Abstract
Background: Nitric oxide synthase (NOS) activity is increased during hypertension and cerebral ischemia. NOS inactivation reduces stroke-induced cerebral injuries, but little is known about its role in blood-brain barrier (BBB) disruption and cerebral edema formation during stroke in acute hypertension. Here, we investigated the role of NOS inhibition in progression of edema formation and BBB disruptions provoked by ischemia/reperfusion injuries in acute hypertensive rats. Methods: Rats were made acutely hypertensive by aortic coarctation. After 7 days, the rats were randomly selected for the recording of carotid artery pressure, or regional cerebral blood flow (rCBF) using laser Doppler. Ishcemia induced by 60-min middle cerebral artery occlusion (MCAO), followed by 12-h reperfusion. A single i.p. dose of L-NAME (1 mg/kg) was injected before MCAO. After evaluation of neurological disabilities, rats were slaughtered under deep anesthesia to assess cerebral infarction volume, edema, or BBB disruption. Results: A 75-85% reduction in rCBF was occurred during MCAO which returned to pre-occluded levels during reperfusion. Profound neurological disabilities were evidenced after MCAO alongside with severe cerebral infarctions (628 ± 98 mm3), considerable edema (4.05 ± 0.52%) and extensive BBB disruptions (Evans blue extravasation, 8.46 ± 2.03 µg/g). L-NAME drastically improved neurological disabilities, diminished cerebral infarction (264 ± 46 mm3), reduced edema (1.49 ± 0.47%) and BBB disruption (2.93 ± 0.66 µg/g). Conclusion: The harmful actions of NOS activity on cerebral microvascular integrity are intensified by ischemia/reperfusion injuries during acute hypertension. NOS inactivation by L-NAME preserved this integrity and diminished cerebral edema.
Key Words: Acute hypertension, Ischemia/reperfusion injury, Nitric oxide synthase (NOX), L-NAME, Blood-brain barrier (BBB)
INTRODUCTION
Arterial hypertension is the leading cause of stroke. Hypertension is recognized as one of the most potent vasculotoxic conditions associated with early death due to cerebral edema and recurrence of stroke [1]. Cerebral circulatory system perfectly ensures circulatory and metabolic homeostasis of the brain in health. One of the most serious complications of abnormal cerebral hemodynamics and fluid dynamics, especially during hypertension, is the development of brain edema [2]. Brain is surrounded by a solid vault that has no extra space for extra fluid accumulation; therefore, edema formation compresses its blood vessel, eventually leading to brain hypoperfusion and tissue demolition [3, 4]. Hypertensive diseases usually induce brain edema by rising capillary pressure in response to high cerebral perfusion pressure, or extensive cerebral microvascular damages due to cerebral hypoxia or ischemia [5, 6].
There is general consensus that the increased NOS activity has detrimental effects on ischemic organs after reperfusion [7, 8]. Previous studies have revealed the beneficial effects of L-NAME on the occurrence of ischemia/reperfusion damages in acute cerebral ischemia in normotensive rats [9, 10]. The present study is based on the hypothesis that 1) acute hypertension, to some extent, increases cerebral capillary pressure, owing to high arterial pressure, cerebral hyperperfusion, and increases the risk of edema formation. 2) Cerebral ischemia damages the microvascular integrity and further worsens the influence of hypertension. Previous studies have demonstrated that the functional reactions of cerebral microvascular beds to increased NOS activity, in acute cerebral ischemia, are disruption of blood-brain barrier (BBB) integrity and cerebral edema independently of changing arterial pressure [9, 11]. The aim of the present study was to analyze the effects of superimposed ischemia/reperfusion injuries on acute cerebral hypertension, and also the participation of NOS activity in the pathogenesis of ischemic stroke outcomes.
MATERIALS AND METHODS
Male normal Sprague Dawley rats (270-330 g) were obtained from Central Animal House Facility of Shiraz University of Medical Sciences (). All the protocols of the study were approved by the Institutional Animal Ethics Committee of Shiraz University of Medical Sciences, which follows the NIH guidelines for care and use of animals (NIH publication No. 85-23, revised in 1996). Animals were housed in standard cages in a room with controlled temperature (22-24°C), humidity (40-60%) and light period (07.00-19.00), while having access to food and water ad libitum.
Induction of acute hypertension. Acute arterial hypertension was induced by abdominal aortic coarctation procedure [12]. In brief, under general anesthesia with ketamine (80 mg/kg) and xylazine (8 mg/kg), the abdomen was opened and abdominal aorta was surgically dissected from the inferior vena cava slightly above the renal arteries. A blunt needle was placed alongside the isolated aorta, both were gently tightened with a stitch (3-0 silk tread), and the needle was removed accordingly. The results of pilot experiments indicated that a 23-gauge needle size produced severe aortic constriction and raised blood pressure of the pre-narrowed segment (carotid pressure) of the aorta 50-70% higher than normal. Finally, the abdomen was sutured and after recovery, the animals were kept in separate cages for 7 days during which they had access to regular rat chow and water ad libitum. Arterial hypertension was assessed in randomly selected rats (n = 10) under anesthesia and via a cannulation of the right common carotid artery and the rest was assigned in sham group for evaluations of brain water content and BBB integrity.
Animal preparation. Animals were fasted overnight prior to use, without deprivation of water. Anesthesia was conducted with an i.p. injection of 400 mg/kg chloral hydrate. The animals were breathing room air, mixed with 3-5% extra oxygen, during surgery and the experimental period. Core temperature was continuously recorded by a rectal probe connected to a thermistor and the body temperature was maintained at 37 ± 1ºC with a heating pad and a lamp. A laser Doppler flowmeter (AD Instrument, model: ML191, Australia) was used to measure the regional cerebral blood flow (rCBF) of the areas of the right hemisphere that gets its blood flow from the right middle cerebral artery (MCA) according to the method described by Lecrux et al. [13] . In brief, the right temporalis muscle was dissected at the middle distance between the eye and the ear. After debridement of soft tissues, the probe of the laser Doppler probe was placed on the temporal bone. Anatomical analysis of probe location revealed that in most cases it was positioned about 1 to 2 mm from the origin of the MCA, or within the area of the ischemic core in the ischemic rats. After placement of the probe, continuous flow recording was performed from 15 min before MCA occlusion (MCAO) until 15 min from the start of reperfusion.
Experimental protocol and groups. After anesthesia, sham rats (n = 17) received a single i.p. injection of the vehicle (1 mL/kg normal saline) and underwent the neck surgery without being exposed to MCAO. In control ischemia (n = 18), surgery performed at the neck region the same as sham, a single i.p. injection of the vehicle (1 mL/kg normal saline) was done at 20 min before MCAO. After 10 min rest brain ischemia achieved by 60-min MCAO was followed by 12 h reperfusion. L-NAME ischemia (n = 17), rats of this group received a single i.p. injection of 1 mg/kg L-NAME (Nω-nitro-L-arginine methyl ester in 1 mL normal saline, Fluka Chemicals) at 20 min before MCAO. Other procedures were followed the same as control ischemia. Then, the rats of each group were separately positioned into three subgroups for the assessments of cerebral infarction volume, cerebral edema and the evaluation of BBB integrity. The number of animals presented in each group is the number of rats that were alive after 14 h of neck surgery (Sham), or 12 h of reperfusion periods (control ischemia and L-NAME ischemia). The collected data of the animals died during 12 h reperfusion period were excluded from the study. The number of dead animals with respect to the total numbers of rats in each group was regarded as the percent of mortality rate, which was 0%, 41%, and 15% in the sham, control ischemia and L-NAME ischemia, respectively. The rats of each group were randomly divided into two subgroups of equal numbers to quantify the extent of cerebral infarction volumes and brain edema.
Neurological assessment. Neurologic functions were quantified at 12 h of reperfusion (just prior to sacrifice) in rats that survived the ischemic trauma and at equivalent time periods in the sham group. A five-point grading scale of neurological deficit scores (NDS) [14,15] was used, in which rats with normal motor function or no observable neurological deficits were assigned as grade 1. Grade 2 was given to the rats that showed flexion of contralateral torso or forelimb upon lifting by their tail, or failure to extend their forepaw when suspended vertically, forelimb flexion and shoulder adduction. Grade 3 was for circling to the contralateral side of the MCA occluded hemisphere when the animal is held by the tail on a flat surface, but with normal posture at rest. Grade 4 was assigned to loss of righting reflex and decreased resistance to lateral push, and finally, grade 5 was given to the rats that have no spontaneous motor activity.
Induction of transient focal cerebral ischemia. For the surgical procedure, all animals were anesthetized with i.p. injection of 400 mg/kg chloral hydrate for only 10 min. After neck surgery, the right common carotid artery was exposed through a midline neck incision. Cerebral ischemia was induced using the intraluminal MCAO method described by Longa et al. [16] and modified by Vakili et al. [17]. The right MCA was occluded with a 19-21 mm poly-l-lysine-coated 3-0 surgical nylon filament. The filament was introduced from the external carotid artery lumen into the internal carotid artery and moved forward to reach and close the origin of MCA. Reperfusion started after 1 h of MCAO by gently removing the filament. In some animals, arterial blood gases were measured 5 min before, 15 min after MCAO and 10 min after reopening. Finally after suturing all incisions and recovery, the animals returned to their cages for recuperation during 12 h reperfusion period. At 12 h after the onset of MCA reopening (reperfusion), after neurological assessment, the animals were deeply anesthetized with sodium thiopental, sacrificed and then the brains were removed for measurements of cerebral infarct size or edema.
Cerebral infarct size. Cerebral infarct size was measured according to the method of Swanson et al. [18]. The brain tissue was sliced into six 2 mm-thick slices in the coronal plane, stained with a 2% solution of 2,3,5-triphenyltetrazolium chloride (Sigma Chemical Co. Germany) for 15-20 min and fixed in formalin buffer for 24 h. Images of the stained sections were taken. Grossly visible infarction zones were quantified using image analysis software (NIH Image Analyzer), and finally cerebral infarction volume was calculated as described previously [15].
Brain edema . The wet/dry technique was used to measure the absolute brain water content (ABWC) of ipsilateral ischemic and contralateral non-ischemic hemispheres [19]. The rats were killed under deep anesthesia, decapitated, their brain removed and placed in the brain-matrix to separate the cerebellum and olfactory bulb. A midline sagittal incision was made to divide the brain into right and left hemispheres in sham or lesioned (right) and non-lesioned (left) hemispheres in the ischemic groups. Each hemisphere was placed in separate pre-weighed container to measure its wet weight (WW). Then, the containers and their tissues were placed in an oven at 110°C for 24 h to evaporate their water, in order to obtain their dry tissue weight (DW). The %ABWC of each hemisphere was obtained separately according to the equation 1 and the percent of edema formation of the lesioned hemisphere was determined (equation 2) by subtracting the %ABWC of non-lesioned (NL) from that of lesioned (L) hemisphere.
(1) ABWC (%) = [(WW - DW)/WW] ×100
(2) Edema (%) = ABWCL – ABWCNL
BBB permeability. After deep anesthesia, a catheter was positioned into the femoral vein for slow Evans blue infusion (4 mL/kg of 2% of Evans blue solution in normal saline, Sigma Chemical Co., ). Five min slow EB infusion was done 60 min after the neck surgery in sham, or 60 min after MCAO in ischemic rats. After closing all incisions and recovery, the animals were placed in their cages for 12 h. Neurological assessments were performed and the rats were deeply anesthetized with sodium thiopental. The chest was opened and a catheter was placed into the left ventricle for 15 min infusion of 300 mL of warm normal saline (37ºC) to completely washout the remnants of EB from general circulation. After decapitation, the brain was gently excised, the cerebellum and olfactory bulb were removed, and with the help of a brain matrix, the rest was divided into the right and the left hemispheres. Each hemisphere was carefully weighed and tissue EB (µg/g wet tissue) was measured by a spectrophotometer (UV 7500, Spectro Lab, England), as described previously [20].
Statistical analysis. All values are presented as means ± SEM. Comparisons between groups were performed by analysis of variance (ANOVA) followed by Tukey’s post-hoc test, and P<0.05 was considered as statistically significance.
RESULTS
Physiological parameters. Table 1 shows arterial blood gas values and pH and body temperature before and during MCA occlusion, and also the first 15 min of reperfusion period. The results indicate that no significant differences exist among them.
Table. 1.
Physiological parameters before, during middle cerebral artery occlusion (MCAO) and during reperfusion in sham, control ischemia and L-NAME treated rats.
|
Groups
|
Sham | Control ischemia | L-NAME ischemia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters |
Before
MCAO |
During
MCAO |
After
MCAO |
Before
MCAO |
During
MCAO |
After
MCAO |
Before
MCAO |
During
MCAO |
After
MCAO |
|||
| pH | 7.393 ± 0.012 | 7.390 ± 0.010 | 7.396 ± 0.003 | 7.393 ± 0.012 | 7.390 ± 0.010 | 7.393 ± 0.012 | 7.406 ± 0.003 | 7.386 ± 0.008 | 7.396 ± 0.003 | |||
| PaCO2 (mmHg) | 39.000 ± 1.000 | 39.000 ± 1.000 | 36.000 ± 1.000 | 37.000 ±1.000 | 38.000 ± 1.000 | 36.000 ± 1.000 | 37.000 ± 1.000 | 38.000 ± 1.000 | 36.000 ± 1.000 | |||
| PaO2 (mmHg) | 81.000 ± 2.000 | 82.000 ± 2.000 | 84.000 ± 1.000 | 78. 000 ± 1.000 | 79.000 ± 2.000 | 84.000 ± 1.000 | 81.000 ± 3.000 | 84.000 ± 2.000 | 85.000 ± 2.000 | |||
| Core Temp(oC) | 37.300 ± 0.100 | 37.700 ± 0.200 | 37.700 ± 0.400 | 37.500 ± 0.200 | 37.700 ± 0.100 | 37.500 ± 0.300 | 37.200 ± 0.000 | 37.700 ± 0.200 | 37.700 ± 0.100 | |||
Data are presented as mean ± SEM. All values are in physiological range and are not significantly different from each other
Mean arterial blood pressur e. The averages of mean arterial blood pressure of normotensive rats before aortic coarctation were 97 ± 2 mmHg seven days after aortic coarctation sustained carotid artery hypertensions with mean of 151 ± 4 mmHg were observed.
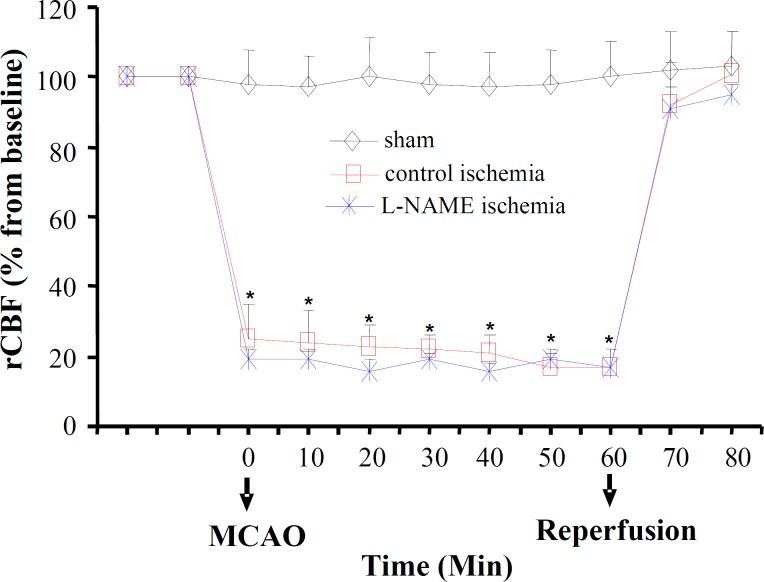
Regional cerebral blood flow . Alterations of rCBF (% from baseline) with time are shown in Figure 1. There was a 75-80% reduction in rCBF in both control ischemia and L-NAME ischemia during MCA occlusion. This reduction returned swiftly back to its pre-occluded level during the first 15 min of reperfusion period.
Fig. 1.
Regional cerebral blood flow (rCBF, % from baseline) in sham operated rats and ischemic rats of control ischemia and L-NAME ischemia groups, before and during MCAO, and the first 15-min reperfusion period. All values are as mean ± SEM. *significantly different from pre-MCAO and from sham (P<0.05).
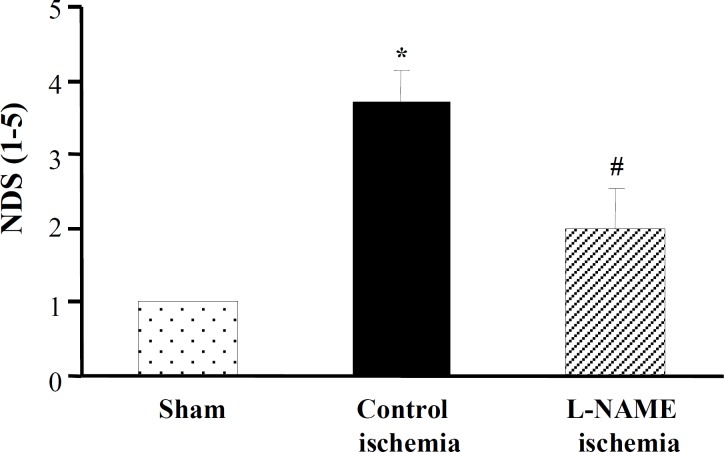
Neurological outcome. Figure 2 represents the neurological disabilities in ischemic rats exposed to 60 min MCAO after 12 h reperfusion. Data of this Figure indicates that there were severe motor disabilities in control ischemia (NDS = 3.7 ± 0.4). Pretreatment with L-NAME before induction of ischemia (L-NAME ischemia) significantly improved neurological disabilities (NDS = 2.0 ± 0.5) such it became insignificant from sham.
Fig. 2.
Neurological deficit score (NDS) in groups of sham, control ischemia and L-NAME ischemia. All values are as mean ± SEM. *significantly different from sham (P<0.05); #significantly different from control ischemia (P<0.05).
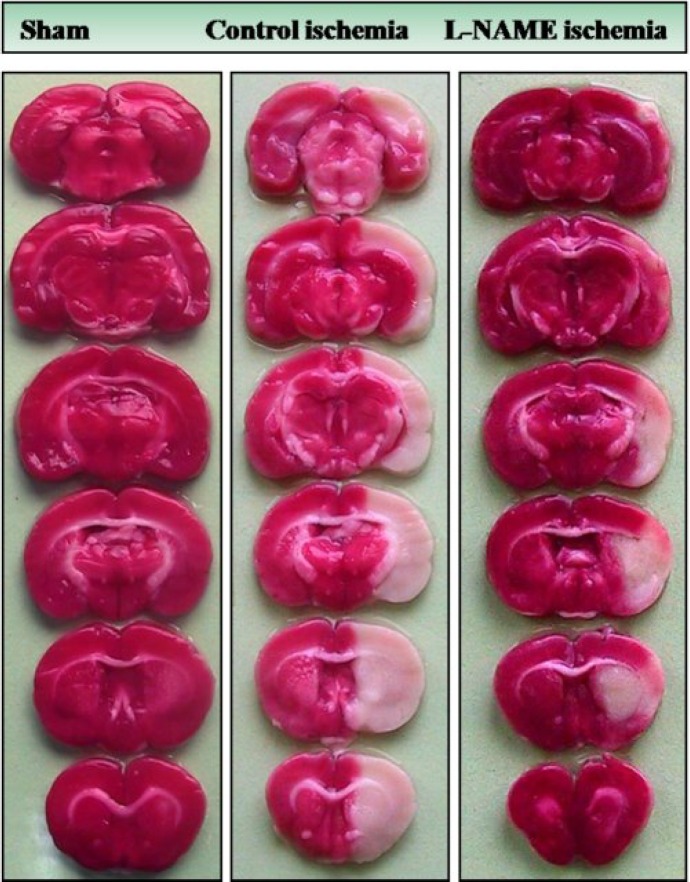
Cerebral infarction . Figure 3 is a photograph of the coronal sections of rat brain stained with triphenyltetrazolium chloride. The uniform dark red color of the slices of right and left hemispheres of sham is an induction that anesthesia and neck surgery did not provoke brain injury. The appearance of white color intermingled with dark red color areas in the right hemispheres of control ischemia and L-NAME ischemia indicates that 60 min right MCAO has induced different magnitudes of cerebral infarctions without affecting the left hemispheres. Quantitative comparisons of the values
Fig. 3.
Photographs illustrating the coronal sections of rat brain slices stained with triphenyltetrazolium chloride 12 h after neck surgery in sham or after 60 min MCAO and 12 h reperfusion in control ischemic and L-NAME pre-treated ischemic rats as described in the text. MCAO induced different magnitudes of infarctions in the right hemispheres without affecting the left sides. Non-ischemic areas are colored deep red, whereas, ischemic areas are white. There is a marked decrease in the ischemic areas of L-NAME ischemic hemisphere
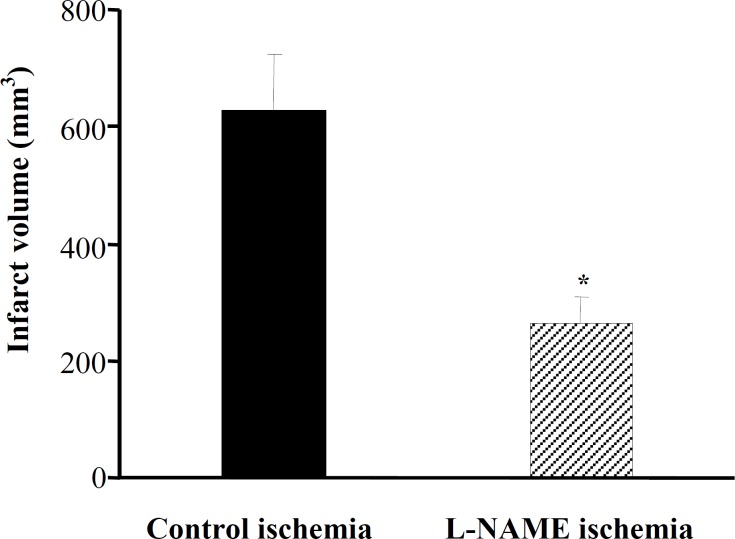
of infarct volumes indicated that ischemia induced severe infarction in the right hemispheres of MCA occluded rats of control ischemia (Fig. 4), whereas there was a 60% reduction in the size of infarction in L-NAME ischemia.
Fig. 4.
Effects of L-NAME on the infarct volume (mm3). All values are mean ± SEM. *significantly different from control ischemia (P<0.05).
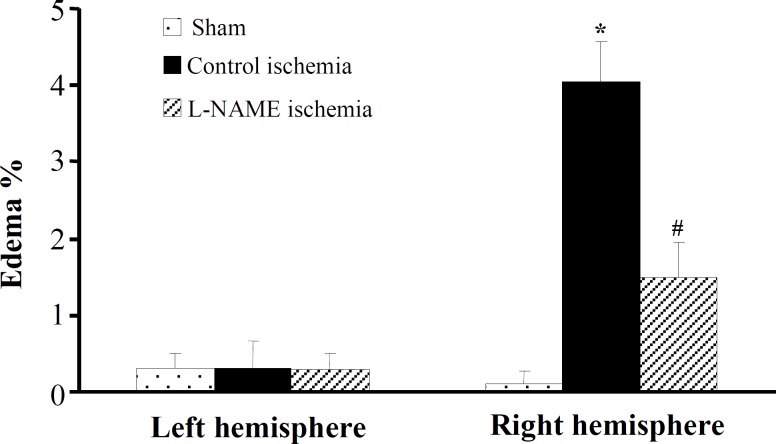
Edema formation . The percent edema formation in the left and the right hemispheres of sham and ischemic groups are presented in Figure 5. The percentage of edema in the left and the right
Fig. 5.
Edema formation (%) of lesioned hemispheres in groups control ischemia and L-NAME ischemia. All values are mean ± SEM. *significantly different from sham and their own left hemispheres (P<0.05); #significantly different from right hemispheres of control ischemia (P<0.05).
hemispheres of sham and non-ischemic left hemispheres of control ischemia and L-NAME ischemia were almost zero. Ischemia-induced edema (4.05 ± 0.52%) in the right hemispheres of control ischemia whereas L-NAME significantly reduced edema formation (1.49 ± 0.47%).
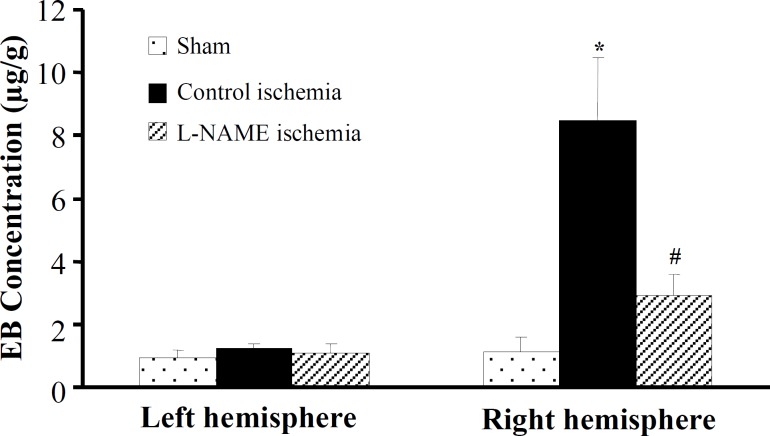
Blood-brain barrier permeability. Figure 6 denotes the quantitative EB extravasations which are used as BBB permeability index. The EB concentrations of both hemispheres of sham and non-ischemic (left) hemispheres of ischemic rats were almost close to zero and they were not significantly different from each other. Ischemia significantly increased the concentrations of EB extravasation of the lesioned (right) hemispheres of control ischemia to 8.461 ± 2.031 µg/g. However, there was a significant reduction (66%) in the concentrations EB extravasation in ischemic rats pretreated with L-NAME (2.93 ± 0.66 µg/g).
Fig. 6.
EB extravasation (µg/g) in the brain tissues of the left and right hemispheres of groups sham, control ischemia and L-NAME ischemia. All values are mean ± SEM. *significantly different from sham and their own left hemispheres (P<0.05); #significantly different from right hemispheres of control ischemia (P<0.05).
DISCUSSION
Cerebral microvascular functions are shown to be changed in stroke-prone spontaneously hypertensive rats preceding the stroke and a pathological damage occurs in arteries of hypertensive subjects [21, 22]. Hypertension is usually associated with micro-vascular injures by generations of various factors (e.g. NO, reactive oxygen species and inflammatory factors) causing endothelial dysfunctions [21, 23]. Changes also occur in blood flow and endothelial cell functions of brain microvasculature during hypertension and increases the chance of edema formation during stroke [22]. In our study, although
we could not measure directly NOS activity, we assume it increases during the hypertension. Earlier reports have shown enhanced NOS expressions and increased NO production in the brain of stroke-prone spontaneously hypertensive rats [24], and in acute hypertension induced by aortic coarctation [12]. From the results of Mayhan et al. [5], our assumption is that 7-day acute arterial hypertension (60-70%) increase in arterial blood compared to baseline, induced by abdominal aortic binding, did not have obvious pathological injuries in the functions of cerebral microvascular beds. This assumption is in accordance with our results because this much increase in arterial blood pressure per se did not lead to extensive EB extravasation in both hemispheres of sham rats (EB concentration was near zero, Fig. 6) and edema formation (Fig. 5).
Reperfusion after ischemia intensifies the increased NO production and augment ischemia injuries [7, 8, 25]. L-NAME is an NOS inhibitor and by lowering NO production protects the brain from ischemia/reperfusion injuries [6, 9]. Chronic L-NAME treatment also increases arterial blood pressure and this may interfere with ischemia injuries [6]. Our assumption is that administration of the 1 mg/kg dose of L-NAME, used in this stud, does not change the sustained hypertension. This seems to be the case because no noticeable differences existed among the values of rCBF in sham-operated rats and untreated or L-NAME pretreated ischemic rats before MCAO or during reperfusion periods (Fig. 1). MCAO similarly declined rCBF (75-85% below the baseline) in ischemic rats, but no similarity was associated with the extent of neurological deterioration outcomes
and infarction volume as thought to be during ischemia. Ischemic animals pretreated with L-NAME had a robust improvement in their neurologic score, as well as significant reductions in their cerebral infarction (Figs.2 and 4). Therefore, we think that in acute hypertension L-NAME pretreatment slows down, or prevents NOS overactivity and consequently protects the brain from ischemia/reperfusion injuries.
The integrity of cerebral microvascular beds saves the brain from various complex insults [26]. Cerebral edema is the commonest by-products of BBB disruption that worsens the side effects of stroke [3, 27]. Disruption of BBB and brain edema formation following ischemic stroke increases intracranial pressure and by compression of brain vascular beds reduces cerebral blood flow [3], and the outcome is the deterioration of neurological disabilities and increased mortality rate [3, 27, 28]. Although, the intensity of BBB disruption has been widely studied in various models of cerebral ischemia, little information exists regarding the functions of NOS activity during cerebral ischemia during acute hypertension. The comparison of the results of the edema formation (Fig. 5) or EB extravasation (Fig. 6) indicates that inhibition of NOS activity prior to ischemia induction significantly reduces the occurrences of cerebral edema formation (Fig. 5) and BBB disruption (Fig. 6) during ischemia/reperfusion in hypertensive rats. Although increased cerebral NOS activity in the ischemic lesion sites are said to amplify NO production and enhance the process of BBB breakdown and edema formation [29], its inhibition with L-NAME without alterations of rCBF and arterial blood pressure lessens BBB disruption (Fig. 6) and edema formation (Fig. 5). Up-regulation of NOS during ischemia, at both levels of mRNA and protein, in the brain endothelial microvasculature in concomitant with BBB breakdown suggests that NO participates in the pathogenesis of vasogenic brain edema [8]. Therefore, the reduced intensity of cerebral ischemia/reperfusion injuries with L-NAME, as seen in this study and reported by others [9, 17, 25], is due to the reduction of NO production in the ischemic tissues.
In conclusion, the results of this study indicates that the inhibition of increased NOS activity protects cerebral microvascular beds of acute hypertensive rats from ischemia/reperfusion injuries and by reducing the formation of cerebral edema, lessens cerebral lesions made by stroke.
ACKNOWLEDGMENTS
The authors are cordially appreciating the financial support (grant No 88-4669) of Vice Chancellor for Research of Shiraz University of Medical Sciences, .
References
- 1.Chamorro A., Vila N., Ascaso C., Elices E., Schonewille W., Blanc R. Blood pressure and functional recovery in acute ischemic stroke. Stroke. 1998;29:1850–1853. doi: 10.1161/01.str.29.9.1850. [DOI] [PubMed] [Google Scholar]
- 2.Euser A.G., Cipolla M.J. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49:334–340. doi: 10.1161/01.HYP.0000255791.54655.29. [DOI] [PubMed] [Google Scholar]
- 3.Unterberg A. W., Stover J., Kress B., Kiening K. L. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Fagan S.C., Hess D.C., Hohnadel E.J., Pollock D.M., Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 5.Mayhan W. G. Regulation of blood-brain barrier permeability. Microcirculation. 2001;8:89–104. doi: 10.1111/j.1549-8719.2001.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 6.Sercombe R., Issertial O., Seylaz J., Pinard E. Effects of chronic L-NAME treatment on rat focal cerebral ischemia and cerebral vasoreactivity. Life Sci. 2001;69:2203–2216. doi: 10.1016/s0024-3205(01)01292-9. [DOI] [PubMed] [Google Scholar]
- 7.Parathath S.R., Parathath S., Tsirka S.E. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J. Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- 8.Han F., Shirasaki Y., Fukunaga K. Microsphere embolism-induced endothelial nitric oxide synthase expression mediates disruption of the blood-brain barrier in rat brain. J. Neurochem. 2006;99:97–106. doi: 10.1111/j.1471-4159.2006.04048.x. [DOI] [PubMed] [Google Scholar]
- 9.Ding-Zhou L., Marchand-Verrecchia C., Croci N., Plotkine M., Margaill I. L-NAME reduces infarction, neurological deficit and blood-brain barrier disruption following cerebral ischemia in mice. Eur. J. Pharmacol. 2002;457:137–146. doi: 10.1016/s0014-2999(02)02686-9. [DOI] [PubMed] [Google Scholar]
- 10.Margaill I., Allix M., Boulu R.G., Plotkine M. Dose- and time-dependence of L-NAME neuroprotection in transient focal cerebral ischaemia in rats. Br. J. Pharmacol. 1997;120:160–163. doi: 10.1038/sj.bjp.0700889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gursoy-Ozdemir Y., Bolay H., Saribas O., Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31:1974–1980. doi: 10.1161/01.str.31.8.1974. [DOI] [PubMed] [Google Scholar]
- 12.Barton C.H., Ni Z., Vaziri N.D. Effect of severe aortic banding above the renal arteries on nitric oxide synthase isotype expression. Kidney Int. 2001;59:654–661. doi: 10.1046/j.1523-1755.2001.059002654.x. [DOI] [PubMed] [Google Scholar]
- 13.Lecrux C., Nicole O., Chazalviel L., Catone C., Chuquet J., MacKenzie E.T., Touzani O. Spontaneously hypertensive rats are highly vulnerable to AMPA-induced brain lesions. Stroke. 2007;38:3007–3015. doi: 10.1161/STROKEAHA.107.491126. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z., Huang P.L., Panahian N., Dalkara T., Fishman M.C., Moskowitz M.A. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 15.Panahpour H., Dehghani G.A. Inhibition of central angiotensin-converting enzyme with enalapril protects the brain from ischemia/reperfusion injury in normotensive rat. Daru. 2010;18:35–40. [PMC free article] [PubMed] [Google Scholar]
- 16.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Vakili A., Nekooeian A.A., Dehghani G.A. L-NAME and 7-Nitroindazole reduces brain injuries in transient focal cerebral ischemia in the rat. Iran J. Med. Sci. 2004;29:109–115. [Google Scholar]
- 18.Swanson R.A., Morton M.T., Tsao-Wu G., Savalos R.A., Davidson C., Sharp F.R. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 19.Gerriets T., Stolz E., Walberer M., Muller C., Kluge A., Bachmann A., Fisher M., Kaps M., Bachmann G. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke. 2004;35:566–571. doi: 10.1161/01.STR.0000113692.38574.57. [DOI] [PubMed] [Google Scholar]
- 20.Belayev L., Busto R., Zhao W., Ginsberg M.D. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 21.Sironi L., Guerrini U., Tremoli E., Miller I., Gelosa P., Lascialfari A., Zucca I., Eberini I., Gemeiner M., Paoletti R., Gianazza E. Analysis of pathological events at the onset of brain damage in stroke-prone rats: a proteomics and magnetic resonance imaging approach. J. Neurosci. Res. 2004;78:115–122. doi: 10.1002/jnr.20219. [DOI] [PubMed] [Google Scholar]
- 22.Lippoldt A., Kniesel U., Liebner S., Kalbacher H., Kirsch T., Wolburg H., Haller H. Structural alterations of tight junctions are associated with loss of polarity in stroke-prone spontaneously hypertensive rat blood-brain barrier endothelial cells. Brain Res. 2000;885:251–261. doi: 10.1016/s0006-8993(00)02954-1. [DOI] [PubMed] [Google Scholar]
- 23.Poulet R., Gentile M.T., Vecchione C., Distaso M., Aretini A., Fratta L., Russo G., Echart C., Maffei A., De Simoni M.G., Lembo G. Acute hypertension induces oxidative stress in brain tissues. J. Cereb. Blood Flow Metab. 2006;26:253–262. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- 24.Qadri F., Arens T., Schwarz E.C., Hauser W., Dendorfer A., Dominiak P. Brain nitric oxide synthase activity in spontaneously hypertensive rats during the development of hypertension. J. Hypertens. 2003;21:1687–1694. doi: 10.1097/00004872-200309000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Gursoy-Ozdemir Y., Can A., Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins B., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 27.Xu M., Su W., Xu Q.P. Aquaporin-4 and traumatic brain edema. Chin J. Traumatol. 2010;13:103–110. [PubMed] [Google Scholar]
- 28.Deb P., Sharma S., Hassan K. M. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Nag S., Picard P., Stewart D.J. Increased immunolocalization of nitric oxide synthases during blood-brain barrier breakdown and cerebral edema. Acta Neurochir. Suppl. 2000;76:65–68. doi: 10.1007/978-3-7091-6346-7_13. [DOI] [PubMed] [Google Scholar]