Abstract
Background: Melatonin has receptors in substantia nigra pars compacta (SNc) and regulates development of dopaminergic (DA) neurons. This study was undertaken to determine ability of melatonin to protect SNc dopaminergic neuron loss induced by estrogen deficiency in ovariectomized rats. Methods: Female rats were randomized into four groups of seven each: control, ethanol sham, ovariectomy (ovx) and ovx with melatonin (ovx + m). In ovx, ovaries were removed. Ovx + m group was intraperitoneally injected with melatonin for 10 days, while the ethanol sham group received only ethanol. All rats were perfused with 4% paraformaldehyde, midbrains removed, fixed and paraffin embedded, then processed for Nissl and tyrosine hydroxylase staining (IHC). Ten sections of SNc in Nissl and IHC staining were analyzed in each animal, Nissl stained and tyrosine hydroxylase (TH) immunoreactive cells were counted in five experimental groups randomly. Data was analyzed using SPSS by ANOVA and t-test. Differences were considered significant for P<0.05. Results: There was less cell number in ovx compared to control and ethanol sham groups significantly (P<0.001). The ovx + m group had more cells than the ovx group in the SNc significantly (P<0.001). Furthermore, there was significant decrease of TH positive cell number in the ovx group compared to control and ethanol sham groups (P<0.05). The number of TH immunoreactive cells was higher in ovx + m compared to the ovx group (P<0.05). Conclusion: These findings can be compared with human and used in clinical application for prevention of DA neuron death of SNc after ovariectomy.
Key Words: Exogenous melatonin, Substantia nigra, Dopaminergic neurons (DA), Ovariectomized rat, Morphometric analysis
INTRODUCTION
The substantia nigra (SN), a part of basal ganglia that located in the mesencephalon (midbrain), plays an important role in reward, addiction and movement. Anatomical studies have found that SN consists of two parts with very different connections and functions, the SN pars compacta (SNc) and SN pars reticulate. The pars compacta serves mainly as an input to the basal ganglia circuit, supplying the striatum with dopamine. The pars reticulata, on the other hand, serves mainly as an output, conveying signals from the basal ganglia to numerous other brain structures. Neurons in the pars compacta are generally dopaminergic (DA) and can be distinguished by their dark appearance to a build-up of melanin.
The neurons in the pars reticulata are mainly GABAergic (γ-Aminobutyric acid-containing neuron) [1-3]. A decrease in the number of the dopaminergic neuronal cells may contribute to the development of the neurodegenerative diseases such as Parkinson's diseases (PD). Gender differences are apparent in the onset and progression of PD [4]. Female animals have greater number of tyrosine hydroxylase (TH) immunoreactive neurons in the SNc than males [5]. TH is an enzyme in the synthesis of dopamine and the antibody for this enzyme is very useful for visualizing DA [6]. Ovariectomy (ovx) diminishes the number of TH-immunoreactive cells in the SNc; an effect which can be reserved by estrogen replacement [5]. Estrogen administration lowers the severity of symptoms of PD in postmenopausal women [4]. Estrogen therapy dramatically improves the effects of estrogen deficiency, but various adverse effects such as breast, endometrial and ovarian cancers are seen with long term estrogen therapy [7-9].
Recently, it has been suggested that melatonin, the pineal gland hormone, regulates the development of dopaminergic system and MT1 (melatonin receptor 1) is expressed in SN area [10]. Melatonin is a radical scavenger and prevents electron leakage from the mitochondrial electron transport chain thereby diminishing free radical generation [11]. Melatonin is not only a radical scavenger but also as a potent antioxidant has neuroprotective activity in animal model of ischemic stroke [11,12] and at pharmacological doses protect neurons in diverse models of neurodegeneration including PD [13]. In addition, melatonin elicits significant functional changes in the nigrostriatal DA system. The studies have shown that environmental stressors such as irradiation inhibit neurogenesis and are associated with the onset of cognitive impairments. Melatonin metabolites act as a protective agent against high energy charged particle radiation-induced oxidative damage to the brain [14].
Melatonin can easily cross the blood brain barrier and lacks side-effect [15]. Therefore, the present study was undertaken to determine the ability of melatonin to protect the SNc DA loss induced by estrogen deficiency in ovariectomized rats. Morphological changes were examined after animal were ovariectomized and treated with melatonin.
MATERIALS AND METHODS
Chemicals. Melatonin was obtained from Sigma (USA). Mouse monoclonal antibody to tyrosine hydroxylase (primary antibody, dilution 1:80) and goat polyclonal secondary antibody to mouse IgG + IgM + IgA (HRP, dilution 1:100) were purchased from Abcam (USA) and DAB (3-3´diamino-benzidine) from Dako (Denmark).
Animals. Three-month old female Sprague-Dawley rats (n = 28, 170-180 g) were purchased from the Pasteur Institute of Iran and housed in a temperature-controlled room at 23 ± 2ºC with 12-hour light/12-hour dark cycle. The animals were fed with pellets purchased from Pars Dam (Tehran, Iran) and were cared for in accordance with principals and guidelines of the Cellular and Molecular Research Center of Tehran University of Medical Sciences.
Experimental design. The rats were randomized into four equal groups of seven each: control, ethanol sham, ovx and ovx with melatonin (ovx + m). For ovx group, fourteen animals were anesthetized with ketamine (50 mg/kg) in combination with xylazine (5 mg/kg). An incision was made in the abdominal wall and the ovaries were removed, then the incision was sutured. After two months, the blood samples were collected and the level of serum estrogen was measured. After approving the reduction of the serum estrogen level in the ovariectomized rats, the half of the ovx rats were intraperitoneally injected with melatonin (20 mg/kg) dissolved in ethanol (8 mg/kg) for 10 days. The ethanol sham group received only ethanol.
Histopathological and immunohistochemical analyses. Seven days after the last injection, all rats were anesthetized and perfused transcardially with 0.1 M PBS (pH 7.4) followed by 4% buffered paraformaldehyde fixative. The brains were removed and postfixed in the same fixative overnight. Then, the midbrains were cut and dehydrated in ascending alcohol series, cleared in xylene, infiltrated with paraffin and embedded in paraffin. The 5-µm coronal sections were serially collected from bregma -4.52 mm to -6.04 mm of midbrains [16] with an interval of 30 µm between each two consecutive sections. The half of the sections was stained for Nissl (cresyl violet), the other half of the sections were processed for immunohistochemistry (IHC). For this purpose, the sections were incubated at 62ºC for 20 minutes, rehydrated in descending alcohols, immersed in 10% H2O2/Methanol to reduce endogenous peroxidase activity for 10 minutes. Then, the sections were washed in Tris buffer [H2NC(CH2OH)3, pH 7.4] and kept in citrate buffer (C6H5Na3O7.2H2O, pH 6) in autoclave to boil for 11 minutes. After cooling, the sections were washed in Tris wash buffered and incubated in BSA for 10 minutes. Afterward, the sections were incubated in the primary antibody for 1 hour. The sections were washed again in Tris wash buffered (pH 7.4), and then incubated in the secondary antibody for 1 hour. The sections were washed in Tris wash buffered (pH 7.4). To visualize the bound antibody, the sections were reacted with DAB for 10 minutes, washed in Tris wash buffered (pH 7.4), and counterstained with immersing in hematoxylin for 10 minutes , then washed in tap water for 3 minutes and dehydrated in ascending alcohols, cleared in xylol and covered with cover slip. For negative control, the sections were processed as described above except that the primary antibody was not used.
Morphometric studies. Ten coronal sections from rostral to caudal of the SNc in Nissl and IHC staining were analyzed in each animal. The picture of each section was taken by an Olympus AX70 microscope and DP11 digital camera under magnification of 40×. An area of 90,000 µm² was measured randomly in the region of SNc in five separate microscopic fields [17,18]. To count the number of neuronal cells in Nissl staining (including dopaminergic cells and interneurons) and IHC (the TH positive cells), the pictures were transferred to the computer using OLYSIA autobiorepot software (Olympus optical co. Ltd, Japan). A grid was superimposed on the picture and the cells with obvious nucleus were counted. Then, a cross was appeared on the counted cells to prevent recounting.
Statistical analysis. Data was analyzed using SPSS software by one way analysis of variance (ANOVA) and t-test. The results are expressed as the mean ± SD and differences were considered significant for P<0.05.
RESULTS
The estrogen level test of the experimental groups demonstrated that the level of estrogen in the control and ethanol sham groups were 78.9 pg/ml, while the estrogen level decreased to 5.5 pg/ml in the ovx group after two months. Figures 1 and 2 show the boundaries of the SNc which were used to measure cell number in the experimental groups in Nissl and IHC staining. Statistical and comparative light microscopic analyses demonstrated that population of Nissl-stained cell number in the ovx group had a prominent decrease compared to the control and ethanol sham groups (P<0.001, Table1 and Fig. 1). The cell number in the control and ethanol sham groups were similar (213 ± 1.92 and 212 ± 2, respectively), but in the ovx group, the number of Nissl-stained cells was 169 ± 1.7. The ovariectomized rats which had received melatonin, had significantly more Nissl-stained cells than the ovx group in the SNc (P<0.001, Table 1 and Fig. 1). The count of DA cells in the SNc of the four groups is summarized in Table 1. There was a significant decrease of TH positive cell number in the ovx group compared to the control and ethanol sham groups (P<0.05, Fig. 2). As it is shown in Table1, the ovx + m group had an apparent more TH positive cells compared to the ovx group (P<0.05, Fig. 2), indicating the resistance of DA cells against estrogen deficiency.
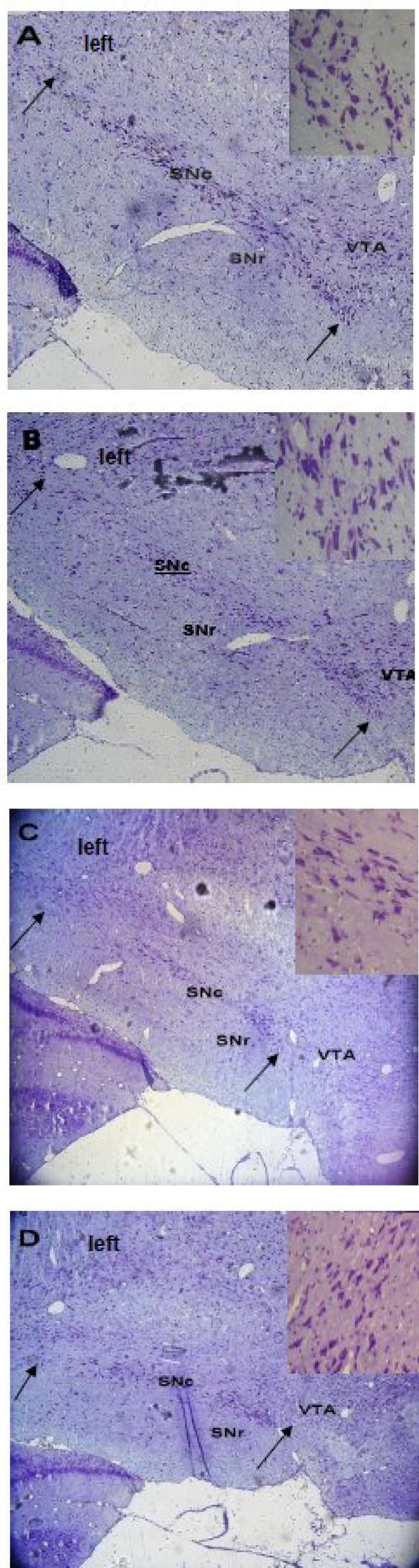
Fig. 1.
. Images of the midbrain coronal sections in the control (A), ethanol sham (B), ovx (C) and ovx + m (D) groups. The arrows show boundaries of the SNc used to measure cell number in the experimental groups. Nissl staining, 10×. A higher magnification of neurons in the SNc is observed on the right corner of each photomicrograph. As shown in the Figure, the ovx group has lesser Nissl-stained cells than the ovx + m group in the SNc (40 ×). All figures are left side of the SNc.
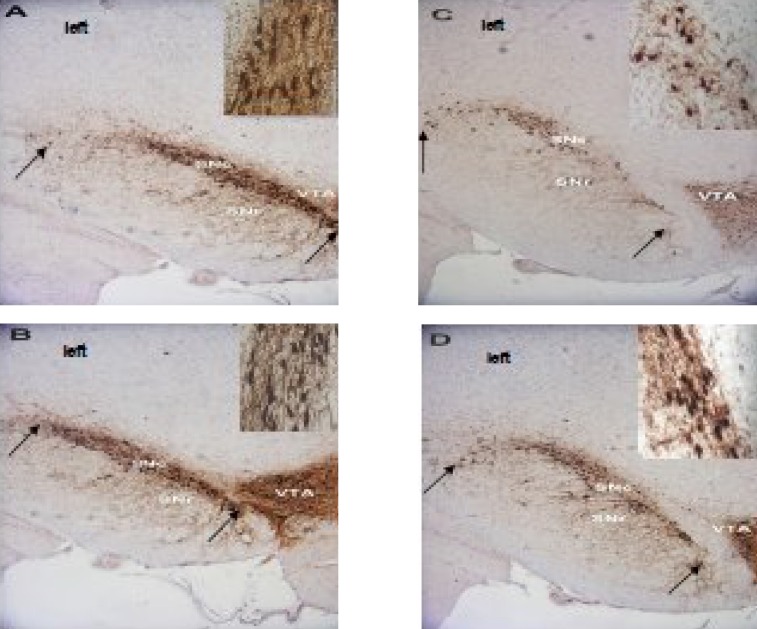
Fig. 2.
TH immunoreactivity in coronal sections of the midbrain showing the region of SNc used for counting the TH positive cells. Photographs are shown from the control (A), ethanol sham (B), ovx (C) and ovx + m (D) groups. The arrows show the boundaries of the SNc used to measure cell number in the experimental groups. IHC staining, 10×. The TH positive neurons in the SNc of each group are shown on the right corner of the photomicrographs with a higher magnification. Note the more neurons in the ovx + m group compared to ovx group (40 ×). All figures are left side of the SNc.
Table 1.
The cell number of Nissl and TH positive (IHC) stained in the SNc of the four experimental groups. Nissl-stained cells included dopaminergic cells and interneurons.
| Groups |
Nissl-stained cell number
mean ± SD |
TH positive (IHC) stained cell number
mean ± SD |
|---|---|---|
| Control | 213 ± 1.92 | 179 ± 1.48 |
| Ethanol sham | 212 ± 2.00 | 177 ± 1.08 |
| Ovx | 169 ± 1.70* | 137 ± 1.63*** |
| Ovx + m | 178 ± 0.90** | 143 ± 1.33**** |
*Significant P<0.001 as compared to the control and ethanol sham groups, **significant P<0.001 as compared to the ovx group, ***significant P<0.05 as compared to the control and ethanol sham groups, ****significant P<0.05 as compared to the ovx group.
DISCUSSION
The present study focused on the effect of melatonin on DA loss in ovariectomized rats using morpho-metric analysis. Studies involving estrogen treatment of ovariectomized rats have attributed to this hormone a neuroprotective effect on the SNc neurons. Estrogen exerts effects on DA that are the target of PD. They induce expression of tyrosine hydroxylase in developing DA neurons potentiate release of dopamine [19-24]. Yet the exact mechanisms implicated remain obscured [25].
Estrogen receptors are thought to be sparse in mouse striatum and SNc. It may act via nuclear receptor independent mechanisms to protect dopamine neurons [26]. One of the findings of this
research was the reduction of estrogen level and SNc DA neuron loss in the ovx group, which is in agreement with the research carried out in primates and 10 days after ovx; estrogen is practically eliminated and dopaminergic cell density decreased significantly [5]. In human studies, Ragonese et al. [23] found that PD was associated with factors that reduced estrogen stimulation during their life.
Therefore, both animal and human studies suggest a beneficial regulatory effect of estrogen on the nigrostriatal dopaminergic system. Estrogen therapy lowers the severity of symptoms of PD in postmenopausal women [4], but in long term treatment causes breast and genital cancers [7-9]. As a result, melatonin, which easily passes the blood brain barrier and lacks of any relevant side-effect, was proposed as a potential agent to prevent loss of DA cells in SNc after ovx or in cases with estrogen deficiency.
Melatonin is one of the most powerful antioxidant acting at various levels from direct radical scavenging to enzymatic regulation of oxidant formation and mitochondrial radical avoidance [27,28]. The prevention of apoptotic or necrotic cell death can be partially attributed to this property, but additional mitochondrial effect concerning the support of electron flux, ATP synthesis can be considered. The phenomenology of its protection is complex and melatonin may have acted on multiple targets [29]. In a neuron which is more vulnerable to decrease of estrogen level, melatonin may be sufficient for rescuing the cell as shown in this research. Melatonin effect was demonstrated by determining tyrosine hydroxylase level and the number of normal DA cells [30]. A significant increase in TH protein at 0.5 and 1 nM of melatonin has been seen and suggested a physiological role of melatonin in modulating TH expression, possibly via the MT1 receptors [31,32]. TH immunostaining was useful for visualizing dopamine synthesizing neuronal cells in SNc [33,34]. By this method, we could recognize apparent decrease in the number of TH positive neurons in SNc of the ovx group, while in the ovx + m group the more neurons were observed. This fact indicates that dopamine synthesizing neuronal cells are protected by melatonin and may be more resistant against estrogen deficiency.
Furthermore, there were more Nissl-stained cells in the ovx + m group compared to the ovx group indicating that there was both a functional and a true neuroprotection by melatonin. In other words, there was an actual increase in SNc cell survival. Our results also indicated that in each group, there were more Nissl-stained cells than TH cells in SNc. Nissl staining has been shown to label all the cells across the entire thickness of the section [35]. IHC did not reveal a distinct population of non-TH positive cells in the SNc. These cells would have been revealed by Nissl staining [36].
The results of this study indicated that SNc dopaminergic cells, protected by melatonin and DA cells, were more resistant to estrogen deficiency in the ovx + m group. Therefore, these findings can be compared with human and have clinical application for prevention of DA neurons death after ovx or menopause.
ACKNOWLEDGEMENTS
This research was supported by Tehran University of Medical Sciences. The authors wish to thank the help of Miss Mitra Farnoodian (Cancer and Oncopathology Research Center of Tehran University of Medical Sciences).
References
- 1.Juraska J.M., Wilson C.J., Groves P.M. The substantia nigra of the rat: a golgi study. J. Comp. Neurol. 1970;172:585–600. doi: 10.1002/cne.901720403. [DOI] [PubMed] [Google Scholar]
- 2.Jeyasingham R.A., Baird A.L., Meldrum A., Dunnett S.B. Differential effects of unilateral striatal and nigrostriatal lesion on grip strength, skilled paw reaching a drug induced rotation in the rat. Brain. Res Bull. 2001;55:541–548. doi: 10.1016/s0361-9230(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 3.Hendou G., Chasserot Golaz S., Kemmel V., Gobaille S., Roussel G., Artault J.C., Andriamampandry C, Aunis D, Maitre M. Immunohistochemical studies of the localization of neurons containing the enzyme that synthesizes dopamine, GABA or γ hydroxybutyrate in the rat substantia nigra and striatum. J. Comp. Neurol. 2000;426:549–560. doi: 10.1002/1096-9861(20001030)426:4<549::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Mayeux R., Denaro J., Hemenegildo N., Marder K., Tang M.X., Cote L.J., Stern Y. A population-based investigation of Parkinson's disease with and without dementia. Relationship to age and gender. Arch. Neurol. 1992;49:492–497. doi: 10.1001/archneur.1992.00530290076015. [DOI] [PubMed] [Google Scholar]
- 5.Leranth C., Roth R.H., Elsworth J.D., Naftolin F., Horvath T.L., Redmond DE. Jr. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J. NeuroSci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross M.E., Reis D.J., Joh T.H. Monoclonal antibodies to tyrosine hydroxylase: Production and characterization. Brain Res. 1981;208:493–498. doi: 10.1016/0006-8993(81)90583-7. [DOI] [PubMed] [Google Scholar]
- 7.Eisen A, Lubinski J., Gronwald J., Moller P., Lynch H.T., Klijn N., Sing C.K., Neuhausen S.L., Gilbert L., Ghaderian P., et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J. Natl. Cancer Inst. 2008;100:1361–1367. doi: 10.1093/jnci/djn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer D.W., Knapp R.C. Review of epidemiologic studies of endometrial cancer and exogenous estrogen. Obstet. Gynecol. 1979;54:521–526. [PubMed] [Google Scholar]
- 9.Lacey J.V., Mink P.J., Lubin J.H., Sherman M.E., Trisi R., Hartge P., Schatzkin A., Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 10.UZ T., Arsalan A.D., Kurtuncu M., Imbesi M., Akhisaroglu M., Dwivedi Y., Pandey G.N., Manev H. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res. Mol. Brain Res. 2005;136:45–53. doi: 10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Reiter R.J., Tan D.X., Manchester L.C., Tamura H. Melatonin defeat neurally derived free radicals and reduces the associated neuromorphological and neurobehavioral damage. J. Physiol. Pharmacol. 2007;6:5–22. [PubMed] [Google Scholar]
- 12.Kilic E., Kilic U., Bacigaluppi M., Guo Z., Ben Abdallah N.M., Wolfer D.P., Reiter R.J., Hermann D.M., Bassetti C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal. Res. 2008;45:142–148. doi: 10.1111/j.1600-079X.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R., McMillan C.R., Tenn C.C., Niles L.P. Physiological neuroprotection by melatonin in a 6-hydroxydopamine model of Parkinson's disease. Brain Res. 2006;1068:230–236. doi: 10.1016/j.brainres.2005.10.084. [DOI] [PubMed] [Google Scholar]
- 14.Manda K., Ueno M., Anzai K. Space radiation-induced inhibition of neurogenesis in the hippocampal dentate gyrus and memory impairment in mice: ameliorative potential of the melatonin metabolite, AFMK. J. Pineal. Res. 2008;45:430–438. doi: 10.1111/j.1600-079X.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 15.Reiter R.J. Melatonin: that ubiquitous by acting pineal hormone. New Physiol. Sci. 1991;6:223–227. [Google Scholar]
- 16.Paxinos G., Watson C. The rat brain in the stereotaxic coordinates. San Diego, USA: Academic Press; 1998. [Google Scholar]
- 17.Post A., Crochemore C., Uhr M., Holsboer F., Behl C. Differential induction of NF-KB activity and neural cell death by antidepressants in vitro. Eur. J. Neurosci. 2000;12:4331–4337. doi: 10.1046/j.0953-816x.2000.01352.x. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto T., Yonetani M., Nakamura H. Selective Brain Hypothermia protects against hypoxia-Ischemic injury in newborn rats by reducing hydroxyl radical production. Kobe J. Med. Sci. 2003;49:83–91. [PubMed] [Google Scholar]
- 19.Sherwin B.B., Henry J.F. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Wise P.M. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–835. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 21.D'Astous M., Morissette M., Di Paolo T. Effect of estrogen receptor agonists treatment in MPTP mice: evidence of neuroprotection by an ER alpha agonist. Neuropharmacology. 2004;47:1180–1188. doi: 10.1016/j.neuropharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Currie L.J., Harrison M.B., Trugman J.M., Bennett J.P., Wooten G.F. Postmenopausal estrogen use effects risk for Parkinson disease. Arch Neurol. 2004;61:886–888. doi: 10.1001/archneur.61.6.886. [DOI] [PubMed] [Google Scholar]
- 23.Ragonese P., D'Amelio M., Salemi G., Aridon P., Gammino M., Epifanio A., Morgante L., Savettieri G. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology. 2004;62:2010–2014. doi: 10.1212/wnl.62.11.2010. [DOI] [PubMed] [Google Scholar]
- 24.Gardiner S.A., Morrison M.F., Mozley P.D., Mozley L.H., Brensinger C., Bilker W., Newberg A., Battistini M. Piolt study on the effect of estrogen replacement therapy on brain dopamine transporter availability in healthy, postmenopausal women. Am. J. Geriatr. Psychiatry. 2004;12:621–630. doi: 10.1176/appi.ajgp.12.6.621. [DOI] [PubMed] [Google Scholar]
- 25.Tripanichkul W., Sripanichkulchai K., Finkelstein D.I. Estrogen down regulates glial activation in male mice following 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine intoxication. Brain Res. 2006;1084:28–37. doi: 10.1016/j.brainres.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Shughrue P.J. Estrogen attenuates the MPTP- induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta) Exp. Neurol. 2004;190:468–477. doi: 10.1016/j.expneurol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues C., Mayo J.C., Saniz R.M., Antolin I., Herrera F., Martin V., Reiter R.J. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal. Res. 2003;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan V. Melatonin oxidative stress and neurodegenerative disease. Indian J. Exp. Biol. 2002;40:668–679. [PubMed] [Google Scholar]
- 29.Benitez-King G., Ramirez- Rodriguez G., Ortiz L., Meza L. The neural cytoskeleton as a potential therapeutical target in neurodegenerative diseases and schizophrenia. Curr. Drug Targets CNS Neurol. Disord. 2004;3:515–533. doi: 10.2174/1568007043336761. [DOI] [PubMed] [Google Scholar]
- 30.Antolin I., Mayo J.C., Saniz R.M., del Brio Mde I., Herrera F., Martin V., Rodrigues C. Protective effect of melatonin in a chronic experimental model of Parkinson's disease. Brain Res. 2002;943:163–173. doi: 10.1016/s0006-8993(02)02551-9. [DOI] [PubMed] [Google Scholar]
- 31.McMillan C.R., Sharma R., Ottenhof T., Niles L.P. Modulation of tyrosine hydroxylase expression by melatonin in human SH-SY5Y neuroblastoma cells. Neurosci Lett. 2007;419:202–206. doi: 10.1016/j.neulet.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Venero J.L., Absi E.H., Cano J., Machado A. Melatonin induces tyrosine hydroxylase mRNA expression in the ventral mesencephalon but not in the hypothalamus. J. Pineal. Res. 2002;32:6–14. doi: 10.1034/j.1600-079x.2002.10813.x. [DOI] [PubMed] [Google Scholar]
- 33.Gaspar P., Berger B., Feburet A., Vigny A., Krieger-poulet M., Borrivoltattorni C. Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group. Neurosci. Lett. 1987;80:257–262. doi: 10.1016/0304-3940(87)90464-2. [DOI] [PubMed] [Google Scholar]
- 34.Hornung J.P., Tork. I., de Tribolet N. Morphology of tyrosine hydroxylase immunoreactive neurons in the human cerebral cortex. Exp. Brain Res. 1989;76:12–20. doi: 10.1007/BF00253618. [DOI] [PubMed] [Google Scholar]
- 35.Mitrofanis J. Calbindin immunoreactivity in a subset of cat thalamic reticular neurons. J. Neurocytol. 1992;21:494–505. doi: 10.1007/BF01186953. [DOI] [PubMed] [Google Scholar]
- 36.Fallon J.H., Loughlin S.E. Monoamine innervation of the forebrain: Collateralization. Brain Res Bull. 1982;9:295–307. doi: 10.1016/0361-9230(82)90143-5. [DOI] [PubMed] [Google Scholar]