Abstract
Background: The aim of the present study was to investigate the in vitro effects of mercury (Hg+2), lead (Pb+2), silver (Ag+2), tin (Sn+2), bismuth (Bi+3) and indium (In+3) ions on sperm creatine kinase. Methods: creatine kinase was isolated from human sperm homogenates after chromatography on a DEAE cellulose column. Results: At 60 µg ml-1 metal concentration, 70% of the creatine kinase activity was inhibited by Hg+2, while at the same concentration, Pb+2, Ag+2, Sn+2, Bi+3 and In+3 caused 68%, 66.5%, 65.7%, 64.7% and 62.7% inhibition, respectively. All six metal ions displayed a competitive type of inhibition mechanism for the isolated creatine kinase as analyzed by Lineweaver-Burk plot. Ki values of Hg+2, Pb+2, Ag+2, Sn+2, Bi+3 and In+3 were calculated and 8.34 mM, 5 mM, 4.54 mM, 3.45 mM, 3.12 mM and 2.63 mM values were obtained, respectively. Conclusion: All the studied metal ions, at levels of 60 µg ml-1, may reduce normal sperm metabolism by inhibition of sperm creatine kinase, which probably is an important cause of infertility in men. However, further investigations, as in vitro and in vivo, are needed to elucidate the exact mechanism of heavy metals on male reproductive functioning at the molecular level.
Key Words: Sperm, Creatine kinase, Infertility, Heavy metals
INTRODUCTION
Heavy metals are well known environmental and industrial pollutants. Recent studies have shown a considerable increase in metal contamination in relation to the worldwide distri-bution and extensive use of these chemical agents [1]. Unlike organic pollutants, metals cannot be degraded easily and accumulate throughout the food chain, producing potential human health risks and ecological disturbances [2]. Animal experiments and epidemiological studies have shown that heavy metals are reproductive toxicants [3]. Toxicological studies have demonstrated that these environment pollutants can accumulate in testes and/or epidi-dymis impairing their endocrine and reproductive function [4, 5]. Heavy metals also seem to have a direct effect on sperm cells by reducing their motility and/or affecting their morphology. These adverse effects have been reported either by epidemiological studies of occupationally exposed individuals [6] or animals [7] and in vitro studies [8], highlighting a positive correlation with high concentrations of metals in semen.
Creatine kinase (EC.2.7.3.2) is widely distributed in tissues which require high energy. It reversibly catalyzes the phosphorylation of creatine with ATP (creatine + ATP ↔ creatine-phosphate + ADP + H+). Its biological role is to provide an ATP buffering system for tissues which require large amounts of energy [9]. Sperm moves using ATP and phosphoryl creatine shuttle as energy sources [10]. Therefore, proper functioning of creatine kinase is a main factor of energy preparation for sperm movement.
Infertility is common in couples of childbearing age. Approximately half of the infertility problems are related to a male factor [11]. According to the previous reports, exposure to heavy metals can affect the male reproductive system [3-6,12]. In recent years, the amount of heavy metals is widely increased in the environment. Rapid industriali-zation, increase in human population, city traffics and increased use of diesel generators/diesel exhaust are believed to be responsible for the increased release of toxic metals into the environment. Exposure to these metals occurs through diet, air, drinking polluted water and ingestion of dust [12,13]. While a great deal of research has been conducted on the toxic effects of heavy metals on male reproductive function [3, 7, 8, 12,13], the literature concerning the effects of heavy metals on sperm creatine kinase activity is limited. Thus, the present study was conducted to compare the in vitro effect of six metal ions (lead [Pb+2], silver [Ag+2], tin [Sn+2], mercury [Hg+2], indium [In+3] and bismuth [Bi+3]) which are widely distributed in the environment, on creatine kinase activity isolated from human sperm.
MATERIALS AND METHODS
Materials . ADP, AMP, NADP, glucose 6-phosphate dehydrogenase, hexokinase, Triton X-100 and DEAE cellulose were obtained from Sigma Chemical Co. (St. Louis, MO, USA). EDTA, Pb+2, Ag+2, Sn+2, Hg+2, In+3, Bi+3 and BSA were obtained from Merck Chemical Co. (Darmstadt, Germany). All other reagents used were of the highest grade and purity available. All metals as chloride salts were dissolved in double distilled-deionized water.
Methods. The semen sample collection and creatine kinase isolation from human sperm were performed according to our previous study [14]. Briefly, the semen samples were analyzed based on WHO [15]. Only samples with the following seminal characteristics were included in the study: volume ≥ 3.0 ml; sperm concentration / ml ≥ 50 × 106; forward motility ≥ 60%; atypical forms ≤ 40%.
Creatine kinase was isolated from the samples, as we previously described [14]. Chromatography fractions were collected and monitored for protein content at 280 nm. In addition, an aliquot form each sample was removed for determination of total protein [16], creatine kinase activity [17] and electrophoresis on SDS-PAGE [18].
Creatine kinase activity in different steps of isolation was measured by Rosalki method [17]. As we mentioned previously [14], this method is based on the ability of creatine kinase to reduce NADP in presence of glucose, hexokinase and glucose 6-phosphate dehydrogenase.
The effects of Pb+2, Ag+2, Sn+2, Hg+2, In+3, Bi+3 chloride on the creatine kinase activity were examined by incubation of 1 unit of creatine kinase (10 μg ml-1) with different concentrations (0-100 μg ml-1) of these metals in Tris buffer (0.1 M, pH 6.8) at 25oC for 10 minutes. Degree of inhibition of creatine kinase activity by different concen-trations of Pb+2, Ag+2, Sn+2, Hg+2, In+3 and Bi+3 was determined at 340 nm as previously described [14, 17]. Then, inhibition kinetic was determined by incubation of 1 unit of creatine kinase with 0.3 mM Pb+2, 0.6 mM Ag+2, 0.5 mM Sn+2, 0.3 mM Hg+2, 0.5 mM In+3 and/or 0.3 mM Bi+3 in presence of concentrations of 0 to 10 mM creatine phosphate (as creatine kinase substrate).
Statistical analysis. Results are presented as mean ± standard deviation (SD). All assays were performed in triplicate and the mean was used for the calculation. Creatine kinase activity in absence (as control) and/or presence of heavy metals was compared using analysis of variance (ANOVA). The statistical significance was accepted when p<0.05. All analyses were carried out using the Statistical Package for the Social Sciences (SPSS) 15.0 software.
RESULTS
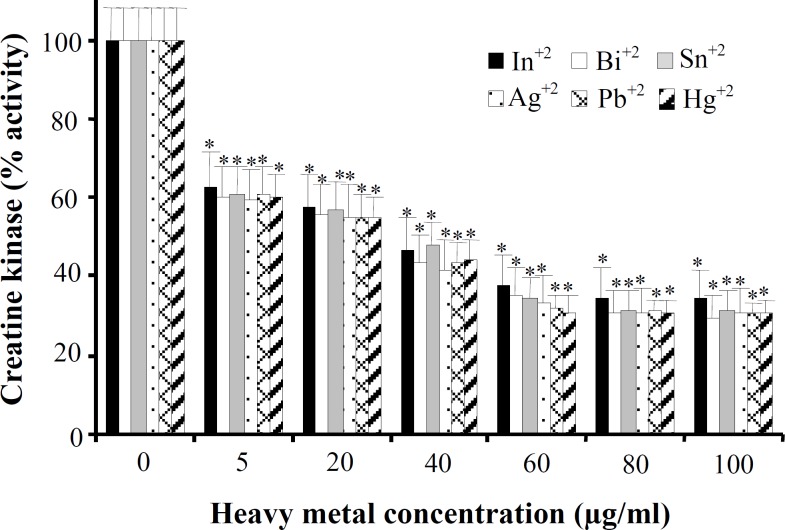
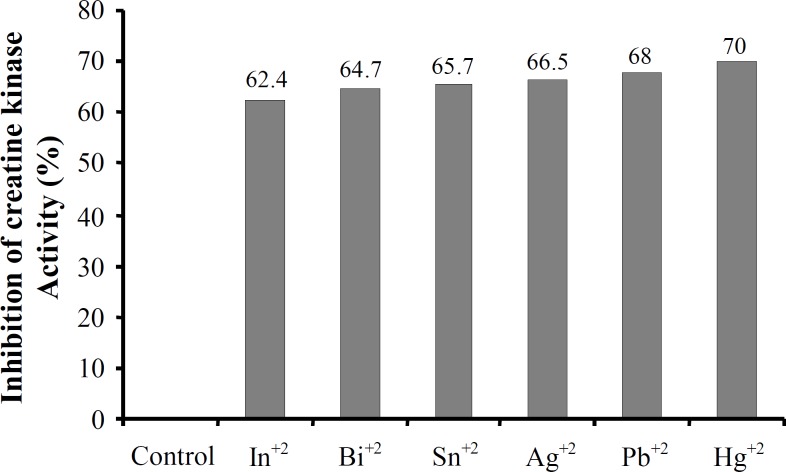
Creatine kinase isolation from human sperm was confirmed according to our previous study [14]. To study in vitro effects of metal compounds on human sperm creatine kinase activity we first examined the effects of six metals ions (In+3, Bi+3, Sn+2, Ag+2, Pb+2 and Hg+2) on activity of this enzyme at six different concentrations: 5, 20, 40, 60, 80 and 100 μg ml-1 (Fig. 1). According to the Figure 1, these metals are significantly able to inhibit human sperm creatine kinase activity in a dose-dependent manner, and the most effect of inhibition was at 60 µg ml-1 concentration. Although all these metals with concentrations of 5 to 100 μg ml-1 in comparison to the control (metal-free) showed almost similar inhibition effect on creatine kinase activity, Hg+2 had the most powerful effect (Fig. 1). This study showed that 60 μg ml-1 concentrations of Hg+2, Pb+2, Ag+2, Sn+2, Bi+3, and In+3 can inhibit human sperm creatine kinase activity by approximately 70%, 68%, 66.5%, 65.7%, 64.7% and 62.4%, respectively (Fig. 2).
Fig. 1.
In vitro effects of lead, silver, tin, mercury, indium and bismuth on creatine kinase activity obtained from human sperm. Samples (10 μg ml-1 creatine kinase equal with 1 unit activity) were incubated with different metal concentrations (5-100 μg ml-1) for 10 min at 25oC. Results are expressed as percentage of the corresponding control activities (unit per liter). Data have represented as the mean ± S.D. of triplicate determinations. *P<0.01 compared with control (in absence of heavy metals).
Fig. 2.
The inhibition percentage of human sperm creatine kinase activity in presence of 60 μg ml-1 indium, bismuth, tin, silver, lead and mercury compared with control (in absence of heavy metals).
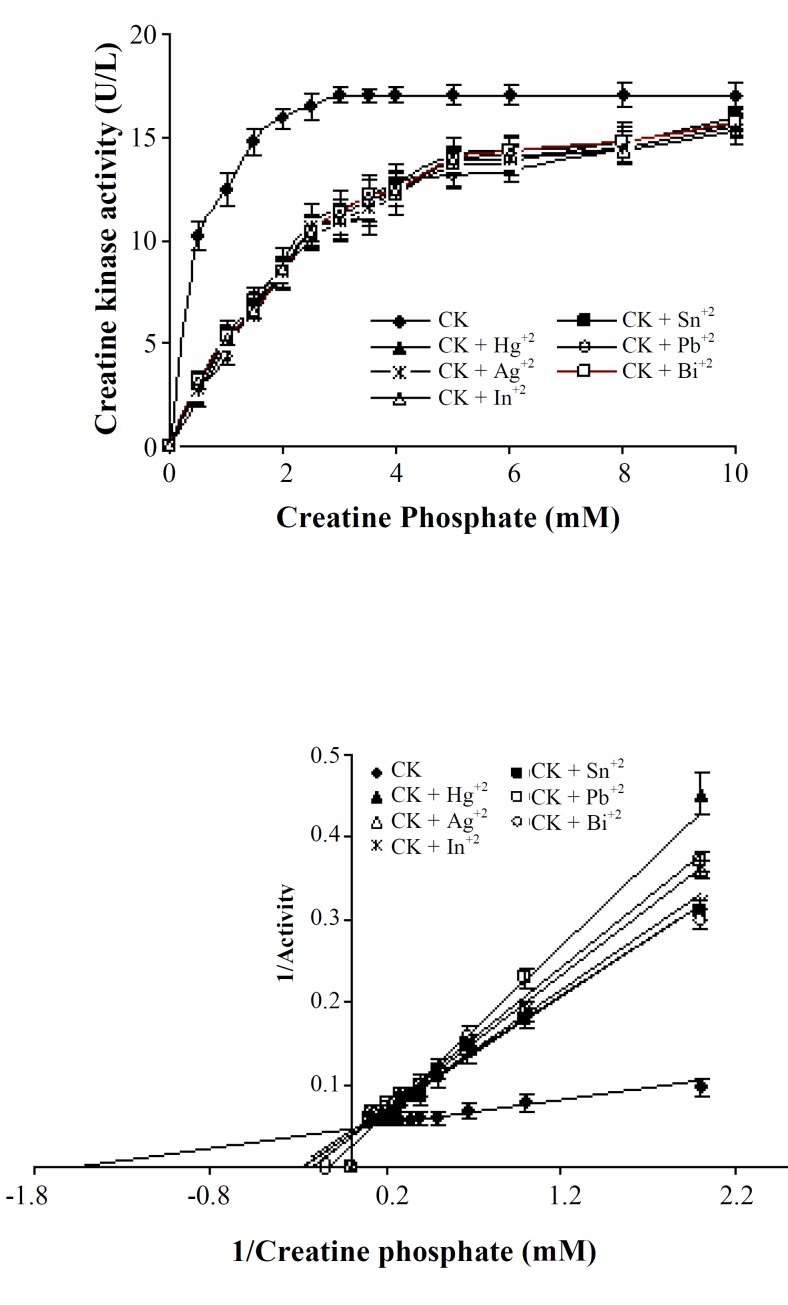
We also investigated the effects of 0.3 mM of Bi+3, Pb+2, Hg+2, 0.5 mM of In+3, Sn+2 and 0.6 mM of Ag+2 on human sperm creatine kinase activity plots (activity vs. substrate concentration) using the Michaelis-Menten equation/model (Fig. 3, upper panel).
Fig. 3.
(Upper panel) Heavy metals-mediated inhibition of human sperm creatine kinase in a vary concentrations of substrate (0-10 mM). Concentrations of 0.3 mM of bismuth (Bi+3), 0.3 mM of lead (Pb+2), 0.3 mM of mercury (Hg+2), 0.5 mM of indium (In+3), 0.5 mM of tin (Sn+2) and 0.6 mM of silver (Ag+2) were used. Results are expressed as mean ± S.D. of triplicate determination. (Lower panel) Lineweaver-Burk plots reflect a probable competitive type inhibition of human sperm creatine kinase activity by heavy metals.
Data from the resulting control curve (creatine kinase activity in absence of heavy metals) match the Lineweaver-Burke model equation yielding an estimated Km for creatine phosphate of 625 μM under these assay conditions (Fig. 3, lower panel). Therefore, these heavy metals can inhibit human sperm creatine kinase activity as in vitro in 0.5 to 10 mM of creatine phosphate (substrate) concentrations (Fig. 3). According to Lineweaver-Burk equation parameters, Ki values for In+3, Bi+3, Sn+2, Ag+2, Pb+2 and Hg+2 were estimated 2.63, 3.12, 3.45, 4.54, 5 and 8.34 mM, respectively (Fig. 3, lower panel). The lower panel of Figure 3 also indicates a probable competitive mechanism for mediated inhibition of these heavy metals.
DISCUSSION
Heavy metals are widely distributed in the environment and these metal ions are present in low concentrations in the semen of healthy and presumably unexposed men [19]. Men working in industries with high exposure to heavy metals have been reported to have elevated levels of these metals in their blood [20]. Gennart et al. [20] found when workers were exposed to high levels of Pb+2, their fertility decreased. Xu et al. [21] reported a significant correlation between cadmium (Cd+2) and sperm concentration. Lerda [22] reported that a decrease in sperm concentration occurred in men with a mean blood Pb+2 concentration of 48.6 μg dl-1 (about 0.0023 mM). The addition of heavy metals to human semen can reduce sperm motility [23, 24]. Young et al. [25] observed that hyper-activation of rabbit sperm was inhibited by heavy metals. Although it is known that heavy metals are associated with an overall reduction in men fertility, the mechanism is not clear yet.
Creatine kinase is an important enzyme which plays a major role in sperm energy homeostasis [11]. Thus, in the present study effects of some heavy metals which are more present in the environment such as Hg+2, Pb+2, Ag+2, Sn+2, Bi+3 and In+3 were evaluated on human sperm creatine kinase activity in an in vitro model. After isolation of creatine kinase from human sperm, the experiments were conducted with various concentrations of these heavy metals (0-100 μg ml-1) on activity of this enzyme, as a necessary key enzyme for sperm normal metabolism. At first, it was observed which all these metals significantly (P<0.01) inhibited creatine kinase activity in a dose-dependent manner.
Maximum inhibition was seen with Hg+2, followed by Pb+2 > Ag+2 > Sn+2 > Bi+3 > In+3. Then, the kinetic analysis of the effect of these metals on human sperm creatine kinase activity showed that all heavy metals in this study could act as competitive inhibitors of human sperm creatine kinase. This study also showed maximum Ki for Hg+2 is 8.34 mM and then followed by Pb+2 (5 mM) > Ag+2 (4.54 mM) > Sn+2 (3.45 mM) > Bi+3 (3.12 mM) > In+3 (2.63 mM), which confirm results of the inhibition
percentage of human sperm creatine kinase activity in the presence of each one of metals above. Previous studies by others suggested that creatine kinase possess two sites, one for the nucleotide (Mg-ATP) and the other for the guanidine substrate (phosphocreatine) [26, 27]. These studies also indicated that the most likely mechanism for phosphorylation of ADP by phosphocreatine involves a highly reactive metaphosphate inter-mediate that is generated in the rate-determination step by proton transfer and cleavage of the P-N bound of phosphocreatine [27, 28]. The most important role which creatine kinase plays in catalysis of this reaction involves protonation of the P-N nitrogen of phosphocreatine.
There is evidence that a sulfhydryl group (cysteine residues) and an imidazole group (histidine residues) are involved in catalysis at the active site of creatine kinase. Either the -SH group or imidazolium group could function as a general acid catalyst for protonation of the P-N nitrogen [28]. According to these reports, presence of Mg+2 and -SH group at creatine kinase active site is necessary for its activity [26-28]. Different reports have shown that heavy metals with different mechanisms can inhibit enzymes. Mercuric chloride and sodium selenite can oxidize -SH groups of different enzymes and thus Pb+2 to the inhibition of a large number of sulfhydryl-containing enzymes [29]. Farina et al. [29] indicated that Hg+2 inhibits the activity of δ-aminolevulinate dehydrogenase in mouse liver, kidney and brain by oxidizing -SH groups located at the active center of the enzyme. Shinozaki and Pritzker [30] and Waalkes [31] reported that Pb+2 and cadmium can inhibit the alkaline phosphatase activity in brain, through Zn+2 substitutions.
Our previous study showed cadmium as an important heavy metal in tobacco probably is able to inhibit activity of this enzyme via displace magnesium ions in human sperm creatine kinase [14]. The results of this study are also in agreement with other findings. We indicated that although human sperm creatine kinase is inhibited by all these metals in approximately the same range, divalent cations, especially, mercury have the most effectiveness on human sperm creatine kinase activity. As mentioned above, mercury ions via SH group oxidation at active site of different enzymes can inhibit their activity. This can be an important reason for more inhibitory effect of mercury compared with other ions in this study. Therefore, inhibition effects of these metals on activity of human sperm creatine kinase may be due to –SH group oxidation at active site or displacement of Mg+2 and/or both.
In conclusion, our results indicated that Hg+2, Pb+2, Ag+2, Sn+2, Bi+3 and In+3 reduce in vitro creatine kinase activity in human sperm, by an apparently competitive inhibition, possibly through displacement of Mg+2 in this enzyme. Creatine kinase has an important role in sperm energy homeostasis [9,10]. Therefore, diminution of creatine kinase activity by these heavy metals may potentially impair sperm functional parameters. However, further in vitro and in vivo investigations are required to confirm the role played by these heavy metals in male infertility.
ACKNOWLEDGMENTS
This work was financially supported by a grant from Physiology Research Center of Ahwaz Jundishapur University of Medical Sciences (Ahwaz, Iran), Project No. PRC-29.
References
- 1.Gosselin M., Bouquegneau J.M., Lefèbvre F., Lepoint G., Pergent G., Pergent-Martini C., Gobert S. Trace metal concentrations in Posidonia oceanica of North Corsica (northwestern Mediterranean Sea): use as a biological monitor? BMC. Ecology. 2006;6:12. doi: 10.1186/1472-6785-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordberg G.F., Jin T., Hong F., Zhang A., Buchet J.P., Bernard A. Biomarkers of cadmium and arsenic interactions. Toxicol. Appl. Pharmacol. 2005;206:191–197. doi: 10.1016/j.taap.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 3.De Celis R., Pedron-Nuevo N., Feria-Velasco A. Toxicology of male reproduction in animals and humans. Arch. Androl. 1996;37:201–218. doi: 10.3109/01485019608988523. [DOI] [PubMed] [Google Scholar]
- 4.Lynch M.L., Huang L.S., Cox C., Strain J.J., Myers G.J., Bonham M.P., Shamlaye C.F., Stokes-Riner A., Wallace J.M., Duffy E.M., Clarkson T.W., Davidson P.W. Varying coefficient function models to explore interactions between maternal nutritional status and prenatal methylmercury toxicity in the Seychelles child development nutrition study. Environ. Res. 2011;111:75–80. doi: 10.1016/j.envres.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson J., Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S. Occupational exposure associated with reproductive dysfunction. J. Occup. Health. 2004;46:1–19. doi: 10.1539/joh.46.1. [DOI] [PubMed] [Google Scholar]
- 7.Benoff S., Auborn K., Marmar J.L., Hurley I.R. Link between low-dose environmentally relevant cadmium exposures and asthenozoospermia in a rat model. Fertil. Steril. 2008;89:e73–e79. doi: 10.1016/j.fertnstert.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousef M.I., Kamel K.I., El-Guendi M.I., El-Demerdash F.M. An in vitro study on reproductive toxicity of aluminum chloride on rabbit sperm: the protective role of some antioxidants. Toxicology. 2007;239:213–223. doi: 10.1016/j.tox.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Ellington W.R. Evolution and physiological roles of phosphagen system. Annu. Rev. Physiol. 2001;63:289–325. doi: 10.1146/annurev.physiol.63.1.289. [DOI] [PubMed] [Google Scholar]
- 10.Miyaji K., Kaneko S., Ishikawa H., Aoyaqi T., Hayakawa K., Hata M. Creatine kinase isoforms in the seminal plasma and the purified human sperm. Arch. Androl. 2001;46:127–134. [PubMed] [Google Scholar]
- 11.Yesilli C., Mungan G., Seckiner I., Akduman B., Acikgoz S., Altan K., Mungan A. Effect of varicocelectomy on sperm creatin kinase, HspA2 chaperone protein (creatine kinase-M type), LDH, LDH-X and lipid peroxidation product levels in infertile men with varicocele. Urology. 2005;66:610–615. doi: 10.1016/j.urology.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 12.Pant N., Upadhyay G., Pandey S., Mathur N., Saxena D.K, Srivastava S.P. Lead and cadmium concentration in the seminal plasma of men in the general population: correlation with sperm quality. Reprod. Toxicol. 2003;17:447–450. doi: 10.1016/s0890-6238(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R., Pant N., Srivastava S.P. Chlorinated pesticides and heavy metals in human semen. Int. J. Androl. 2000;23:145–149. doi: 10.1046/j.1365-2605.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghaffari M.A., Abroumand M., Motlagh B. In vitro inhibition of human sperm creatine kinase by nicotine, cotinine and cadmium, as a mechanism in smoker men infertility. Inter. J. Fertil. Stril. 2008;2:125–130. [Google Scholar]
- 15.Laboratory manual for the examination of human semen and semen cervical mucus interaction. 4th ed. New York, USA: 1999. [Google Scholar]
- 16.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Rosalki S.B. An improved procedure for serum creatine phosphokinase determination. J. Lab. Clin. Med. 1967;69:696–705. [PubMed] [Google Scholar]
- 18.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Noack-Fuller G., De Beer C., Seibert H. Cadmium, lead, selenium and zinc in semen of occupationally unexposed men. Andrologia. 1993;25:7–12. doi: 10.1111/j.1439-0272.1993.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 20.Gennart J.P., Buchet J.P., Roels H., Ghyselen P., Ceulemans E., Lauwerys R. Fertility of male workers exposed to cadmium, lead or manganese. Am. J. Epidemiol. 1992;135:1208–1219. doi: 10.1093/oxfordjournals.aje.a116227. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., Chia S.E., Tsakok M., Ong C.N. Trace elements in blood and seminal plasma and their relationship to sperm quality. Reprod. Toxicol. 1993;7:613–618. doi: 10.1016/0890-6238(93)90038-9. [DOI] [PubMed] [Google Scholar]
- 22.Lerda D. Study of sperm characteristics in persons occupationally exposed to lead. Am. J. Ind. Med. 1992;22:567–571. doi: 10.1002/ajim.4700220411. [DOI] [PubMed] [Google Scholar]
- 23.Holland M.K., White I.G. Heavy metals and spermatozoa. 1. Inhibition of the motility and metabolism of spermatozoa by metals-related to copper. Fertil. Steril. 1980;34:483–489. doi: 10.1016/s0015-0282(16)45142-3. [DOI] [PubMed] [Google Scholar]
- 24.Benoff S., Hauser R., Marmar J. L., Hurley I. R., Napolitano B., Centola G. M. Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers) Mol. Med. 2009;15:248–262. doi: 10.2119/molmed.2008.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young R.J., Bodt B.A., Heitkamp D.H. Action of metabolic ions on the precocious development of rabbit sperm motion patterns that is characteristic of hyperactivated motility. Mol. Reprod. Dev. 1995;4:239–248. doi: 10.1002/mrd.1080410215. [DOI] [PubMed] [Google Scholar]
- 26.Morrison J.F., James E. The mechanism of the reaction catalyzed by adenosine triphosphate-creatine phosphotransferase. Biochem. J. 1965;97:37–52. doi: 10.1042/bj0970037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe G., Sproat B.S. Evidence for an associative mechanism in the phosphoryl transfer step catalyzed by rabbit muscle creatine kinase. J. Biol. Chem. 1980;255:3944–3951. [PubMed] [Google Scholar]
- 28.Haake P., Allen G.W. Studies on phosphorylation by phosphoroguanidinates. The mechanism of action of creatine: ATP transphorylase (creatine kinase) Proc. Natl. Acad. Sci. UAS. 1971;68:2691–2693. doi: 10.1073/pnas.68.11.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farian M., Brandao R., deLara F.S., Pagliosa L.B., Soares F.A., Souza D.O., Rocha J.B.T. Profile of non-protein thiols, lipid peroxidation and δ-aminolevulinate dehydrogenase activity in mouse kidney and liver in response to acute exposure to mercuric chloride and sodium Selenite. Toxicology. 2003;184:179–187. doi: 10.1016/s0300-483x(02)00576-0. [DOI] [PubMed] [Google Scholar]
- 30.Shinozaki T., Pritzker K.P. Regulation of alkaline phosphatase: implications for calcium pyrophosphate dehydrate crystal dissolution and other alkaline phosphatase functions. J. Rheumatol. 1996;23:677–683. [PubMed] [Google Scholar]
- 31.Waalkes M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000;79:241–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]