Abstract
Background: Spinal cord injury (SCI) stimulates an inflammatory reaction that causes substantial secondary damage inside the injured spinal tissue. The purpose of this study was to determine the anti-inflammatory effects of epigallocatechin gallate (EGCG) on traumatized spinal cord. Methods: Rats were randomly divided into four groups of 12 rats each as follow: sham-operated group, trauma group, and EGCG-treatment groups (50 mg/kg, i.p., immediately and 1 hour after SCI). Spinal cord samples were taken 24 hours after injury and studied for determination of myeloperoxidase (MPO) activity, histopathological assessment and immunohistochemistry of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), Nitrotyrosine, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and poly(ADP-ribose) polymerase (PARP). Results: The results showed that MPO activity was significantly decreased in EGCG-treatment groups. Attenuated TNF-α, IL-1β, Nitrotyrosine, iNOS, COX-2, and PARP expression could be detected in the EGCG treated rats. Also, EGCG attenuated myelin degradation. Conclusion: On the basis of these findings, we propose that EGCG may be effective in protecting rat spinal cord from secondary damage by modulating the inflammatory reactions.
Key Words: Epigallocatechin gallate (EGCG), Spinal cord trauma, Inflammation
INTRODUCTION
Neurological damages after traumatic spinal cord injury (SCI) result from both primary mechanical injury and secondary degeneration process. The outcome of SCI depends on the extent of secondary damage mediated by a series of cellular, molecular and biochemical cascades including calcium ion influx, oxygen free radical-induced lipid peroxidation, inflammatory reaction, autoimmune response, vascular events, and apoptosis [1].
In recent years, much attention has been focused on secondary injury, because it appears to be susceptible to therapeutic interventions that may include the use of free radical scavengers and anti-inflammatory agents. The chemical composition of green tea contains many polyphenolic compounds, generally known as catechins. Catechins have many actions such as free radical scavenging/antioxidant actions, preventing lipid peroxidation due to oxidative stress, modulating apoptotic pathways, prooxidant properties, and anti-inflammatory effects [2]. (-)-Epigallocatechin gallate (EGCG) is the most abundant composition of the tea catechins and thought to be responsible for the majority of biological activity of green tea extracts [3]. EGCG has been shown to have some protective effects against neuronal damage after transient ischemia [2], oxidative damage on periventricular white matter in hydrocephalic rats [4], suppression of disease progression of amyotrophic lateral sclerosis [5], acute hypoxi [2], iron-induced oxidative stress [2], Alzheimer׳s and Parkinson's diseases [6], aging [7], and cancer [8]. Also, we recently showed that EGCG attenuated neuronal apoptosis and improved locomotor function after SCI in rats [9], but the mechanisms behind these actions have not been fully elucidated.
The aim of the current study was to investigate the potential anti-inflammatory effect of EGCG after experimental contusion injury. A better under-standing of these mechanisms might lead to the introduction of the preventive and therapeutic strategies in clinical practice. Accordingly, in this work, we evaluated the effect of EGCG on the activity and expression of the inflammatory criteria in the traumatized spinal tissue of rat.
MATERIALS AND METHODS
Animals. Male adult Spargue-Dawley rats (250-300 g, the Pasteur Institute of Iran, ) were used in this study. The animals were kept under standard conditions according to the Guidelines of the University׳s Animal Care Codes to minimize the animal's suffering.
Contusive SCI using the weigh dropping technique. The animals were anesthetized with ketamine (75 mg/kg i.p.) and xylazine (10 mg/kg i.p.). Laminectomy was performed at T9 level vertebra; the dorsal surface of the cord was then subjected to weight drop impact using a 10-g weight dropped from a height of 2.5 cm in order to produce contusive SCI. Following the surgery, the recovery of the animals was assisted by administering lactated ringer׳s solution (12-25 ml) subcutaneously immediately after surgery and then by cefazolin (50 µg/kg, Jaber Ibn Hayan, ) which was administered twice daily for 3 days. The urinary bladders were pressed three times a day until the function was retained. The rats were randomly allocated in four groups, each containing 12 rats: (i) sham-operated group, which underwent laminectomy alone; (ii) trauma group, which underwent laminectomy followed by SCI and received saline (vehicle); (iii and iv) EGCG treatment groups, which underwent laminectomy followed by SCI and received a 50- mg/kg single dose of EGCG (Sigma, USA) i.p. immediately (EGCGI) and 1 hour (EGCGII) after trauma, respectively. Each group of animals was divided into 2 subgroups: (A) for biochemical analysis (n = 6), and (B) for histopathological assessment and immunohistochemistry (n = 6).
Biochemical analysis. Six rats from each group were euthanized, and 1.5 cm traumatized spinal cord specimens were removed for biochemical analysis 24 hours after SCI. The obtained samples were thoroughly cleaned of blood and the meninges were carefully removed. Then, the tissue samples were immediately frozen and stored in a -70°C freezer for assays of tissue myeloperoxidase (MPO) activity, an indicator of polymorphonuclear leukocyte accumulation, as previously described by Mullane [10]. MPO activity was expressed as units of MPO/mg of proteins.
Immunohistochemistry. For immunohisto-chemistry, the sections were incubated in normal serum (in order to block non-specific site), and then with anti-iNOS (inducible nitric oxide synthase) rabbit polyclonal antibody (1:50 in PBS, v/v, Abcam, England), anti-COX-2 (cyclooxygenase-2) rabbit polyclonal antibody (1:100 in PBS, v/v, Abcam), anti-PARP [poly(ADP-ribose) polymerase] rabbit polyclonal antibody (1:100 in PBS, v/v, Abcam), anti-nitrotyrosine rabbit polyclonal antibody (1:50 in PBS, v/v, Millipore, France), Biotin anti-mouse/rat interleukin-1β (IL-1β) antibody (10 µg/ml in PBS, w/v, Biolegend), and finally with anti-rat in PBS, w/v, R&D) at 4°C overnight. The sections were washed with PBS, incubated with Ultratek HRP (anti-polyvalent) (ScyTek, USA), streptavidin-HRP (Millipore, France), or with HRP conjugated rabbit anti-goat secondary antibody (Millipore, France), and then demonstrated with diaminobenzidine tetrahydro-chloride for 10 minutes. After wards, they were counterstained with hematoxylin, dehydrated, and mounted. For negative controls, primary antibodies were omitted. For quantitative analysis, immune-histochemical photographs (n = 5 photos from each samples, collected from all rats in each experimental group) were assessed by densitometry using MacBiophotonics ImageJ 1.41a software on a personal computer.
Histopathological assessment. For histopatho-logical assessment, eight-micrometer tissue sections were deparaffinized with xylene, stained with Luxol fast blue (used to assess demyelination), and studied using light microscopy (Leica DME).
Statistical analysis. Statistical analysis was carried out using SPSS package. Results were presented as mean values (± SEM). The KS test was used in order to evaluate the normality of the data. Also, the Tukey׳s multiple comparison tests and the analysis of the variance were used in order to compare each two groups and compare the data among the groups, respectively. A value of P<0.05 was considered significant.
RESULTS
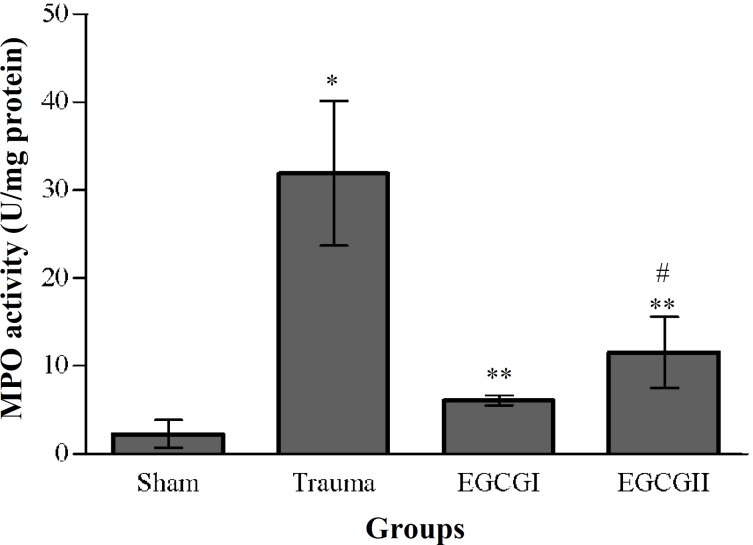
Effects of EGCG on neutrophil infiltration. The histogram of the MPO activity for all groups at 24 hours post-injury has been shown in Figure 1. Induction of SCI in trauma produced a significant elevation (P<0.01) in MPO activity compared to the sham-operated group. The MPO activity in EGCG-treatment groups were significantly lower than trauma group (P<0.05), while the differences between EGCG1 and EGCG2 were not significant (P>0.05).
Fig. 1.
Effects of EGCG on myeloperoxidase (MPO) activity. The histogram shows the activity of MPO at 24 hours after SCI. MPO activity was expressed as units of MPO/mg of proteins.*P<0.01 versus sham, **P<0.05 versus trauma, #P>0.05 versus EGCGI group.
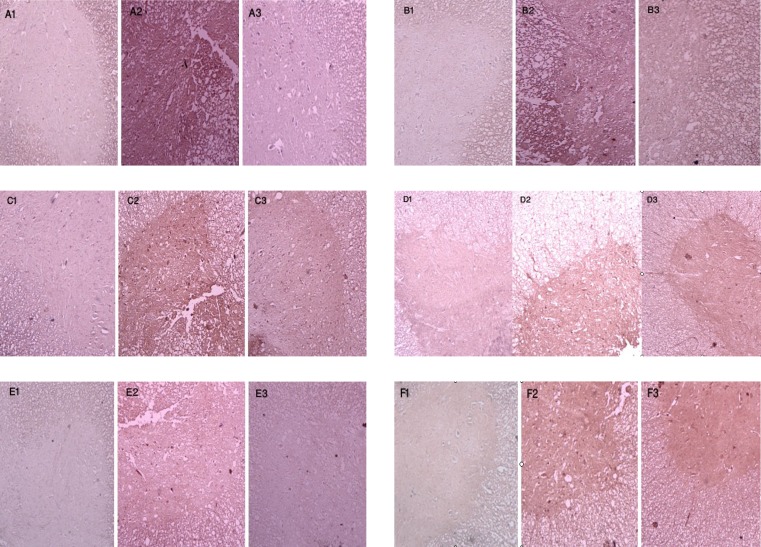
Effects of EGCG on expression of TNF-α, IL-1β, iNOS, COX-2, PARP, and nitrotyrosine. Figure 2 shows the immunohistochemical staining of TNF-α (A), IL-1β (B), iNOS (C), COX-2 (D), PARP (E), and nitrotyrosine (F), respectively. Almost no positive reaction could be detected in sham-operated groups for all of the antigens (A1, B1, C1, D1, E1 and F1), whereas the spinal cord sections of traumatized rats exhibited an increased positive staining for these antigens (A2, B2, C2, D2, E2 and F2). EGCG treatment significantly reduced the degree of positive staining for all of the antigens (A3, B3, C3, D3, E3 and D3). The quantitative analysis of the antigen expression in experimental groups has been shown in Table 1.
Fig. 2.
Immunohistochemical expression of TNF-α, IL-1β, iNOS, COX-2, PARP, and nitrotyrosine. Light photomicrographs show TNF-α reactivity (A1, sham; A2, trauma; A3, EGCG), IL-1β reactivity (B1, sham; B2, trauma; B3, EGCG), iNOS reactivity (C1, sham; C2, trauma; C3, EGCG), COX-2 reactivity (D1, sham; D2, trauma; D3, EGCG), PARP reactivity (E1, sham; E2, trauma; E3, EGCG), and nitrotyrosine reactivity (F1, sham; F2, trauma; F3, EGCG) 24 hours after injury (counterstained with hematoxylin, magnification × 100). The positive staining of the antigens has been presented by brown color.
Table 1.
Densitometry analysis of immunohistochemical photographs for TNF-α, IL-1β, iNOS, COX-2, PARP, and nitrotyrosine.
| Experimental group | TNF-α | IL-1β | iNOS | COX-2 | PARP | Nitrotyrosine |
|---|---|---|---|---|---|---|
| Sham | 0.0044 ± 0.0018 | 0.0000 ± 0.0000 | 0.0129 ± 0.0079 | 0.0794 ± 0.0316 | 0.0007 ± 0.0004 | 0.0002 ± 0.0001 |
| Trauma | 0.4736 ± 0.0407* | 0.4197 ± 0.0767* | 1.8342 ± 0.1899* | 1.7451 ± 0.2671* | 0.1876 ± 0.0142* | 0.4219 ± 0.0836* |
| EGCGI | 0.0303 ± 0.0229** | 0.0166 ± 0.0095** | 1.0151 ± 0.1309** | 0.3136 ± 0.0506** | 0.0191 ± 0.0043** | 0.1166 ± 0.0148** |
| EGCGII | 0.0341 ± 0.0092**# | 0.0243 ± 0.0049**# | 0.7950 ± 0.2144**# | 0.1532 ± 0.0362**# | 0.0167 ± 0.0078**# | 0.0713 ± 0.0077**# |
Data are expressed as a percentage of total tissue area. *P<0.001 versus sham; **P<0.001 versus trauma; #P>0.05 versus EGCGI group
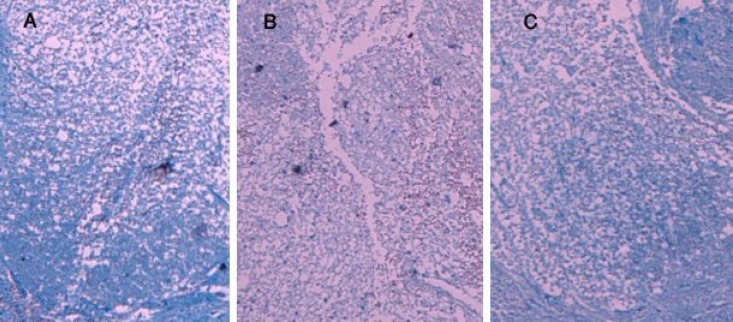
Effects of EGCG on myelin preservation. Myelin structure of the spinal cord was clearly stained by Luxol fast blue in sham group (Fig. 3A), whereas, a significant loss of myelin in dorsal funiculus, site of contusion, was observed in the spinal cord sections of traumatized rats (Fig. 3B). In EGCG-treated rats, myelin degradation was attenuated in the dorsal funiculus (Fig. 3C).
Fig. 3.
Effects of EGCG on myelin preservation. Photomicrographs of horizontal sections through the lesion epicenter were taken 24 hours after SCI in sham (A), trauma (B), and EGCG (C) groups (stained with Luxol fast blue, magnification ×100).
DISCUSSION
Secondary auto-destructive processes of SCI have a highly debilitating pathology, considered to be a number of interrelated processes. Injury to the spinal cord provokes a local inflammatory response which amplifies the secondary damage. The inflammatory response involves noncellular and cellular components. It is well documented that the potent pro-inflammatory cytokines including TNF-α and IL-1β, which are synthesized immediately after injury, play detrimental roles in post-traumatic injury associated with SCI [11]. TNF-α and IL-1β are involved in a wide range of events, including neuronal and glial secondary cell death [12], vascular permeability [13], recruitment of inflammatory cells [14], induction of iNOS [15] and cyclooxigenase-2 (COX-2) [16], and glutamate excitotoxicity [17] in the injury site. In this regard, it has been well demonstrated that the blocking of TNF-α or IL-1β confers neuroprotection and improves functional recovery following experimental SCI [18]. On the other hand, TNF-α and IL-1β play a central role in the induction of iNOS [15]. iNOS is a one of the three distinct enzymes that produces nitric oxide, a free radical gas molecule which is known to have a crucial role in the development of the secondary inflammatory response and apoptosis following traumatic SCI [19]. In this regard, some studies have clearly demonstrated that attenuation of iNOS expression is secondary to a reduced formation of endogenous TNF-α and IL-1β [18]. Similar to iNOS, the expression of COX-2, a enzyme which is involved in the generation of some inflammatory mediators, is also mediated by TNF-α and IL-1β [16]. COX inhibitors can improve functional outcome after spinal cord contusion [20]. In this study, we
demonstrated that EGCG treatments attenuated significantly expression of TNF-α and IL-1β, and consequently expression of iNOS and COX-2. These results are in agreement with our previous study in which treatment with EGCG improved motor function after SCI. Although the most famous and widely renowned properties of catechins have long been attributed to the antioxidant and free radical scavenging effects [2], emerging evidences have shown the neuroprotective effects of the catechins against neurodegenerative or neuroinflammatory diseases such as Alzheimer׳s disease [6], Parkinson's disease [6], and multiple sclerosis disease [21].
There is substantial evidence that the anti-inflammatory effects of EGCG, the most effective catechins, may be due in part to inhibition of iNOS [2]. In this regard, in vitro studies have shown that EGCG inhibited the induction of iNOS mRNA after treatment with TNF-α and IL-1 [22]. Moreover, it has been well established that EGCG inhibits iNOS activity and expression following brain damage [23]. Catechins also enhanced the production of IL-10, an anti-inflammatory cytokine [24]. Another study has also shown that the production of eicosanoids by COX, a major pathway leading to the endpoint of inflammation, reduced significantly postischemia by catechins [25].
Peroxynitrite, a cytotoxic molecule produced in the spinal cord tissue following trauma, contributes to the post-traumatic inflammatory reaction including tyrosine nitration and lipid peroxidation [26], and also cause DNA damage [27] resulting in the activation of PARP. On the other hand, overactivation of PARP, a nuclear enzyme which is activated by strand break in DNA, results in depletion of NAD and ATP and ultimately cell death [28]. It has been clearly demonstrated that SCI induced PARP activation, and treatment with PARP inhibitors significantly reduced the development of inflammation and apoptosis in the traumatized tissue [29]. In this regard, we have previously identified that EGCG treatment induced up-regulation of antiapoptotic Bcl-2 and down-regulation of proapoptotic Bax in injured spinal tissue [9]; this phenomenon might be due in part to reductional expression of PARP, which is observed in this study. Koh et al. [30] showed that EGCG inhibited many points of the apoptotic pathway such as poly (ADP-ribose) polymerase cleavage. Meanwhile, our present immunohistochemical findings have shown that nitrotyrosine formation, a relatively specific marker for the detection of the endogenous peroxynitrite formation, decreased in EGCG treatment groups. It has been documented that catechins can scavenge peroxynitrite by preventing tyrosine nitration [31].
Post-traumatic inflammation is characterized in part by the accumulation of activated leukocytes, especially neutrophils, within the injured tissue following SCI in rat [32]. Some evidences suggested that these cells play an important role in the pathogenesis of secondary degeneration such as lipid peroxidation and myelin vesiculation [33]. We have observed in the present study that the MPO activity reduced significantly in treated rats when compared with non-treated rats. Moreover, in this study, reduction of demyelination was observed in EGCG treatment groups. The current results are in agreement with our previous study in which lipid peroxidation decreased in EGCG treatment groups [9]. In this regard, it has been documented that administration of EGCG to rats exposed to ultraviolet prevented infiltration of neutrophils and lymphocytes [34]. Moreover, induction of vascular adhesion molecule-1 by TNF-α and IL-1 is prevented with EGCG, which subsequently reduced monocytes adhesion [35].
Finally, our results showed that administration of EGCG immediately and 1 h after SCI, significantly attenuated inflammatory responses. These findings have not only helped to achieve a better understanding of the neuroprotective mechanisms of EGCG, but also offered the possibilities of potential therapeutic use in SCI.
ACKNOWLEDGEMENTS
This work was supported by , Lorestan (No 100.185384).
References
- 1.Amar A.P., Levy M.L. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland B.A., Rahman R.M., Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 2006;17:291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Kimura M., Umegaki K., Kasuya Y., Sugisawa A., Higuchi M. The relation between single/double or repeated tea catechin ingestions and plasma antioxidant activity in humans. Eur. J. Clin. Nutr. 2002;56:1186–1193. doi: 10.1038/sj.ejcn.1601471. [DOI] [PubMed] [Google Scholar]
- 4.Etus V., Etus T., Belce A., Ceylan S. Green tea polyphenol (-)-epigallocatechin gallate prevents oxidative damage on periventricular white matter of infantile rats with hydrocephalus. Tohoku J. Exp. Med. 2003;200:203–209. doi: 10.1620/tjem.200.203. [DOI] [PubMed] [Google Scholar]
- 5.Koh S.H., Lee S.M., Kim H.Y., Lee K.Y., Lee Y.J., Kim H.T., Kim J., Kim M.H., Hwang M.S., Song C., Yang K.W., Lee K.W., Kim S.H., Kim O.H. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci. Lett. 2006;395:103–107. doi: 10.1016/j.neulet.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb O., Mandel S., Amit T., Youdim M.B. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004;15:506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.He M., Zhao L., Wet M.J., Yao W.F., Zhao H.S., Chen F.J. Neuroprotective effects of (-)-epigallocatechin-3-gallate on aging mice induced by D-galactose. Biol. Pharm. Bull. 2009;32:55–60. doi: 10.1248/bpb.32.55. [DOI] [PubMed] [Google Scholar]
- 8.Khan N., Adhami V.M., Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutr. Cancer. 2009;61:836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalatbary A.R., Tiraihi T., Beigi Boroujeni M., Ahmadvand H., Tavafi M., Tamjidipoor A. Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 2010;1306:168–175. doi: 10.1016/j.brainres.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 10.Mullane K. Neutrophil-platelet interactions and post-ischemic myocardial injury. Prog. Clin. Biol. Res. 1989;301:39–51. [PubMed] [Google Scholar]
- 11.Hayashi M., Ueyama T., Nemoto K., Tamaki T., Senba E. Sequential mRNA expression for immediate early genes, cytokines and neurotrophins in spinal cord injury. J. Neurotrauma. 2000;17:203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y.B., Yune T.Y., Baik S.Y., Shin Y.H., Du S., Rhim H., Lee E.B., Kim Y.C., Shin M.L., Markelonis G.J., Oh T.H. Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp. Neurol. 2000;166:190–195. doi: 10.1006/exnr.2000.7494. [DOI] [PubMed] [Google Scholar]
- 13.Schnell L., Fearn S., Schwab M.E., Perry V.H., Anthony D.C. Cytokine-induced acute inflammation in the brain and spinal cord. J. Neuropathol. Exp. Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Pineau I., Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 15.Matsuyama Y., Sato K., Kamiya M., Yano J., Iwata H., Isobe K. Nitric oxide: a possible etiologic factor in spinal cord cavitation. J. Spinal Disord. 1998;11:248–252. [PubMed] [Google Scholar]
- 16.Tonai T., Taketani Y., Ueda N., Nishisho T., Ohmoto Y., Sakata Y., Muraguchi M., Wada K., Yamamoto S. Possible involvement of interleukin-1 in cyclooxygenase-2 induction after spinal cord injury in rats. J. Neurochem. 1999;72:302–309. doi: 10.1046/j.1471-4159.1999.0720302.x. [DOI] [PubMed] [Google Scholar]
- 17.Chao C.C., Hu S., Ehrlich L., Peterson P.K. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav. Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 18.Genovese T., Mazzon E., Crisafulli C., Di Paola R., Muia C., Bramanti P., Cuzzocrea S. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J. Pharmacol. Exp. Ther. 2006;316:1006–1016. doi: 10.1124/jpet.105.097188. [DOI] [PubMed] [Google Scholar]
- 19.Genovese T., Mazzon E., Mariotto S., Menegazzi M., Cardali S., Conti A., Suzuki H., Bramanti P., Cuzzocrea S. Modulation of nitric oxide homeostasis in a mouse model of spinal cord injury. J. Neurosurg. Spine. 2006;4:145–153. doi: 10.3171/spi.2006.4.2.145. [DOI] [PubMed] [Google Scholar]
- 20.Resnick D.K., Graham S.H., Dixon C.E., Marion D.W. Role of cyclooxygenase 2 in acute spinal cord injury. J. Neurotrauma. 1998;15:1005–1013. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- 21.Aktas O., Prozorovski T., Smorodchenko A., Savaskan N.E., Lauster R., Kloetzel P.M., Infante-Duarte C., Brocke S., Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 22.Tedeschi E., Menegazzi M., Yao Y., Suzuki H., Forstermann U., Kleinert H. Green tea inhibits human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1alpha activation. Mol. Pharmacol. 2004;65:111–120. doi: 10.1124/mol.65.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland B.A., Shaw O.M., Clarkson A.N., Jackson D.N., Sammut I.A., Appleton I. Neuroprotective effects of (-)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB J. 2005;19:258–260. doi: 10.1096/fj.04-2806fje. [DOI] [PubMed] [Google Scholar]
- 24.Crouvezier S., Powell B., Keir D., Yaqoob P. The effects of phenolic components of tea on the production of pro- and anti-inflammatory cytokines by human leukocytes in vitro. Cytokine. 2001;13:280–286. doi: 10.1006/cyto.2000.0837. [DOI] [PubMed] [Google Scholar]
- 25.Hong J.T., Ryu S.R., Kim H.J., Lee J.K., Lee S.H., Kim D.B., Yun Y.P., Ryu J.H., Lee B.M., Kim P.Y. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res. Bull. 2000;53:743–749. doi: 10.1016/s0361-9230(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard-Fillion B., Souza J.M., Friel T., Jiang G.C., Vrana K., Sharov V., Barron L., Schoneich C., Quijano C., Alvarez B., Radi R., Przedborski S., Fernando G.S., Horwitz J., Ischiropoulos H. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J. Biol. Chem. 2001;276:46017–46023. doi: 10.1074/jbc.M105564200. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad R., Rasheed Z. Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immuno-pharmacol. Immunotoxicol. 2009;31:388–396. doi: 10.1080/08923970802709197. [DOI] [PubMed] [Google Scholar]
- 28.Scott G.S., Szabo C., Hooper D.C. Poly(ADP-ribose) polymerase activity contributes to peroxynitrite-induced spinal cord neuronal cell death in vitro. J. Neurotrauma. 2004;21:1255–1263. doi: 10.1089/neu.2004.21.1255. [DOI] [PubMed] [Google Scholar]
- 29.Genovese T., Mazzon E., Muia C., Patel N.S.A., Threadgill M.D., Bramanti P., De Sarro A., Thiemermann C., Cuzzocrea S. Inhibitors of poly(ADP-ribose) polymerase modulate signal transduction pathways and secondary damage in experimental spinal cord trauma. J. Pharmacol. Exp. Ther. 2005;312:449–457. doi: 10.1124/jpet.104.076711. [DOI] [PubMed] [Google Scholar]
- 30.Koh S.H., Kim S.H., Kwon H., Park Y., Kim K.S., Song C.W., Kim J., Kim M.H., Yu H.J., Henkel J.S., Jung H.R. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Brain Res. Mol. Brain Res. 2003;118:72–81. doi: 10.1016/j.molbrainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Pannala A.S., Rice-Evans C.A., Halliwell B., Singh S. Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochem. Biophys. Res. Commun. 1997;232:164–168. doi: 10.1006/bbrc.1997.6254. [DOI] [PubMed] [Google Scholar]
- 32.Carlson S.L., Parrish M.E., Springer J.E., Doty K., Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- 33.Popovich P.G., Wei P., Stokes B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Katiyar S.K., Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukoc. Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 35.Ludwig A., Lorenz M., Grimbo N., Steinle F., Meiners S., Bartsch C., Stangl K., Baumann G., Stangl V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]