INTRODUCTION
It is strongly accepted that the surface of a biomaterial is a place where first interaction between biomaterial and biological system takes place. Therefore, the surface properties of a biomaterial play an important role in determining its biocompatibility, and strongly influence biological response to it. It also determines its long-term performance in vivo. Hence, it is important to design biomaterials with proper surface properties. Obviously, biomaterials must also possess bulk properties that meet other requirements such as mechanical strength in order to function properly in the biological environment. Indeed, it is difficult to design biomaterials that fulfill both needs; however, a common approach is suggested to fabricate biomaterials with adequate and acceptable bulk properties. In addition, the modification of biomaterial surface is needed to enhance its biocompatibility [1-3].
A new nanocomposite (NC) polymer based on incorporation of polyhedral oligomeric silses-quioxane (POSS) into poly (carbonate‐urea) urethane (PCU) has been recently patented by University College London (UCL-NanoTM) [4]. This NC is used for biomedical applications due to its proper properties such as appropriate mechanical properties, non‐biodegradation, long-term bio-stability, and its good biocompatibility as it is tested in a sheep model for the period of 3 years [5-9]. UCL‐NanoTM is generally considered as a biocompatible polymer with acceptable cell adhesion on it, of course, it would be an ideal material if the rate of cell adhesion and proliferation increases over it. In fact, in order to reach this goal, some surface modifications are needed. Several surface modification techniques have been developed to improve cell adhesion and proliferation on the surface of polymeric biomaterials [10, 2].
The protein adsorption to polymer surface and/or coating substrates with extracellular matrix (ECM) proteins such as fibronectin, laminin, collagen, or vitronectin is one way to enhance the cell affinity toward the polymers. The reason behind this is that these methods rely on creating the new chemical groups on the polymer surface which favors cell adhesion. As it is known in natural tissue, cells are surrounded with ECM, then if the surface of biomaterial contains binding sites similar to the natural ECM, cells will interact with the material in a comparable way, theoretically cells will recognize the implant as if it was a part of the body [11, 12]. Accordingly, in order to enhance cell adhesion and proliferation, the immobilization of collagen as main part of ECM on the surface of substrate was studied extensively [13, 14]. It was found that these collagen -immobilized surfaces can improve attachment and proliferation of different types of cells [15-18]. With this in mind, in the present study, the effects of plasma and collagen immobilization on the cell adhesion and proliferation of UCL‐NanoTM were investigated.
In this study, collagen type I was directly reacted with plasma activated NC (P-NC) without using low molecular weight reagents such as acrylic acid. Plasma treatment is widely used to enhance cell adhesion on surfaces [19-21], but the effect of plasma treatment is not permanent and it reduces by time [22, 23]. In this work, it was hypothesized that the hydroperoxide groups which were introduced by low temperature plasma on the surface might be used as a promoter to graft the collagen layer on the surface of NC. The immobilization of collagen and other natural polymers such as gelatin with one-step plasma method has been done elsewhere in order to increase cell adhesion [13, 24, 25]. The modified surfaces of NC films were characterized by water contact angle (WCA) measurement, water uptake, Fourier transform infrared spectroscopy in attenuated total reflection mode (ATR-FTIR) and scanning electron microscopy (SEM). Furthermore, the cellular behaviors of human umbilical vascular endothelial cells (HUVEC) such as attachment, growth and proliferation, in contact with surfaces of NC were also evaluated in vitro by optical microscopy and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test.
MATERIALS AND METHODS
The NC film (type: PQ 070409-2% POSS, thickness: 0.1 mm) was made in the UCL. Collagen type I was obtained from Sigma Aldrich, UK. NC sheets were cleaned with 70% (v/v) ethanol in an ultrasonic water bath for 5 min. The samples were washed with deionized water (dH2O) for 5 min and dried at room temperature for 24 h before plasma treatment. The low pressure plasma system (Nano-RF, Diener Electronic GmbH, Germany) was used to treat NC films (20 × 20 mm2) with plasma reactor operating at a frequency of 40 kHz, a gas pressure of 0.2 mbar, an oxygen flow rate of 50 sccm and a power of 60 W and the plasma treatment time was 120 s.
According to our previous study, plasma treatment for 120 s with 60 W plasma power results is super-hydrophile surface [26, 27]. Collagen solution was added drop-wise on activated NC film (P-NC) surface. The concentration and pH of collagen solution were 1 mg.mL-1 and 3.0, respectively. The sample was put for collagen immobilization at 37°C for 12 h. Free collagen was removed from the sample by rinsing with 0.1 N acetic acid solution and then with water [13]. The collagen-grafted NC (COL-P-NC) films were stored at 4°C before use.
The graft thickness and surface morphology of COL-P-NC films were evaluated using a SEM, Vega-II XMU (Tescan U.S.A Inc). The films were mounted on SEM stubs and coated by vapor deposition using a sputter coater with a gold (Au) target for conductance and high resolution imaging. The optimum parameters for SEM imaging used an electron accelerating voltage of 20 kV with a working distance about 15 mm.
Surface hydrophilicity of NC, P-NC and COL-P-NC films was evaluated by measuring the contact angle formed between water drops and the surface (immediately after plasma treatment for P-NC and 24 h after collagen grafting for COL-P-NC), and the amount of their absorbed water. To measure WCA, a droplet of water (approximately 5 μl) was placed on the dry film surface and the contact angles were determined by goniometry at room temperature after 1 min using a Krüss G10 goniometer (Krüss GmbH, Germany) with image capture software. The test was done on 5 different places of the surface, and average values were obtained. To determine the surface water uptake, the samples were dried in a vacuum oven at ambient temperature and weighted; after that, they were immersed into distilled water for 30 s and their weight increase was determined to obtain the surface water uptake as shown below:
Surface water uptake = Wa-Wb/A (1)
Where Wa is the weight after immersing in water, Wb is weight before immersing into water, and A is the surface area. The data obtained are the mean values of five readings. The presence of grafted collagen and the changes in chemical structure of NC films were investigated using a Bruker (IFS-48) ATR-FTIR spectrophotometer with a KRS-5 prism (Bruker Optik GmbH, Germany). The incident angle was 45° and progressive scanning measurements for untreated and collagen-grafted films ranged from 4000-650 cm-1.
The HUVEC from the National Cell Bank of Iran (Pasteur Institute of Iran) was used to evaluate the cell attachment and proliferation. The NC films (NC, P-NC and COL-P-NC) were washed with phosphate buffer saline (PBS) sterile and then placed in a 24-well tissue culture plate on sterile cabinet. HUVEC suspension (2 ml , 5 × 104 cells/ml) in medium DMEM (GIBCO™, USA) containing 10% fetal calf serum (GIBCO™, USA) was added to each well and cultured under a humidified atmosphere of 5% CO2/95% air in the incubator at 37°C. After 24 h, the samples were removed from the well, washed with PBS twice, fixed with ethanol and then stained with hematoxylin-eosin (H & E). All the samples were air-dried and the morphology of attached cells on the films was examined by an optical microscope (Nikon, Japan).
The proliferation rate of the HUVEC on different samples was measured using MTT assay [28,29]. Briefly, on the first day, NC, P-NC and COL-P-NC films (five sample of each) were put into a 96-well plate and 5 × 103 cells were added to each well. After the incubation for 72 h, the medium was eliminated and 100 μL of a 0.5 mg/mL solution of MTT in PBS (Sigma, USA) was added to each well following the incubation at 37°C for 4 h. The purple formazan crystals (formed in the mitochondria of the cells) were detected and later dissolved by addition of 100 μL isopropanol (Sigma, USA) to each well. The plates were then incubated at 37°C for 15 min prior to absorb the measurements. The optical density was recorded on a multi-well microplate reader (ICN, Switzerland) at 545 nm.
RESULTS
The schematic reactions of the plasma treatment and collagen grafting are shown in Figure 1. The presence and thickness of the grafted layer was observed by comparison between cross-sectional view of SEM micrograph for NC and COL-P-NC (Fig. 2). Surface morphology and topography of NC films was investigated after O2 plasma treatment and collagen graft using SEM. Figure 3 highlights SEM micrographs of NC, P-NC and COL-P-NC captured at magnification ×5000.
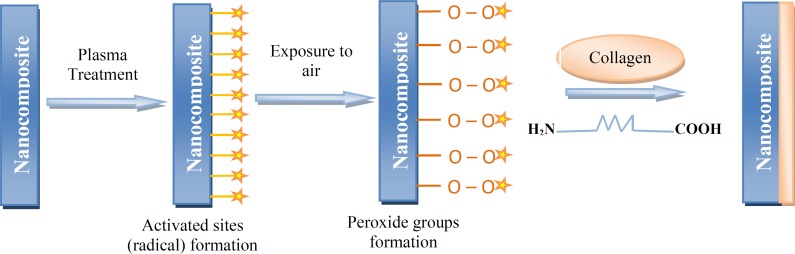
Fig. 1 .
Schematic illustration of collagen immobilization on the surface of nanocomposite (◊ indicates radical).
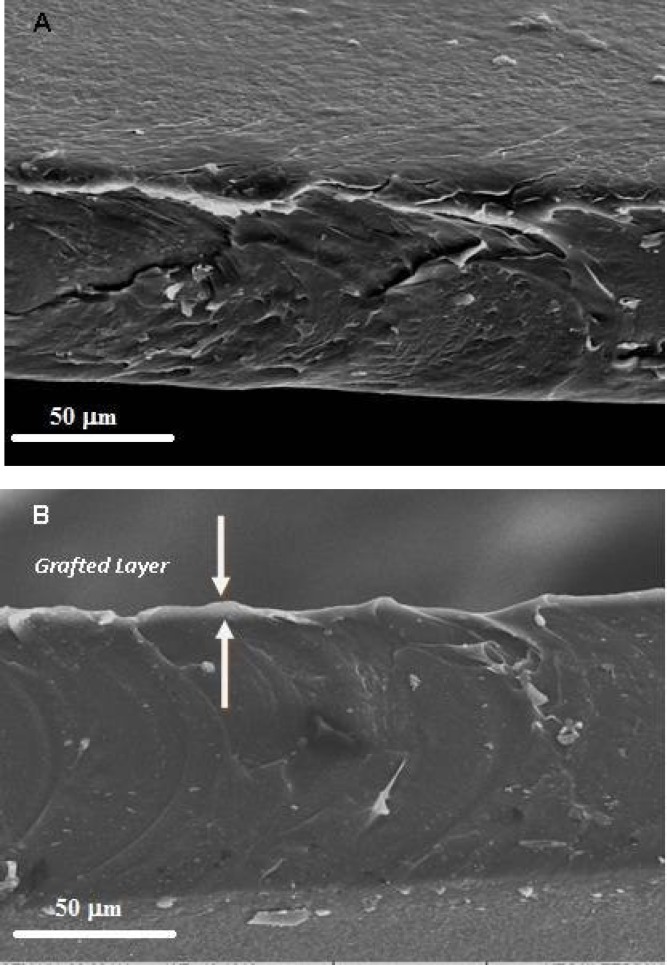
Fig. 2.
SEM cross-sectional micrograph of (A) NC and (B) COL-P-NC (magnification ×1000). The thickness of the grafted layer is about 3-5 µm.
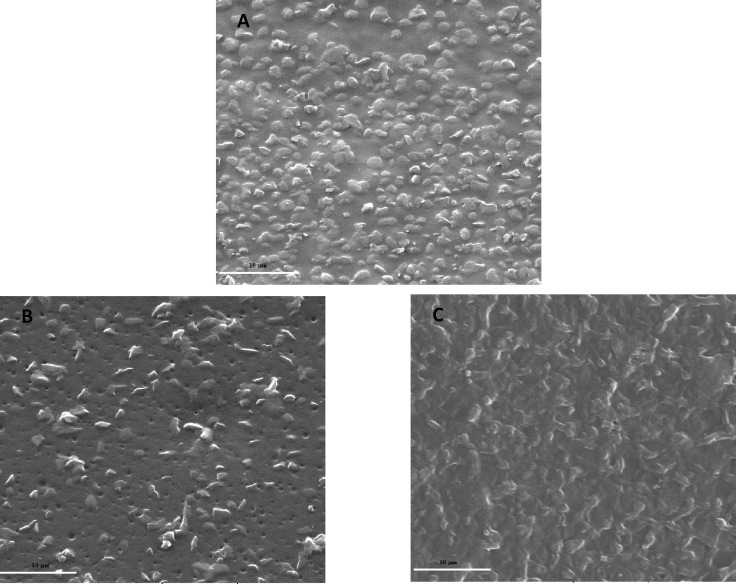
Fig. 3.
SEM micrographs of (A) NC, (B) P-NC and (C) COL-P-NC films (magnification ×5000).
The effect of surface modification on the hydrophilicity was illustrated by WCA and surface water uptake as presented in Table 1. The average of WCA of NC surface was markedly decreased after oxygen plasma treatment and collagen immo-bilization (Table 1). Moreover, surface water uptake was also significantly increased due to either plasma treatment or collagen immobilization. These two experiments proved enhancement in the hydro-philicity of NC.
Table 1 .
Water contact angle and surface water uptake for NC, P-NC and COL-P-NC
| Sample | Water contact angle (°) | Surface water uptake (µg/cm 2 ) |
|---|---|---|
| NC | 92 ± 4 | 0 |
| P-NC | 6 ± 2* | 200 ± 25* |
| COL-P-NC | 52 ± 5* | 240 ± 15* |
* P<0.05 in comparison with NC (n = 5).
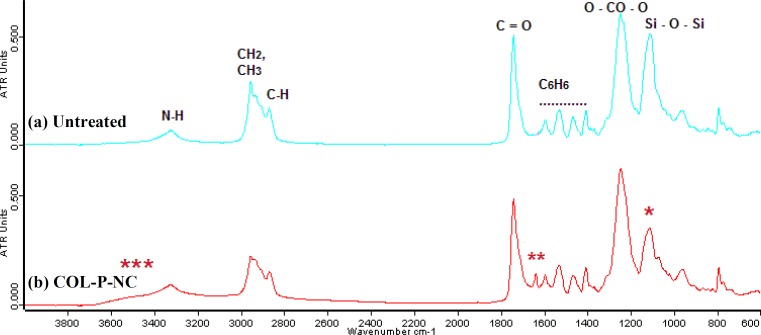
Figure 4 represents the ATR-FTIR spectra of untreated and collagen-grafted NC. As it is marked in the Figure 4, three notable changes have been happened to the spectra after collagen grafting.
Fig. 4.
ATR-FTIR spectra of (a) untreated nanocomposite and (b) collagen-grafted nanocomposite. *** broad peak which is located at 3000-3600 cm-1 can be ascribed to the stretching of the hydroxyl groups (-OH) or NH groups from grafted collagen groups; ** a new peak at 1648 cm-1 which might belong to vibration of amide groups because of collagen immobilization; * is a decrease in strength of Si-O-Si bands, (between 900-1100 cm-1) which may be attributed to the breaking of covalent bonds and chain scission events as a result of plasma treatment before collagen graft.
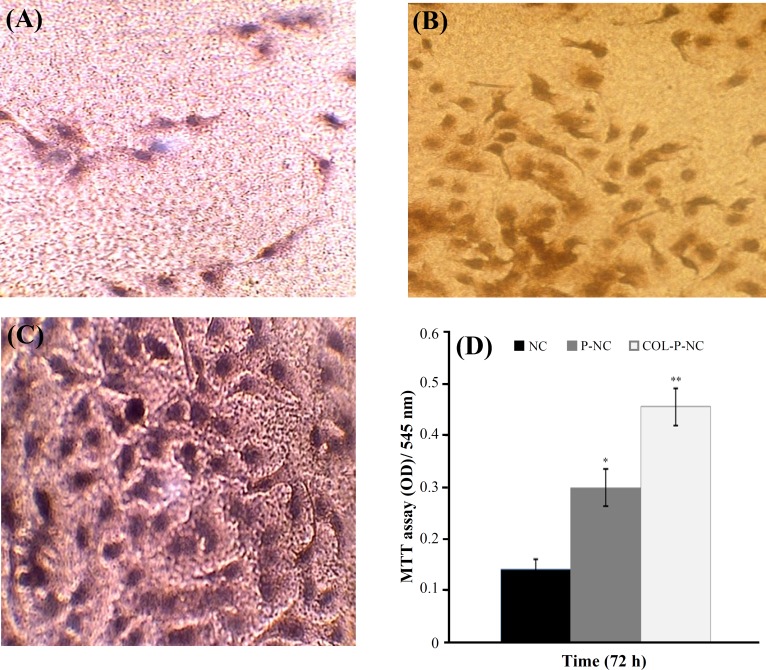
Cell adhesion and growth onto the surface of COL-P- NC, P-NC, and NC films were used to study the interaction of the surface with the cells. The morphology of cells after 24 h incubation with HUVEC is shown in Figure 5. As displayed in Figure 5, the number of attached cells to P-NC (Fig. 5B) and COL-P-NC (Fig. 5C) was significantly larger than NC with P<0.05 (Fig. 5A). Furthermore, the number of attached cells on COL-P-NC was more than P-NC (P<0.05), and they were distributed everywhere on the substrate. The same results can be also obtained from quantitative experiment-MTT test as seen in Figure 5D. Each material was cut into 5 pieces, and the average value was used to evaluate the number of living cells attached to the substrates. The results showed that the number of living cells on COL-P-NC was much larger than those on NC and P-NC.
Fig. 5.
Optical micrographs of the HUVEC response to (A) NC, (B) P-NC, (C) COL-P-NC after 24 h in culture at magnification ×400. (D) The metabolic activity of HUVEC through the MTT assay on NC, P-NC, and COL-P-NC films over a 3-day period. *P<0.05 when compared with NC and **P<0.005 when compared with both NC and P-NC using the student's t-test (n = 5).
DISCUSSION
As it comes from Figure 1, the radicals are formed on the surface of NC films. When the films were exposed to air, the produced radicals, immediately reacted with oxygen and peroxide radicals, were formed. Collagen solution was added drop-wise on P-NC surface and a layer of collagen was grafted on the NC surface. The reasons behind plasma pre-treatment of the films before grafting are: (1) producing polar groups (hydroperoxide groups) on the chemically inert surface of NC so that it would physically react with collagen, and (2) the production of peroxide groups which either may act as initiators in the grafting step and increase in hydrophilicity [30].
Figure 2 clearly indicates that the grafted layer was restricted to the surface region of the film with a thickness of about 3-5 µm. The untreated and O2 treated films (Fig. 3A and 3B) were similar in appearance with micron scale surface protrusions as a result of the integration of cross-linked POSS within PCU. After plasma treatment, the surface structures appeared to be abraded with tiny surface pores ranging from 0.25 to 1 µm in diameter, which are occurred due to the interaction of reactive O2 gas with the NC surface. However, after grafting of collagen, the surface of NC films became rougher than that of control and plasma-treated films (Fig. 3C).
As presented in Table 1, the oxygen plasma treatment for 120 s with power of 60 W resulted in a drastic decrease in average WCA from 92° to 6°, indicating that the material is becoming super-hydrophile. The changing of WCA of the collagen-grafted sample was from 92° to 52°, also implied that collagen was successfully immobilized onto NC surface, which caused the surface to be more hydrophile. The same results could be also obtained from water uptake test as shown in Table 1. It was observed that hydrophobic surface of NC did not
show any increase in its weight after it was immersed into water for 30 s, and its superficial water was dried by filter paper, meaning that the water just was on its surface and was not absorbed. Therefore, the amount of water uptake for NC was zero. Unlike untreated NC, the surfaces of P-NC and COL-P-NC are able to absorb water within 30 s immersion into the water and their water uptake amount was 200 and 240 (µg/cm2), respectively. The reason behind this increase in hydrophilicity is that the bombardment of the NC film by energetic particles during plasma activation breaks covalent bonds which lead to the formation of surface radicals (peroxide radicals). This phenomenon is followed by hydrogen abstraction from the side chains and adding functional groups such as carboxyl or hydroxyl groups to polymer surfaces. These formed hydroperoxide or other polar groups caused an increase in the hydrophilicity [31].
The ATR-FTIR spectra of untreated NC and COL-P-NC have been compared in Figure 4. ATR-FTIR spectrum of untreated NC correlates with the vibration modes of individual functional groups of the NC polymer backbone (Figure 4a). For example, the peak at 3321 cm-1 could be identified as -NH vibrations, presumably from urea and urethane components of the polymer. Larger peak at 2953 cm-1 corresponds to -CH vibrations from the methylene segments within the polymer chain, and the side peak at 2868 cm-1 to -CH vibrations from -NH groups. Peaks at 1738 cm-1 was identified as -C = O vibration arising from carbonate and urethane segments. The -C = C bonds of 4,4’- methylenebis (phenyl isocyanate) (MDI, hard crystalline segment) were evident at 1529 cm-1. Peaks at 1244 cm-1 were assigned to O-CO-O vibrations from the long chain polymer backbone. The peak at 1110 cm-1 could be assigned to POSS containing siloxane bonding (Si-O-Si) [32]. Comparison between spectra of Figure 4a and 4b show that the ATR-FTIR spectrum of collagen-grafted NC is not significantly different from untreated NC. As it is noted in literature, poly-peptide chains of collagen with different types more or less contain some free -COOH as well as -NH2 groups on the residues of amino acids such as aspartic acid, glutamic acid and lysine etc., respectively. It is mentioned that typical carbonyl absorption band of carboxyl groups in grafted copolymers is usually found between 1710 and 1730 cm-1 [33]. Therefore, there is a possibility that the intense carbonyl group signal of the polyurethane group in the backbone of NC overlaps with the much lower intensity peak of the correspondent COOH group [34]. Hence, the carbonyl peak at 1730 cm-1 from collagen carboxyl groups is masked with inherent vibration of carbonyl functional groups in NC backbone. Comparing Figure 4a and 4b emphasizes that there are three remarkable differences in COL-P-NC spectrum with untreated NC. First, a broader peak which is located at 3000-3600 cm-1 can be ascribed to the stretching of the hydroxyl groups (-OH) or NH groups from grafted collagen groups [34]. Second, a new peak at 1648 cm-1 which might belong to vibration of amide groups because of collagen immobilization. Third one is a decrease in strength of Si-O-Si bands, (between 900-1100 cm-1) which may be attributed to the breaking of covalent bonds and chain scission events as a result of plasma treatment before collagen graft [35].
The effects of the two different surfaces i.e. the plasma treated and collagen immobilized on the HUVEC behavior were observed by the light microscope, and are shown in Figure 3. It is known that the cellular behavior on a biomaterial is an important factor to determine its biocompatibility. The whole process of adhesion and spreading of cells after contact with biomaterials consist of cell attachment, filopodia growth, cytoplasmic webbing and flattening cell mass, and the ruffling peripheral cytoplasm, which progress in a sequential fashion [36]. As seen in Figure 5, the cell attachment onto the surface of untreated NC is negligible, while improvement in attachment and growth of endothelial cells was observed onto oxygen plasma treated and collagen-grafted NC surfaces (Fig. 3). The same results can also be obtained from quantitative experiment-MTT test, as shown in Figure 5d. Each material was cut into 5 pieces, and average value was used to evaluate the number of living cells attached to the substrates. The results showed that the number of living cells on COL-P-NC was larger than those on NC and P-NC (P<0.001) and even so close to the number of living cells in contact with polystyrene tissue culture plate (as negative control). Although P-NC showed more cell adhesion in both qualitative and quantitative experiments than NC (P<0.01), it suggested that the collagen immobilization was beneficial to the attachment of endothelial cells. That is to say that COL-P-NC had better cell compatibility in comparison with NC and P-NC.
As reported in the literature, cell adhesion onto biomaterial surface depends on the wettability of the surface. Neither substrates with high WCA (super-hydrophobic) nor substrates with low WCA (super-hydrophile) are favorable to the process of the cell adhesion. Surfaces with moderate hydrophilicity were found the best support to the cell adhesion [37]. According to our observation, COL-P-NC with WCA of 52° showed most cell adhesion meanwhile NC and P-NC which had extremely hydrophobic and hydrophile surfaces (WCA 94° and 6°, respectively) did not show much cell adhesion. As seen in this study, oxygen plasma treatment slightly increased the number of attached cells; the morphological changes during plasma treatment might be reason for this positive effect on cell growth and proliferation [38, 36]. Hence, it is concluded that the oxygen plasma-treated and collagen-grafted NC films with submicrometer or nanometer scales roughness provide an environment for better cell attachment and growth. Obviously, the presence of collagen as a main component of ECM was able to encourage cell adhesion and proliferation too [39, 37]. Therefore, collagen-grafted surface showed more biocompatibility performance in comparison with just plasma treatment.
In conclusion, surfaces of this new generation of NC have been successfully modified with both plasma treatment and collagen immobilization. The results of surface modification have shown that the cell adhesion and proliferation have been significantly improved. These changes in cell adhesion and proliferation could be explained by changes in wettability, surface morphology or creating new functional groups on the surface. The increase in cell adhesion and proliferation has significant application in tissue engineering and development of hybrid medical devices such as small diameter bypass graft, the main interest of this consortium.
ACKNOWLEDGEMENTS
We are grateful for the support and help given by Mr. Radfar and Dr. Bonakdar during the period of this study, and Mr. Stephan Kamali for English editing. We should mention that the nanocomposite in development of cardiovascular application is funded by EPSRC and Welcome Trust.
References
- 1.Ikada Y. Surface modification of polymers for medical applications. Biomaterials. 1994;15:725–736. doi: 10.1016/0142-9612(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 2.Inagaki N. Plasma Surface Modification and Polymerization. Lancaster, USA: TechnomicPublication Co; 1996. pp. 43–88. [Google Scholar]
- 3.Hoffman A. S., Frederick B. D. R., Schoen J. Biomaterials Science: An Introduction to Materials in Medicine. Academic Press; 2004. [Google Scholar]
- 4.Salacinski, H.J., Handcock, S., Seifalian A.M., inventors. Polymer for use in conduits and medical devices. World Pat WO2005070998. 2005
- 5.Olbrich M., Punshon G., Frischauf I., Salacinski H.J., Rebollar E., Romanin C., Seifalian A.M., Heitz J. UV surface modification of a new nanocomposite polymer to improve cytocompatibility. J. Biomater. Sci. Polym. Ed. 2007;18:453–468. doi: 10.1163/156856207780425059. [DOI] [PubMed] [Google Scholar]
- 6.Ghanbari H., Viatge H., Kidane A.G., Burriesci G., Tavakoli M., Seifalian A.M. Polymeric heart valves: new materials, emerging hopes. Trends Biotechnol. 2009;27:359–367. doi: 10.1016/j.tibtech.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Kannan R.Y., Salacinski H.J., Edirisinghe M.J., Hamilton G., Seifalian A.M. Polyhedral oligomeric silsesquioxane-polyurethane nanocomposite microvessels for an artificial capillary bed. Biomaterials. 2006;27:4618–4626. doi: 10.1016/j.biomaterials.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Bakhshi R., Eaton-Evans J., Edirisinghe M., Darbyshire A., You Z., Seifalian A.M., Hamilton G. A Novel Nanocomposite Polymer for the Development of a New Aortic Stent Graft. Br. J. Surg. 2009;96:5–15. [Google Scholar]
- 9.Seifalian A.M., Salacinski H.J., Tiwari A., Edwards A., Bowald S., Hamilton G. In vivo biostability of a poly(carbonate-urea) urethane graft. Biomaterials. 2003;24:2549–2557. doi: 10.1016/s0142-9612(02)00608-7. [DOI] [PubMed] [Google Scholar]
- 10.Huang N., Yang P., Leng Y.X., Wang J., Sun H., Chen J.Y., Wan G.J. Surface modification of biomaterials by plasma immersion ion implantation. Surf. Coat Tech. 2004;186:218–226. [Google Scholar]
- 11.Kurihara, H., Nagamune, T. Cell adhesion ability of artificial extracellular matrix proteins containing a long repetitive Arg-Gly-Asp sequence. J. Biosci. Bioeng. 2005;100:82–87. doi: 10.1263/jbb.100.82. [DOI] [PubMed] [Google Scholar]
- 12.Shin H., Jo S., Mikos A. G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 13.Li Y.H., Huang Y.D. The study of collagen immobilization on polyurethane by oxygen plasma treatment to enhance cell adhesion and growth. Surf. Coat Tech. 2007;201:5124–5127. [Google Scholar]
- 14.Yang J., Bei J., Wang S. Enhanced cell affinity of poly (,-Lactide) by combining plasma treatment with collagen anchorage. Biomaterials. 2002;23:2607–2614. doi: 10.1016/s0142-9612(01)00400-8. [DOI] [PubMed] [Google Scholar]
- 15.Shariati S.R.P., Shokrgozar M.A., Vossoughi M., Eslamifar A. In vitro co-culture of human skin keratinocytes and fibroblasts on a biocompatible and biodegradable scaffold. Iran. Biomed. J. 2009;13:169–177. [PubMed] [Google Scholar]
- 16.Bisson I., Hilborn J.N., Wurm F., Meyrat B., Frey P. Human urothelial cells grown on collagen adsorbed to surface-modified polymers. Urology. 2002;60:176–180. doi: 10.1016/s0090-4295(02)01642-4. [DOI] [PubMed] [Google Scholar]
- 17.Bisson I., Kosinski M., Ruault S., Gupta B., Hilborn J.N., Wurm F., Frey P. Acrylic acid grafting and collagen immobilization on poly(ethylene terephthalate) surfaces for adherence and growth of human bladder smooth muscle cells. Biomaterials. 2002;23:3149–3158. doi: 10.1016/s0142-9612(02)00061-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y., Chan-Park M.B. Density quantification of collagen grafted on biodegradable polyester: its application to esophageal smooth muscle cell. Anal. Biochem. 2007;363:119–127. doi: 10.1016/j.ab.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Wan Y.Q., Yang J., Bei J., Wang S. Cell adhesion on gaseous plasma modified poly-(L-Lactide) surface under shear stress field. Biomaterials. 2003;24:3757–3764. doi: 10.1016/s0142-9612(03)00251-5. [DOI] [PubMed] [Google Scholar]
- 20.Alves C.M., Yang Y., Marton D., Carnes D.L., Ong J.L., Sylvia V.L., Dean D.D., Reis R.L., Agrawal C.M. Plasma surface modification of poly(D,L-lactic acid) as a tool to enhance protein adsorption and the attachment of different cell Types. J. Biomed. Mater. Res. B Appl. Biomater. 2008;87:59–66. doi: 10.1002/jbm.b.31068. [DOI] [PubMed] [Google Scholar]
- 21.Hanson A.D., Wall M.E., Pourdeyhimi B., Loboa E.G. Effects of oxygen plasma treatment on adipose-derived human mesenchymal stem cell adherence to poly(L-lactic acid) scaffolds. J. Biomater. Sci. Polym. Ed. 2007;18:1387–1400. doi: 10.1163/156856207782246812. [DOI] [PubMed] [Google Scholar]
- 22.Sanchis M.R., Calvo O., Fenollar O., Garcia D., Balart R. Characterization of the surface changes and the aging effects of low-pressure nitrogen plasma treatment in a polyurethane film. PolymTest. 2008;27:75–83. [Google Scholar]
- 23.Shen F., Zhang E., Wei Z. Surface bio-modification of poly(Hydroxybutyrate-co-hydroxy-hexanoate) and its aging effect. Colloid Surf. B. Biointerfaces. 2009;73:302–307. doi: 10.1016/j.colsurfb.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Tabata Y., Lonikar S.V., Horii F., Ikada Y. Immobilization of collagen onto polymer surfaces having hydroxyl groups. Biomaterials. 1986;7:234–238. doi: 10.1016/0142-9612(86)90110-9. [DOI] [PubMed] [Google Scholar]
- 25.Lee M.R., Kwon K.W., Jung H., Kim H.N., Suh K.Y., Kim K., Kim K.S. Direct differen-tiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–4366. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Mirzadeh H., Bagheri S.H. Comparison of the Effect of Excimer Laser Irradiation and RF Plasma Treatment on Polystyrene Surface. Radiat. Phys. Chem. 2007;76:1435–1440. [Google Scholar]
- 27.Solouk A., Cousin B.G., Mirzadeh H., Solati- Hashjin M., Najarian S., Seifalian A.M. Surface modification of POSS-nanocomposite biomaterials using reactive oxygen plasma treatment for cardiovascular surgical implants application. J. Biotech. Appl. Biochem. 2011 doi: 10.1002/bab.22. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Bonakdar S., Emami S.H., Shokrgozar M.A., Farhadi A., Ahmadi S.A.H., Amanzadeh A. Preparation and characterization of polyvinyl alcohol hydrogels crosslinked by biodegradable polyurethane for tissue engineering of cartilage. Mater. Sci. Eng. 2010;C30:636–643. [Google Scholar]
- 29.Zali H., Rezaei-Tavirani M., Kariminia A., Yousefi R., Shokrgozar M.A. Evaluation of growth inhibitory and apoptosis inducing activity of human calprotectin on the human gastric cell line (Ags) Iran. Biomed. J. 2008;12:7–14. [PubMed] [Google Scholar]
- 30.Karkhaneh A., Mirzadeh H., Ghaffariyeh A.R. Simultaneous graft copolymerization of 2-hydroxyethyl methacrylate and acrylic acid onto polydimethylsiloxane surfaces using a two-step plasma treatment. J. Appl. Polym. Sci. 2007;105:2208–2217. [Google Scholar]
- 31.Khorasani M.T., Mirzadeh H. Effect of oxygen plasma treatment on surface charge and wettability of PVC blood bag/in vitro assay. Radiat. Phys. Chem. 2007;76:1011–1016. [Google Scholar]
- 32.Solouk A., Solati-Hashjin M., Najarian S., Mirzadeh H., Seifalian A.M. Opti-mization of acrylic acid grafting onto POSS-PCU nanocomposite using response surface methodology. Iran. Polym. J. 2011;20:91–107. [Google Scholar]
- 33.Gupta B., Plummer C., Bisson I., Frey P., Hilborn J. Plasma-induced graft poly-merization of acrylic acid onto poly(ethylene terephthalate) films: characterization and human smooth muscle cell growth on grafted films. Biomaterials. 2002;23:863–871. doi: 10.1016/s0142-9612(01)00195-8. [DOI] [PubMed] [Google Scholar]
- 34.Choi H.S., Kim Y.S., Zhang Y., Tang S., Myung S.W., Shin B.C. Plasma-induced graft co-polymerization of acrylic acid onto the polyurethane surface. Surf. Coat. Tech. 2004;182:55–64. [Google Scholar]
- 35.Augustine B. H., Hughes W. C., Zimmermann K. J., Figueiredo A. J., Guo X., Chusuei C. C., Maidment J. S. Plasma Surface Modification and Characterization of POSS-Based Nanocomposite Polymeric Thin Films, Langmuir,' 23: 4346-4350. Langmuir. 2007;23:4346–4350. doi: 10.1021/la063180k. [DOI] [PubMed] [Google Scholar]
- 36.Dadsetan M., Mirzadeh H., Sharifi N., Daliri M. Cell behavior on laser surface-modified polyethylene terephthalate in vitro. J. Biomed. Mater. Res. A. 2001;57:183–189. doi: 10.1002/1097-4636(200111)57:2<183::aid-jbm1157>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Sun B., Ziemer K.S., Barabino G.A., Carrier R.L. Chemical and physical modifications to poly(dimethylsiloxane) surfaces affect adhesion of caco-2 cells. J. Biomed. Mater. Res. A. 2010;93:1260–1271. doi: 10.1002/jbm.a.32621. [DOI] [PubMed] [Google Scholar]
- 38.Parvin A., Mirzadeh H., Khorasani M.T. Physicochemical and biological evaluation of plasma-induced graft polymerization of acrylamide onto polydimethylsiloxane. J. Appl. Polym. Sci. 2008;107:2343–2349. [Google Scholar]
- 39.Keshvari H., Mirzadeh H., Mansoori P. Collagen immobilization onto acrylic acid laser-grafted silicone for using as artificial skin: in vitro. Iran Polym. J. 2008;17:171–182. [Google Scholar]