IQR 137–632) before disease progression (table 2). Only 14 patients, five on zoledronic acid and nine on clodronic acid, had less than 12 months' follow-up.
Table 2.
Treatment status
| Zoledronic acid (n=981) | Clodronic acid (n=979) | ||
|---|---|---|---|
| Follow-up (years) | 3·7 (2·8–4·7) | 3·8 (2·9–4·7) | |
| Still receiving bisphosphonate | 111 (11%) | 132 (13%) | |
| Administration of bisphosphonate not confirmed | 54 (6%) | 36 (4%) | |
| Discontinued study before disease progression | 235 (24%) | 185 (19%) | |
| Disease progression or death | 581 (59%) | 626 (64%) | |
| Time on treatment (days) | |||
| Intensive pathway | 396 (152–737) | 409 (152–727) | |
| Non-intensive pathway | 320 (138–520) | 306 (111–505) | |
Data are median (IQR) or number (%).
Our assumption that the intensity of induction chemotherapy would have no interaction with differences in the endpoint of overall survival with zoledronic acid versus clodronic acid was valid (p=0·74 for the intensive pathway, p=0·13 for the non-intensive pathway), enabling the data to be analysed as a factorial trial, as per the prespecified statistical plan. Proportional hazards were assessed by plotting HRs over time for each pathway and the model remained valid, with the possible exception of overall survival in the non-intensive pathway. In this pathway, although the hazards separated significantly from proportionality for type of induction chemotherapy (p=0·011), the hazards did not separate significantly from proportionality (p=0·30) during the first 4 months of treatment, wherein the main effect on overall survival with zoledronic acid was recorded.
Overall survival significantly improved with zoledronic acid compared with clodronic acid during the full follow-up period (figure 2A) and during the first 4 months of treatment (figure 2B). During the full follow-up period, zoledronic acid reduced mortality by 16% relative to clodronic acid (figure 3). Other significant predictors of favourable overall survival were haemoglobin of 115 g/L or greater for men and of 95 g/L or greater for women (HR 0·75, 95% CI 0·65–0·86; p<0·0001), corrected serum calcium of less than 2·6 mmol/L (0·69, 0·59–0·80; p<0·0001), serum creatinine of less than 140 μmol/L (0·75, 0·64–0·87; p=0·0003), and platelet count of 150×109/L or higher (0·77, 0·64–0·93; p=0·0056).
Figure 2.
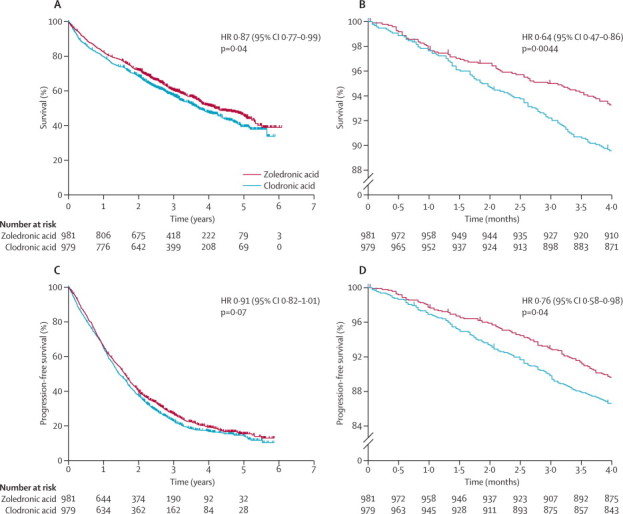
Kaplan-Meier curves for patients randomised to zoledronic acid and clodronic acid for overall survival during the full follow-up period (A), overall survival during the first 4 months of treatment (B), progression-free survival during the full follow-up period (C), and progression-free survival during the first 4 months of treatment (D)
HR=hazard ratio.
Figure 3.
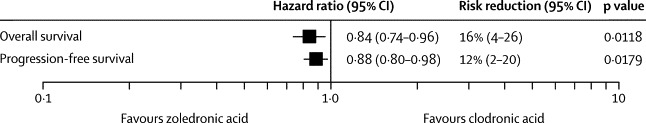
Hazard ratios for overall survival and progression-free survival for zoledronic acid versus clodronic acid during the full follow-up period
Although the study was not powered to detect a significant difference between drug groups in the intensive and non-intensive pathways, overall survival was longer with zoledronic acid than with clodronic acid, although not significantly so, in both the intensive (HR 0·84, 95% CI 0·68–1·03; p=0·0854) and non-intensive pathways (0·83, 0·69–1·00; p=0·0492; table 3). Overall, zoledronic acid extended median overall survival by 5·5 months to 50·0 months (IQR 21·0 to not reached) compared with clodronic acid (44·5 months, IQR 16·5 to not reached; figure 2A). During the first 4 months of treatment, overall survival curves clearly separated between the drug groups (figure 2B). When stratification by treatment centre was omitted, the benefit of zoledronic acid to overall survival remained significant compared with clodronic acid (HR 0·87, 95% CI 0·77–0·99, p=0·037). For patients in the intensive and non-intensive pathways combined, more early deaths related to treatment regimen or multiple myeloma occurred with clodronic acid than with zoledronic acid (p=0·0008; table 4). During this time, significantly fewer patients on zoledronic acid had died of renal failure (p<0·0001), and fewer had died of infection, although the difference was not significant (p=0·08), than did those on clodronic acid (table 4).
Table 3.
Overall and progression-free survival (intention-to-treat population)
|
Intensive pathway |
Non-intensive pathway |
Overall |
||||
|---|---|---|---|---|---|---|
| Zoledronic acid (n=555) | Clodronic acid (n=556) | Zoledronic acid (n=426) | Clodronic acid (n=423) | Zoledronic acid (n=981) | Clodronic acid (n=979) | |
| Overall survival | NR (61·0–NR) | 62·5 (53·5–NR) | 33·5 (29·5–36·5) | 29·5 (25·5–34·0) | 50·0 (46·0–60·5) | 44·5 (42·0–51·5) |
| Progression-free survival | 25·0 (23·5–28·5) | 25·0 (22·5–27·0) | 13·5 (12·0–14·0) | 12·5 (11·5–13·5) | 19·5 (18·0–21·0) | 17·5 (16·5–19·5) |
Data are median months (95% CI). NR=not reached.
Table 4.
Primary causes of deaths within the first 120 days after randomisation
|
Intensive pathway |
Non-intensive pathway |
Overall |
|||||
|---|---|---|---|---|---|---|---|
| Zoledronic acid (n=33) | Clodronic acid (n=44) | Zoledronic acid (n=32) | Clodronic acid (n=57) | Zoledronic acid (n=65) | Clodronic acid (n=101) | ||
| Related to multiple myeloma or treatment | 27 | 37 | 20 | 47 | 47 | 84 | |
| Overwhelming cancer load | 7 | 6 | 6 | 10 | 13 | 16 | |
| Infection | 14 | 19 | 13 | 23 | 27 | 42 | |
| Renal failure | 0 | 5 | 0 | 11 | 0 | 16 | |
| Other | 6 | 7 | 1 | 3 | 7 | 10 | |
| Not related to multiple myeloma or treatment | 6 | 7 | 12 | 10 | 18 | 17 | |
Zoledronic acid significantly improved progression-free survival by 12% (figure 3), and extended median progression-free survival by 2·0 months to 19·5 months (IQR 9·0–38·0) compared with clodronic acid (17·5 months, IQR 8·5–34·0). Although the difference between drug groups was not significant for the full follow-up period (figure 2C), early separation was recorded between the progression-free survival curves (figure 2D). Progression-free survival was non-significantly extended with zoledronic acid as compared with clodronic acid in both the intensive (HR 0·90, 95% CI 0·78–1·05) and non-intensive pathways (0·87, 0·74–1·01). We did not record any significant interactions between the four regimens of first-line bisphosphonate treatment plus induction chemotherapy and the differences in overall and progression-free survival with zoledronic acid versus clodronic acid.
611 patients had a skeletal-related event before disease progression (27% [265/981] on zoledronic acid; 35% [346/979] on clodronic acid; p=0·0004). In an exploratory analysis, we generated a Cox model including first skeletal-related event as a time-dependent covariate, which showed that improvement in overall survival with zoledronic acid versus clodronic acid remained significant (HR 0·85, 95% CI 0·74–0·97; p=0·018).
Overall response rates were higher with intensive than with non-intensive induction chemotherapy, irrespective of bisphosphonate treatment (table 5).19 For non-intensive therapy, zoledronic acid had a significantly increased rate of complete or very good partial response, and had a higher rate of complete response than did clodronic acid, although the difference was not significant. For intensive therapy, rates of complete or very good partial response were not significantly different between drug groups.
Table 5.
Response rates after induction therapy (intention-to-treat population)
|
Intensive pathway |
Non-intensive pathway |
|||||
|---|---|---|---|---|---|---|
| Zoledronic acid (n=555) | Clodronic acid (n=556) | p value | Zoledronic acid (n=426) | Clodronic acid (n=423) | p value | |
| CR, VGPR, or PR | 432 (78%) | 422 (76%) | 0·43 | 215 (50%) | 195 (46%) | 0·18 |
| CR or VGPR* | 200 (36%) | 193 (35%) | 0·63 | 85 (20%) | 60 (14%) | 0·018 |
| CR* | 78 (14%) | 69 (12%) | 0·42 | 39 (9%) | 27 (6%) | 0·13 |
CR=complete response. PR=partial response. VGPR=very good partial response.
Exploratory analyses.
The profile of adverse events was as expected for patients with multiple myeloma receiving bisphosphonate treatment plus induction chemotherapy (table 6). Occurrence of most adverse events was similar between the zoledronic acid and clodronic acid groups in both the intensive and non-intensive pathways. Overall, although more patients on zoledronic acid than on clodronic acid had serious adverse events, rates of treatment-emergent serious adverse events did not differ significantly between these drug groups. Of the treatment-emergent serious adverse events, significantly more patients on zoledronic acid developed bone and musculoskeletal disorders than did those on clodronic acid, and a lower rate of infections was recorded in the zoledronic acid group, but the difference was not significant. Both findings might be related to immune-system activation during an acute-phase reaction.
Table 6.
Adverse events (safety population)
|
Intensive pathway |
Non-intensive pathway |
Overall p value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Zoledronic acid (n=555) | Clodronic acid (n=556) | p value | Zoledronic acid (n=428)† | Clodronic acid (n=423) | p value | ||||
| Acute renal failure | 29 (5%) | 33 (6%) | 0·70 | 28 (7%) | 27 (6%) | 1·0 | 0·78 | ||
| Osteonecrosis of the jaw | 21 (4%) | 2 (<1%) | <0·0001 | 14 (3%) | 1 (<1%) | 0·0009 | <0·0001 | ||
| Thromboembolic events | 104 (19%) | 82 (15%) | 0·08 | 53 (12%) | 35 (8%) | 0·06 | 0·01 | ||
| Any serious adverse event‡ | 327 (59%) | 280 (50%) | 0·0046 | 212 (50%) | 198 (47%) | 0·45 | <0·0001 | ||
| No suspected association with study drugs | 245 (44%) | 214 (38%) | 0·059 | 155 (36%) | 161 (38%) | 0·62 | 0·30 | ||
| Any treatment-emergent serious adverse events§ | 158 (28%) | 141 (25%) | 0·25 | 90 (21%) | 85 (20%) | 0·80 | 0·27 | ||
| Blood and lymphatic system disorders (haematological disorders) | 30 (5%) | 26 (5%) | 0·59 | 10 (2%) | 8 (2%) | 0·81 | 0·55 | ||
| Cardiovascular disorders | 40 (7%) | 42 (8%) | 0·91 | 33 (8%) | 24 (6%) | 0·27 | 0·60 | ||
| Endocrine, metabolism, and nutrition disorders | 6 (1%) | 2 (<1%) | 0·18 | 2 (<1%) | 1 (<1%) | 1·0 | 0·23 | ||
| Gastrointestinal disorders | 15 (3%) | 12 (2%) | 0·57 | 9 (2%) | 18 (4%) | 0·081 | 0·41 | ||
| Infection | 52 (9%) | 62 (11%) | 0·37 | 16 (4%) | 28 (7%) | 0·06 | 0·07 | ||
| Musculoskeletal, connective tissue, and bone disorders | 6 (1%) | 2 (<1%) | 0·18 | 11 (3%) | 0 | 0·0009 | 0·0007 | ||
| Nervous system disorders | 10 (2%) | 8 (1%) | 0·64 | 8 (2%) | 7 (2%) | 1·0 | 0·73 | ||
| Renal and urinary disorders | 7 (1%) | 8 (1%) | 1·0 | 4 (1%) | 6 (1%) | 0·54 | 0·55 | ||
| Skin and subcutaneous disorders | 6 (1%) | 3 (1%) | 0·34 | 4 (1%) | 6 (1%) | 0·54 | 1·0 | ||
p value for comparison of zoledronic acid (n=983) versus clodronic acid (n=979).
Two patients on zoledronic acid who were excluded from the non-intensive pathway because no consent was received were included in the safety population.
Irrespective of suspected association with study drugs; patients who had more than one type of adverse event have been listed against all relevant types of events, but patients who had more than one occurrence of the same type of event are recorded only once.
Suspected association with study drugs.
Rates of acute renal failure were low and similar for patients treated with zoledronic acid and clodronic acid in both the intensive and non-intensive pathways (table 6). Thromboembolic events were more common in the intensive pathway than in the non-intensive pathway, which was most probably caused by the administration of induction chemotherapy. Although thromboembolic events were more common in the overall population of patients on zoledronic acid than in those on clodronic acid, rates were not significantly different in each pathway separately. Confirmed osteonecrosis of the jaw was uncommon, but rates were higher for zoledronic acid than for clodronic acid (4% [35/983] vs <1% [3/979]; table 6). Recovery data for osteonecrosis of the jaw were available for nine patients on zoledronic acid and one on clodronic acid, and showed complete healing for three patients on zoledronic acid, improvement for two patients on zoledronic acid, and no change for four patients on zoledronic acid and one patient on clodronic acid. Dental surgery or trauma was known to precede osteonecrosis of the jaw in six patients on zoledronic acid.
Discussion
Bisphosphonates are the standard of care for prevention of skeletal-related events and treatment of hypercalcaemia in patients with advanced cancer.1,3 In this large-scale, randomised trial of bisphosphonates in addition to standard induction chemotherapy for multiple myeloma, zoledronic acid was superior to clodronic acid across several endpoints. Zoledronic acid significantly improved overall survival and progression-free survival, with extension of overall survival by 5·5 months and progression-free survival by 2·0 months, and significantly reduced the proportion of patients with a skeletal-related event. Moreover, zoledronic acid improved overall survival independently of the reduction in skeletal-related events, suggesting that the drug has underlying antimyeloma effects, which is consistent with higher (albeit not statistically significantly so) overall response rates in patients on zoledronic acid than in those on clodronic acid. Indeed, Child and colleagues established that response to induction chemotherapy for multiple myeloma significantly correlates with overall survival.20 Differences in overall survival between the zoledronic acid and clodronic acid groups emerged within the first few months of treatment, supporting possible synergy of zoledronic acid with first-line myeloma therapies or improved antimyeloma effects of zoledronic acid when used early in the disease course.
Although improvements in disease outcomes with zoledronic acid are consistent with results from small controlled studies of zoledronic acid in newly diagnosed multiple myeloma (n=94) or early stage multiple myeloma (n=140),21,22 zoledronic acid had no significant benefits to disease outcome in asymptomatic myeloma (n=163).23 This discrepancy is probably attributable to reduced risk of disease progression in asymptomatic disease and decreased statistical power with endpoints for disease outcomes. Indeed, most guidelines do not recommend bisphosphonates for smouldering myeloma because this disease has a fairly indolent nature.24,25 Additionally, most patients in our trial continued to receive bisphosphonate therapy until disease progression whereas, in other studies, the duration of bisphosphonate treatment with respect to disease course is uncertain.
Although existing practice at research institutes participating in our trial is to treat with bisphosphonates at least until disease progression, the optimal duration of bisphosphonate treatment needs further analysis. Society treatment guidelines are inconsistent with regard to the optimal duration of bisphosphonate therapy, especially in patients with overall disease remission.3,26 Indeed, in a study of maintenance therapy in patients who did not have disease progression within the first 2 months after autologous stem-cell transplantation (n=597), pamidronic acid did not improve event-free survival or overall survival versus no maintenance, whereas the combination of pamidronic acid and thalidomide improved both outcomes compared with no treatment or pamidronic acid alone.27 In our study, although the benefits to overall survival of zoledronic acid versus clodronic acid were most pronounced early in the study, the overall survival curves separated throughout the study, suggesting potential for continuing benefit.
We used clodronic acid and zoledronic acid in our study because both are approved in the UK for prevention of skeletal-related events in multiple myeloma. Although zoledronic acid and clodronic acid are licensed for malignant bone lesions, not all patients had detectable bone disease at study entry. However, because bone lesions develop in virtually all patients during the disease course, bisphosphonate therapy was given to all patients. Although MRC Myeloma IX is not a placebo-controlled trial, the patterns of skeletal-related events were consistent with those in previous placebo-controlled studies in multiple myeloma—eg, in Berenson and colleagues' trial,9 44% of patients on placebo and 28% of those on pamidronic acid had a skeletal-related event within 1 year. Similarly, in the 25-month randomised trial of pamidronic acid versus zoledronic acid,12,28 Rosen and colleagues reported skeletal-related events in 47% of patients on zoledronic acid versus 49% of those on pamidronic acid at 13 months' follow-up (a duration similar to the median time on study drug in our trial).
All intravenous bisphosphonates are associated with effects on renal function that are dependent on dose and rate of infusion.29 Our study adhered strictly to the renal safety protocols specified in prescription information for zoledronic acid. The risk of renal failure was not significantly different between zoledronic acid and clodronic acid, despite use of thalidomide, a potentially nephrotoxic agent. This finding is consistent with a report by Spencer and colleagues,30 in which renal safety profiles did not differ significantly between zoledronic acid alone and zoledronic acid plus thalidomide in patients with multiple myeloma who were receiving maintenance therapy after autologous stem-cell transplantation. In our study, thromboembolic events were more common in the zoledronic acid group than in the clodronic acid group in both the intensive and non-intensive pathways, but differences were not significant. Further analyses of confounding variables (eg, indwelling-catheter use) are needed to assess these differences fully.
Our study provides important prospective data on occurrence of osteonecrosis of the jaw in patients with multiple myeloma, with adherence to best practice throughout the study. Although osteonecrosis of the jaw was more common in patients on zoledronic acid than in those on clodronic acid, the rates compare favourably with those summarised in guidelines from the American Society of Clinical Oncology for bisphosphonate use in multiple myeloma,26 and most events were manageable and of low grade. According to reports in 2008–09, risk of osteonecrosis of the jaw can be further mitigated with dental hygiene programmes before start of bisphosphonates, and by use of prophylactic antibiotic therapy for oral surgery during bisphosphonate treatment.31–33 Implementation of such procedures might further reduce risk of osteonecrosis of the jaw in all patients, and might have prevented some of the events recorded in our trial.
As with most large trials, the MRC Myeloma IX trial does not provide definitive answers to all the questions it sought to address. The regimens and practices common at the start of the study have since developed. Nonetheless, the study drugs were integrated into the latest chemotherapy regimens, which remain cornerstones of antimyeloma therapy. Because the trial was designed to compare several treatment options, some statistically significant effects could have been chance findings. However, we believe that our findings are robust because significant differences in the main findings had low p values, and we used in-depth and comprehensive statistical models and analyses, such as exploratory analyses showing a significant benefit to overall survival with zoledronic acid during the first 4 months. Another limitation of any study of oral therapies is the potential for poor compliance. We are not able to distinguish the effects of poor compliance in our study from the inherently lower efficacy of the oral bisphosphonate clodronic acid versus zoledronic acid. However, clinically the end result is the same, supporting preferential use of zoledronic acid. The intravenous bisphosphonate pamidronic acid, a bisphosphonate with antiresorptive activity intermediate between clodronic acid and zoledronic acid,4 is also approved for prevention of skeletal-related events in this setting. Therefore, the MRC Myeloma IX trial leaves several questions unanswered, including whether pamidronic acid might also offer benefits to progression-free survival and overall survival versus clodronic acid.
Adverse events in our trial were consistent with the established tolerability profiles of zoledronic acid and clodronic acid in multiple myeloma. One exception was that renal adverse events occurred at a similar rate for both bisphosphonates, despite the fact that one drug was given orally and the other intravenously, suggesting that the aetiology of these events could stem from the underlying disease. Indeed, fewer early deaths from renal failure occurred in the zoledronic acid group than in the clodronic acid group.
The improvement in overall survival with zoledronic acid remained significant after adjustment for the reduction in risk of skeletal-related events. These data add to growing clinical evidence supporting anticancer benefits with zoledronic acid in patients with newly diagnosed cancers.14,21 According to preclinical studies of zoledronic acid, this improvement could occur via several potential mechanisms of action, including proapoptotic effects on cancer cells, cytotoxic synergy with chemotherapeutic agents, antiangiogenesis, interference with adhesion of cancer cells, and stimulation of host anticancer immune responses.4,7,8 The improvements in complete or very good partial response in our trial support synergy between zoledronic acid and induction therapy. Although we have not definitively identified the underlying mechanism of action, the early improvement in overall survival with zoledronic acid compared with clodronic acid supports early use of zoledronic acid in multiple myeloma.
Acknowledgments
Acknowledgments
Financial support for the MRC Myeloma IX trial was obtained from the UK MRC, with additional funding in the form of unrestricted educational grants from Novartis, Schering Health Care, Chugai, Pharmion, Celgene, and Ortho Biotech, mainly to support trial coordination and the laboratory studies. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank all the patients, investigators, and staff who participated in the MRC Myeloma IX Trial; staff at the Clinical Trials Research Unit, University of Leeds, Leeds, UK, for trial coordination and data management; staff at the Department of Immunology, University of Birmingham, Birmingham, UK; staff at Wessex Regional Genetics Laboratory, University of Southampton, Southampton, UK; staff at Haematological Malignancy Diagnostic Service, Leeds Teaching Hospitals NHS Trust, Leeds, UK; staff at Institute of Cancer Research, London, UK, for central laboratory investigations; David Bowen for independent safety oversight; the MRC Leukaemia Data Monitoring and Ethics Committee; the MRC Leukaemia Trial Steering Committee; the National Cancer Research Institute Haematological Oncology Clinical Studies Group; Myeloma UK; the support of the National Institute for Health Research, through the National Cancer Research Network; and Catherine Browning (ProEd Communications, Beachwood, OH, USA) for her medical editorial assistance with this report. Lead investigators in the 20 centres with the highest trial enrolment were N H Russell (Nottingham University Hospitals, Nottingham, UK), Gordon Cook (Leeds Teaching Hospitals NHS Trust, Leeds, UK), Huw Roddie (Western General Hospital, Edinburgh, UK), Claudius Rudin (Royal Devon and Exeter Hospital, Exeter, UK), D W Milligan (Birmingham Heartlands Hospital, Birmingham, UK), John Snowden (Royal Hallamshire Hospital, Sheffield, UK), Haz Sayala (Hull and East Yorkshire Hospitals NHS Trust, Hull, UK), Patrick Chu (Royal Liverpool University Hospital, Liverpool, UK), David Wright (Mid Yorkshire NHS Trust, Wakefield, UK), K Gelly (Ninewells Hospital, Dundee, UK), Deborah Turner (Torbay Hospital, Torquay, UK), Helen Jackson (University Hospital of Wales, Cardiff, UK), Jenny Craig (Addenbrooke's Hospital, Cambridge, UK), Jane Tighe (Aberdeen Royal Infirmary, Aberdeen, UK), Salim Shafeek (Worcestershire Royal Hospital, Worcester, UK), Jeffrey Neilson (Russells Hall Hospital, Dudley, UK), James Cavet (Christie Hospital, Manchester, UK), A McKernan (Royal Derby Hospital, Derby, UK), Anton Kruger (Royal Cornwall Hospital, Truro, UK), M P Macheta (Blackpool Victoria Hospital, Blackpool, UK), Angela Wood (James Cook University Hospital, Middlesbrough, UK), and Alastair Smith (Southampton General Hospital, Southampton, UK). The findings of this study have been previously published in 2010 as abstracts for the American Society of Clinical Oncology, European Hematology Association, Cancer Induced Bone Disease conference of the Cancer and Bone Society, European Society for Medical Oncology, and American Society of Hematology.
Contributors
GJM, JAC, and GHJ were chief investigators. GJM, FED, KC, SEB, and JAC designed the trial and developed the protocol. WMG, KC, and AJS developed the statistical analysis plan. GJM, FED, RGO, AJA, JB, HR, CR, GC, GHJ, and JAC participated in recruitment of patients. SEB, MTD, RGO, and SF verified response data. FED and GHJ reviewed safety data. FED, MTD, RGO, AJA, and FR did central laboratory investigations. SEB and NN-C coordinated the data collection, and regulatory and governance requirements. GJM, WMG, KC, SEB, AJS, NN-C, and JAC contributed to writing of the report, generation of tables and figures, or data interpretation, or a combination. GJM developed an early draft, and all authors reviewed and approved the submitted report.
Conflicts of interest
GJM has participated in advisory boards for, received payment for lectures and development of educational presentations from, and received travel support from Celgene, Novartis, Merck, and Johnson and Johnson. FED has participated in advisory boards and spoken at meetings for Celgene, Ortho Biotech, and Novartis; and has received travel support to attend meetings from Celgene and Ortho Biotech. KC and SEB's institutions have received support for travel to meetings for this study from Celgene and OrthoBiotech. RGO has received speakers fees and travel support to attend scientific meetings from Celgene and Ortho Biotech. JB has received an honorarium for membership of an advisory board for Novartis. GHJ has received honoraria from Celgene and Janssen-Cilag for speaking at educational meetings. JAC has received honoraria and travel support from SAN GmbH (Science Agency and Network) for presentations which included aspects of results from the MRC Myeloma IX trial. All other authors declare that they have no conflicts of interest.
References
- 1.Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma. 2009;9:278–288. doi: 10.3816/CLM.2009.n.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berenson JR, Rajdev L, Broder M. Bone complications in multiple myeloma. Cancer Biol Ther. 2006;5:1082–1085. doi: 10.4161/cbt.5.9.3307. [DOI] [PubMed] [Google Scholar]
- 3.Terpos E, Sezer O, Croucher PI. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20:1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 4.Green JR. Bisphosphonates: preclinical review. Oncologist. 2004;9(suppl 4):3–13. doi: 10.1634/theoncologist.9-90004-3. [DOI] [PubMed] [Google Scholar]
- 5.Corso A, Ferretti E, Lazzarino M. Zoledronic acid exerts its antitumor effect in multiple myeloma interfering with the bone marrow microenvironment. Hematology. 2005;10:215–224. doi: 10.1080/10245330500094714. [DOI] [PubMed] [Google Scholar]
- 6.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34:453–475. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Croucher PI, De Hendrik R, Perry MJ. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 8.Guenther A, Gordon S, Tiemann M. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer. 2010;126:239–246. doi: 10.1002/ijc.24758. [DOI] [PubMed] [Google Scholar]
- 9.Berenson JR, Lichtenstein A, Porter L. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 10.Lipton A, Cook RJ, Coleman RE. Clinical utility of biochemical markers of bone metabolism for improving the management of patients with advanced multiple myeloma. Clin Lymphoma Myeloma. 2007;7:346–353. doi: 10.3816/CLM.2007.n.011. [DOI] [PubMed] [Google Scholar]
- 11.McCloskey EV, Dunn JA, Kanis JA, MacLennan IC, Drayson MT. Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol. 2001;113:1035–1043. doi: 10.1046/j.1365-2141.2001.02851.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosen LS, Gordon D, Kaminski M. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 13.Eidtmann H, de Boer R, Bundred N. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 14.Gnant M, Mlineritsch B, Schippinger W. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 15.Dearnaley DP, Mason MD, Parmar MKB, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–876. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palumbo A, Gay F. How to treat elderly patients with multiple myeloma: combination of therapy or sequencing. Hematology Am Soc Hematol Educ Program. 2009;2009:566–577. doi: 10.1182/asheducation-2009.1.566. [DOI] [PubMed] [Google Scholar]
- 17.San-Miguel JF, Mateos MV. How to treat a newly diagnosed young patient with multiple myeloma. Hematology Am Soc Hematol Educ Program. 2009;2009:555–565. doi: 10.1182/asheducation-2009.1.555. [DOI] [PubMed] [Google Scholar]
- 18.Weitzman R, Sauter N, Eriksen EF. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients—May 2006. Crit Rev Oncol Hematol. 2007;62:148–152. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Harousseau JL, Miguel JS. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Child JA, Morgan GJ, Davies FE. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 21.Aviles A, Nambo MJ, Neri N, Castaneda C, Cleto S, Huerta-Guzman J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol. 2007;24:227–230. doi: 10.1007/BF02698044. [DOI] [PubMed] [Google Scholar]
- 22.Sezer O, Jakob C, Aldaoud A, et al. Zoledronic acid therapy versus control in patients with multiple myeloma in stage I (Durie & Salmon): results of a phase III study of the DSMM and OSHO. 15th Congress of the European Hematology Association; Barcelona, Spain; Jun 10–13, 2010. Abstr 0361.
- 23.Musto P, Petrucci MT, Bringhen S. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–1595. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 24.Fonseca R, Bergsagel PL, Drach J. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders J, Crawford B, Gibson J, Joy Ho P, Iland H, Joshua D. Is there a case for the early use of bisphosphonates in smouldering myeloma and MGUS? (Bisphosphonates in SMM & MGUS) Int J Lab Hematol. 2007;29:395–397. doi: 10.1111/j.1365-2257.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 26.Kyle RA, Yee GC, Somerfield MR. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 27.Attal M, Harousseau JL, Leyvraz S. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 28.Rosen LS, Gordon D, Kaminski M. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 29.Lipton A. The safety of zoledronic acid. Expert Opin Drug Saf. 2007;6:305–313. doi: 10.1517/14740338.6.3.305. [DOI] [PubMed] [Google Scholar]
- 30.Spencer A, Roberts A, Kennedy N. Renal safety of zoledronic acid with thalidomide in patients with myeloma: a pharmacokinetic and safety sub-study. BMC Clin Pharmacol. 2008;8:2. doi: 10.1186/1472-6904-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimopoulos MA, Kastritis E, Bamia C. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20:117–120. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 32.Montefusco V, Gay F, Spina F. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk Lymphoma. 2008;49:2156–2162. doi: 10.1080/10428190802483778. [DOI] [PubMed] [Google Scholar]
- 33.Ripamonti CI, Maniezzo M, Campa T. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20:137–145. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]


