Abstract
Background: Dendritic cells (DC) induce tumor or pathogen-specific T cell responses in humans. Several laboratories have developed culture systems, including maturation factors for human DC from peripheral blood monocytes. We comprehensively compared standard maturation stimulus, an autologous monocyte-conditioned medium (MCM), with heparin for their ability to promote uniformly mature DC that elicit T cell responses. Methods: A short (4-day) priming of plastic adherent monocytes with granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-4 with or without heparin was followed by 48-hour incubation in MCM to generate fully mature and stable DC. Phenotypic and functional analyses were carried out using anti-CD14 and anti-CD83 monoclonal antibodies, and mixed lymphocyte reaction, respectively. Results: We found that fully matured DC with a large amount of cytoplasm and copious dendritic projections were visible at the end of culturing period in the presence of MCM, heparin and MCM plus heparin. Thus, DC generated with these maturation factors are non-adherent and have typical satellite morphology. Flow cytometric analysis using anti-CD14 (monocyte marker) and anti-CD83 (mature DC marker) revealed that expression of CD14 decreased in MCM plus heparin-treated DC, and the expression of CD83 was increased when heparin and MCM used as a maturation factor. Functionally, MCM and MCM plus heparin-treated DC showed stronger mixed leukocyte reaction than heparin alone. Conclusion: These results support the use of the MCM with heparin as maturation factor that could result in functionally mature monocyte-derived DC in comparison to either MCM or heparin alone.
Key Words: Dendritic cell (DC), Maturation, Monocyte Conditioned Medium (MCM), Heparin
Introduction
Ralph Steinman first described Dendritic cells (DC) [1] as subpopulation of cells in the spleen with a striking dendritic shape. These cells were non-phagocytic, loosely adherent, and of low buoyant density [1]. It was soon appreciated that these bone marrow-derived cells existed in all lymphoid and most non-lymphoid tissues. DC were described as cells that constitutively expressed both major histo-compatibility complex class I and class II antigens, and most importantly, stimulated naive CD4+ and CD8+ T cells to respond to nominal and alloantigens more effectively than any other previously described antigen presenting cell [2]. Several reviews on DC and their role in immune regulation have been appeared recently [3, 4].
Over the past years, several in vitro methods have been described for generation and differentiation of DC from peripheral blood monocytes, mostly based on culturing them in a medium containing granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-4 [5,6]. These cells show functional and phenotypic characteristics, which is typical of the immature DC. These cells should undergo another differentiation step as maturation. DC maturation, first proposed as a critical step in immunogenicity 25 years ago, which is now accepted as a crucial component of the induction of most adaptive immune responses. The term of maturation refers to an intricate differentiation process whereby DC responds rapidly to an environmental stimulus and become capable of eliciting adaptive immunity. The type of stimulus determines the program of DC differentiation and the subsequent host immune response [7].
DC can be differentiated into mature cells by various stimulations, such as TNF-α, LPS, -conditioned medium (MCM), PPD, CD40L and platelet-derived cytokines [4, 8-11]. MCM, which is produced by culturing monocytes on immobilized human γ-globulin, contains a cocktail of cytokines; each has different roles in DC maturation [9,12]. Furthermore, it has been recently discovered that heparin together with GM-CSF and IL-4 was able to consistently induce the differentiation of majority of monocytes to CD1a+ DC in the presence or absence of autologous serum or plasma. It has been also found that heparin-treated DC is more potent than heparin-untreated DC in priming naive CD4+ T cells [13,14].
Here, we describe that heparin can induce stable maturation of DC, and MCM augments heparin-treated DC maturation in terms of phenotypic and functional characteristics.
MATERIALS AND METHODS
Media and reagents. Complete medium, including RPMI-1640 (Gibco, Germany), supplemented with 10% human AB serum (Blood Transfusion Organization, Tehran, Iran), 2.5 × 10-5 M 2-ME, 2 mM L-glutamine (Sigma Chemical Co., Munich, Germany), 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma Chemical Co., Munich, Germany) was used to culture cells from peripheral blood mononuclear cells (PBMC). Recombinant human GM-CSF (Novartis-Basel, Switzerland), and IL-4 (Sigma Chemical Co., Munich, Germany), were used to derive immature DC from peripheral blood monocytes, and heparin and monocyte conditioned medium (MCM) were used as maturation factor. MCM was prepared as described elsewhere. [9]. Briefly, PBMC were layered onto the human Ig-coated bacteriologic plates for 1 hour, non-adherent cells were washed away and Ig-adherent cells were incubated in fresh complete medium with 1% autologous plasma at 37ºC for no more than 24 hours. The medium was collected and frozen at -20ºC until use. Isolation of T cells was carried out using anti-CD3 magnetic bead cell sorting technique (Miltenyi Biotec, Germany) and heparin (Sigma Chemical Co., Munich, Germany) was used as maturation factor at concentration of 50 U/ml at the beginning of culture. Allogeneic mixed lymphocyte reaction (MLR) was performed by [3H] thymidine (Amersham Pharma, London, UK) uptake test.
Generation of immature DC. DC were generated from peripheral blood monocytes of five volunteers as described elsewhere [15]. Briefly, complete RPMI medium, supplemented with 1000 U/ml recombinant human GM-CSF and 800 U/ml recombinant human IL-4, was added to the adherent cell population (enriched monocytes) and the cells were incubated at 37°C and 5% CO2. After 3 days, the cells were fed again with the same doses of IL-4 and GM-CSF. On the day 5 of the culture, aggregates of loosely adherent immature DC were apparent which were used for the maturation experiments. Heparin was added at the beginning of culture to two sets of experiments.
Maturation induction. To induce maturation, heparin was used at concentration of 50 U/ml at the beginning of culture. Furthermore, two groups of DC (heparin-treated and heparin-untreated) were stimulated on day 5 with 25% v/v of MCM. Mature DC, which were harvested to review their morphology by light and phase contrast microscopy as well as phenotypic and functional analyses, were appeared on day 7.
Antibodies and flow cytometry. Immunophenotyping of monocyte-derived DC was performed by direct immunofluorescence staining of cell surface antigens using FITC or RPE-conjugated mouse monoclonal antibodies against CD14 and CD83 and the appropriate isotype-matched controls (Serotech, London, UK). The cells were harvested by replacement of culture medium with 5 ml phosphate-buffered saline (PBS) containing 0.5 mM EDTA, and were then resuspended and washed by ice cold FACS buffer (PBS containing 0.1% NaN3, 5 mM EDTA and 2% FCS). After washing, cells were aliquoted at 105 cells per tube and incubated for 30 minutes on ice in 1% normal mouse serum, then, cells washed again and 10 l of each conjugated monoclonal antibody was added to the cell pellet and the tubes were incubated on ice in the dark for 45 minutes. Finally, cells were washed and resuspended in 300-500l of FACS buffer and analyzed immediately. Data acquisition was carried out on a PARTEC flow cytometer (PARTEC, Germany) and analyzed using FlowMax software. Data was expressed as percentage of positive cells in comparison to the cells stained with isotype-matched controls.
Allogeneic mixed lymphocyte reaction . Allogeneic MLR test for T cell stimulatory function of mature DC was performed as described [15]. Briefly, the DC were irradiated with 30Gy and added in graded doses (1:5, 1:10, and 1:20) as stimulators for 2 × 105 magnetic bead cell sorting isolated allogeneic T cells in 96-well, U-bottomed plates (Costar, Cambridge, USA). Cultures were established at a final volume of 200 µl of complete medium, supplemented with 10% AB serum. Phytohemagglutinin-stimulated T cells (2.5%, Sigma Chemical Co., Munich, Germany) and DC or T cells alone served as positive and negative controls, respectively. Proliferation was determined on day 5 with addition of 1 Ci/ml of [3H] thymidine to triplicate wells for last 18 hours. Proliferative responses were measured by a liquid scintillation counter (Wallac Inc., Turku, Finland) and expressed as mean counts per minute and the stimulation index (SI) was obtained for triplicate wells as follow:
 |
Fig. 1.
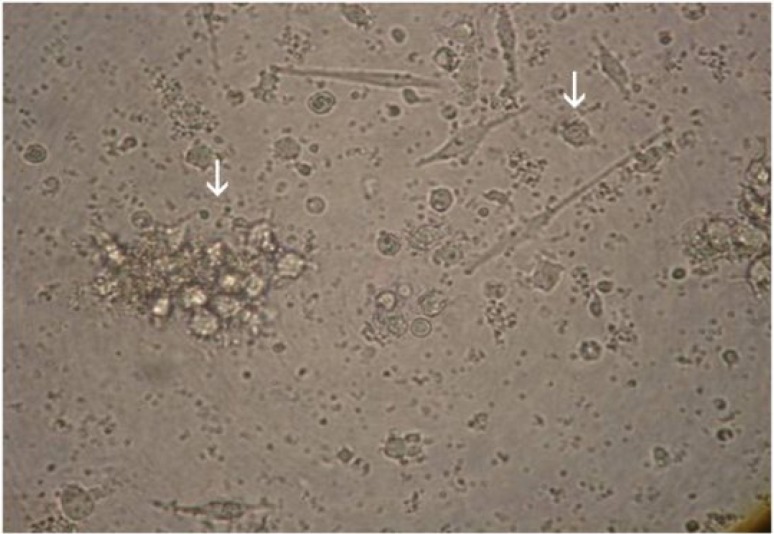
Monocyte-derived dendritic cells differentiated with heparin, MCM or both. Peripheral blood mononuclear cells from five volunteers were incubated at 37oC for 2 h and the adherent cells were cultured in the presence of GM-CSF and IL-4 and/or heparin. Immature DC were stimulated with MCM as maturation factors and mature DC were harvested on day 7. Most of the mature DC were appeared as single cells or loosely adherent aggregates (white arrows) reviewed by light microscopy (magnification ×400).
Statistical analysis. The data depicted in each figure corresponds to one representative experiment of at least five independently performed experiments. One-way ANOVA test was used for statistical analysis of differences among experimental groups. Difference at P value <0.05 was statistically considered significant.
RESULTS
DC morphology. DC were generated from peripheral blood monocytes of five healthy volunteers. Three days after culturing the plastic adherent monocytes in the presence of GM-CSF and IL-4, clusters of non-adherent cells appeared and increased in size thereafter. Heparin and MCM were added at the beginning of the culture and day five, respectively. Eighty percent of cells appeared to be loosely adherent to each other in clumps or isolated floating cells with typical dendritic morphology with typical dendritic morphology by day 7. These cells exhibited typical cytologic features of DC, i.e., large irregular cells with numerous cell membrane processes as viewed by light microscopy (Fig. 1). The yield ranged from 3-10% (mean ± SEM = 6 ± 0.5%) of plated PBMC and the viability of harvested DC was more than 98% as determined by trypan blue exclusion test. Our results showed no morphological differences on DC matured with heparin, MCM or both.
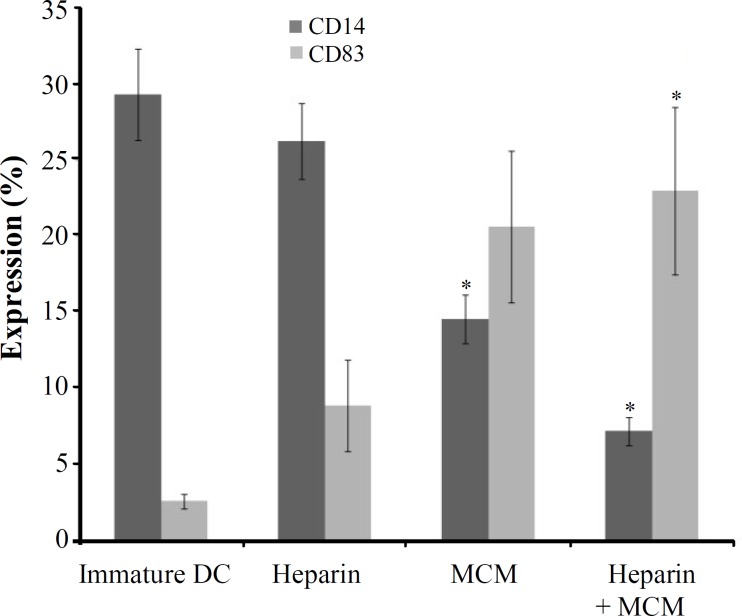
DC phenotype. Phenotypic characteristics of DC stimulated with heparin were examined and compared with those of the DC stimulated with MCM, or heparin plus MCM, using anti-CD14 and anti-CD83 monoclonal antibodies (Fig. 2). Flow cytometric analysis of DC revealed significant differences in the expression of surface molecules crucially used as DC markers. Compared to heparin-treated DC, MCM or MCM and heparin-treated DC showed a substantially reduced expression of CD14 significantly (P = 0.05). Our results showed that MCM plus heparin induced significantly higher levels of expression of maturation marker (CD83) compared to heparin alone (P = 0.05); however, in comparison to MCM alone, MCM plus heparin induced a higher level of CD83 expression non-significantly (Fig. 2).
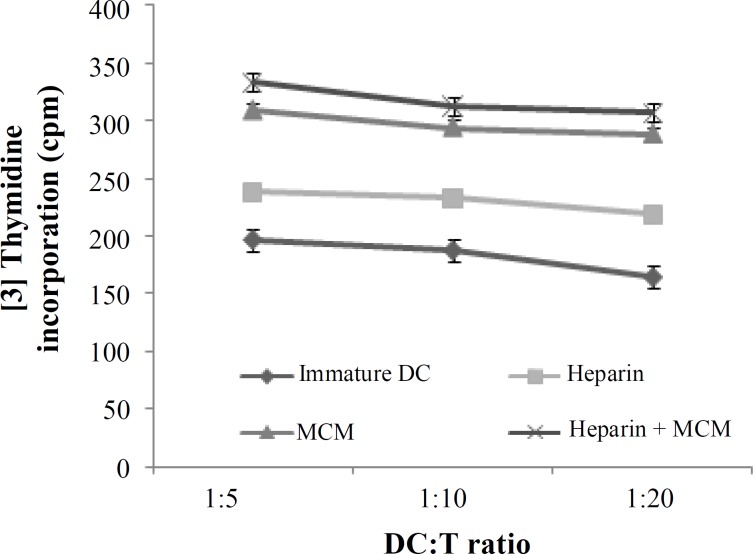
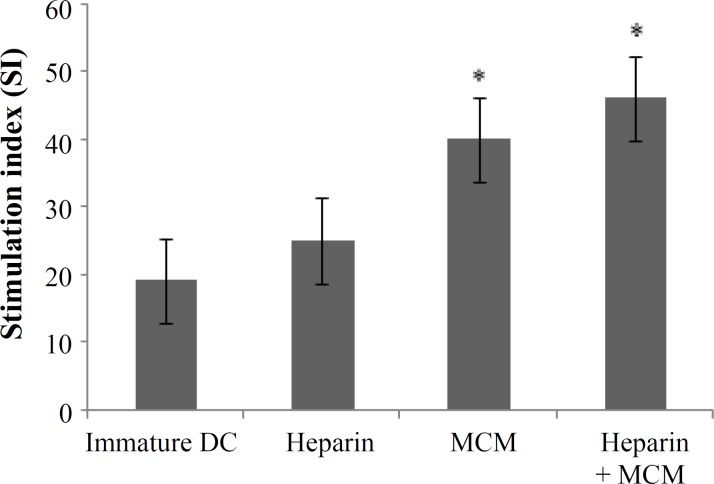
Mixed lymphocyte reaction. To address whether heparin or MCM-stimulated DC would be able to induce a proliferative response in allogeneic MLR, T cells were stimulated with heparin or MCM or both treated DC at a ratio of 1:5, 1:10, and 1:20. Our results showed that T cell proliferative responses were elicited in all three respective ratios for three examined groups (Fig. 3). However, proliferation rate and stimulation indices were higher in MCM or heparin plus MCM-treated groups compared to heparin group significantly (P = 0.05) (Figs. 3 and 4).
Fig. 2.
Flow cytometric analysis of expression of CD14 and CD83 as monocyte and dendritic cell surface markers, respectively. Monocyte-derived DC differentiated in the presence of heparin, MCM or heparin plus MCM were harvested on day 7 and analyzed by FACS using anti-CD14 and anti-CD83 monoclonal antibodies (*P<0.05).
Fig. 3.
Mixed leukocyte reaction induced by DC differentiated in the presence of heparin, MCM or heparin plus MCM. Allogeneic T cells were stimulated with heparin and/or MCM-matured DC at ratios of 1:5, 1:10, and 1:20 for 5 days. Uptake of [3H] thymidine during the last 18 h of incubation was then measured. The results of the T cell proliferation response from five subjects are expressed as a mean of triplicates.
DISCUSSION
Mature human DC has been shown to play a unique role in antitumor immunity [16, 17]. The generation of stable mature DC appears to be important for DC- based immune therapies. Given the unique properties and potent ability of mature DC generated in vitro to stimulate naive T cells, it is not surprising that different maturation agents such as poly(I:C), MCM, TNF-α, IL-1 and IL-6 have already been considered for in vitro DC maturation protocols [9, 18, 19]. Upon our experiences, above-mentioned agents as well as fibroblast, epithelial and in particular T cell conditioned media lead to DC maturation and immune response polarization to various extents (unpublished data).
Fig. 4.
Heparin and/or MCM-matured dendritic cells were analyzed functionally as stimulator of allogeneic mixed leukocyte reaction. Stimulation indices were obtained as explained in Materials and Methods section and expressed as mean of triplicates (*P<0.05).
Heparin is known to have multifaceted functions, and plays critical roles in regulation of blood coagulation [20, 21] and hemopoiesis [22, 23]. Growing evidence has supported that heparin could modulate immune responses such as langerhans cells trafficking in epidermis [24], transendothelial migration of DC [25] and generation of DC which are potent stimulators of T-cell proliferation [26]. Furthermore, it is reported that there are specific binding sites for heparin on monocytes [27]. However, there have been a few reports regarding the effect of heparin on the differentiation of DC from monocytes [14].
In the present study, we compared heparin with the MCM as a well-known maturation factor on the process of monocyte-derived DC maturation. Heparin was added to plastic adherent monocytes at the beginning of DC culture and MCM was added on day five. Then, mature DC were compared in terms of morphology, phenotype and function on day seven.
Previous studies [28] as well as our experience [5, 15] have described the mature DC as non-adherent, large irregular veiled cells with numerous cell membrane processes. Morphological examination of floated cells using inverted or phase-contrast light microscopy after treatment with heparin, MCM or both revealed no differences among these three kinds of maturation protocols (Fig. 1). When adherent monocytes were used for production of DC, some cells other than monocytes were present in the culture, which may affect phenotype and function of the resultant DC. Since these cells are present in all three tested groups, their effects can be considered the same. Therefore, we precede the work with comparison of phenotypic and functional properties.
It is believed that CD14 and CD83 are the main cell surface markers that characterize in vitro the generation of immature and mature DC from peripheral blood monocytes, respectively. In the present study, we used these markers to compare the phenotype of DC generated in the presence of heparin and/or MCM. It is well known that in vitro conversion of peripheral blood monocytes to immature DC accompanied by down regulation of CD14 expression. The most reduction in expression of CD14 was appeared when MCM was added to heparin-treated DC; However, heparin alone had the least effect on elimination of CD14 expression and there was not significant differences between immature and mature DC in this case (Fig. 2). It is noteworthy that our previous study had shown that CD14 was expressed as low as 10% on immature heparin-untreated DC (data not shown). Furthermore, others have shown that immature DC was negative for this surface marker when heparin is used at the beginning of the culture [14]. Altogether, it is found that heparin plus MCM is more effective than each of these factors alone in the generation of immature DC from CD14 positive monocytes.
It is frequently reported that MCM is required to ensure the development of fully differentiated DC from GM-CSF and IL-4-primed progenitor cell cultures [9], so we examined the expression of CD83 as a unique DC maturation marker on heparin and MCM-treated DC. In spite of CD14, heparin plus MCM-treated DC expressed the higher level of CD83 than heparin-treated cells significantly (P = 0.05, Fig. 2). In consistent with others, we previously found that MCM was a potent agent required to induce the development of fully differentiated CD83 + DC [15]; however, in this study, it is revealed that heparin could augment the effect of MCM on the expression of CD83. In consistent to results found by Xia and Kao [14], we showed that heparin could not augment the expression of CD83 by itself, but it plays a role in the presence of appropriate maturation factors such as LPS, IFN-γ, or MCM. The fairly lower level of CD83 expression in the presence of heparin and MCM may be due to interactions between heparin and cytokines contents of MCM, the case that should be more elucidated in the future.
The choice of a maturating agent for DC not only should be based on its impact on cell-surface phenotype (loss of CD14 and acquisition of CD83 expression), but also should take into account the nature of the functional state of DC, which have an instructive role for the immune response, including autologous or allogeneic mixed leukocyte reaction. It is reported that various maturation factors, such as MCM, poly(I:C), cytokines cocktail as well as heparin-matured DC are powerful stimulator of MLR [9, 14, 29]. Therefore, in the present study, we compared the potential of heparin or MCM-treated DC in stimulation of allogeneic lymphocytes in the ratios of 1:5, 1:10, and 1:20. We found that both MCM and heparin were capable to induce allogeneic MLR in all three examined ratios; however, the addition of MCM to heparin-treated DC augmented their stimulatory capacity significantly (P = 0.05, Figs. 3 and 4). In consistent with our results, it is reported that heparin-treated DC compared to heparin-untreated DC are more potent in priming allogeneic and autologous T cells to proliferate in allogeneic mixed lymphocytic culture [14].
Collectively, we investigated the effect of heparin on the differentiation of DC from monocytes, and compared it with MCM as a well-defined maturation agent. We found that heparin had dramatic effect on the promotion of monocytes to differentiate into mature DC and its effect is augmented by addition of MCM to the heparin-treated DC. It is reported that specific heparin-binding sites are known to be present on monocytes [26]. Thus, the effect of heparin on the differentiation of DC alone or in cooperation with MCM is most likely mediated by the direct binding of heparin to monocytes. This conclusion is further supported by the finding that heparin-binding proteins, such as protamine sulfate and PF-4 could neutralize the effect of heparin and prevent the differentiation of monocytes into DC [14]. Furthermore, it is equally likely that other components, not yet defined, which present in MCM might also be critical for this cooperative effect.
In conclusion, since DC play a pivotal role in immune responses, a great deal of research effort has been focused on the potential clinical application of these cells to cancer immune therapy [30]. Our findings indicate that the yields, viability, and morphology of heparin, MCM, and heparin plus MCM-treated DC were equally comparable and the latest combination may be more efficacious than DC treated with either agent alone for the induction of immune responses. Because most maturation agents considered for clinical DC-based vaccination protocols are MCM, and heparin is widely used in clinical applications, this simple method can easily be adapted for the generation of large amounts of mature DC from monocytes and considered as a reliable approach for immune therapy.
Acknowledgment
We grateful to Mr. Azizi and Mr. Meshki at the Institute of Biotechnology of Urmia University (IBUU), Mr. Jabbari at the Urmia Research Center of Hygiene School of Tehran University of Medical Science (TUMS), Mr. Heidar Sani at the Veterinary School of Urmia University (VSUU), and the staff of Radiotherapy Department of Omid Hospital, Urmia, Iran.
References
- 1.Steinman, R.M., Adams, J.C., Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. IV. Identification and distribution in mouse spleen. J. Exp. Med. 1975;141:804–820. [PMC free article] [PubMed] [Google Scholar]
- 2.Mellman, I. Antigen processing and presentation by dendritic cells: cell biological mechanisms. Adv. Exp. Med. Biol. 2005;560:63–67. doi: 10.1007/0-387-24180-9_9. [DOI] [PubMed] [Google Scholar]
- 3.Lipscomb, M.F., Masten, B.J. Dendritic cells: Immune regulators in health and disease. Physiol. Rev. 2002;82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 4.Merad, M., Manz, M.G. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagheri, K., Delirezh, N., Moazzeni, S.M. PPDextract induces the maturation of human monocyte-derived dendritic cells. Immunopharmacol. Immunotoxicol. 2008;30:91–104. doi: 10.1080/08923970701812654. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs, B., Wuttke, M., Papewalis, C., Seissler, J., Schott, M. Dendritic cell subtypes and in vitro generation of dendritic cells. Horm. Metab. Res. 2008;40:99–107. doi: 10.1055/s-2007-1022561. [DOI] [PubMed] [Google Scholar]
- 7.Münz, C., Steinman, R.M., Fujii, S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granucci, F., Ferrero, E., Foti, M., Aggujaro, D., Vettoretto, K., Ricciardi-Castagnoli, P. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1999;1:1079–1084. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 9.Reddy, A., Sapp, M., Feldman, M., Subklewe, M., Bhardwaj, N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–3646. [PubMed] [Google Scholar]
- 10.Tuyaerts, S., Aerts, J.L., Corthals, J., Neyns, B., Heirman, C., Breckpot, K., Thielemans, K., Bonehill, A. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol. Immunother. 2007;56:1513–1537. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamzeh-Cognasse, H., Cognasse, F., Palle, S., Chavarin, P., Olivier, T., Delezay, O., Pozzetto, B., Garraud, O. Direct contact of platelets and their released products exert different effects on human dendritic cell maturation. BMC Immunol. 2008;9:54–69. doi: 10.1186/1471-2172-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jongmans, W., Tiemessen, D.M., van Vlodrop, I.J., Mulders, P.F., Oosterwijk, E. Th1-polarizing capacity of clinical-grade dendritic cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J. Immunother. 2005;28:480–487. doi: 10.1097/01.cji.0000171290.78495.66. [DOI] [PubMed] [Google Scholar]
- 13.Tang, W., Sun, W.H., Wang, X.Y., Sun, X.L. Effect of heparin on immunologicfunctions of dendritic cells from patients with chronic hepatitis B. Zhoghua Gan Zang Bing Za Zhi. 2006;14:233–234. [PubMed] [Google Scholar]
- 14.Xia, C.Q., Kao, K.J. Heparin induces differentiation of CD1a+ dendritic cells from monocytes: phenotypic and functional characterization. J. Immunol. 2002;168:1131–1138. doi: 10.4049/jimmunol.168.3.1131. [DOI] [PubMed] [Google Scholar]
- 15.Delirezh, N., Moazzeni, S.M., Shokri, F., Shokrgozar, M.A., Atri, M., Kokhaei, P. Autologous dendritic cells loaded with apoptotic tumor cells induce T cell-mediated immune responses against breast cancer in vitro. Cell Immunol. 2009;257:23–31. doi: 10.1016/j.cellimm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Curigliano, G., Spitaleri, G., Dettori, M., Locatelli, M., Scarano, E., Goldhirsch, A. Vaccine immunotherapy in breast cancer treatment: promising, but still early. Expert. Rev. Anticancer Ther. 2007;7:1225–1241. doi: 10.1586/14737140.7.9.1225. [DOI] [PubMed] [Google Scholar]
- 17.Saito, H., Frleta, D., Dubsky, P., Paluka, A.K. Dendritic cell-based vaccination against cancer. Hematol. Oncol. Clin. North Am. 2006;20:689–710. doi: 10.1016/j.hoc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Sato, M., Takayama, T., Tanaka, H., Konisha, J., Suzuki, T., Kaiga, T., Tahara, H. Generation of mature dendritic cells fully capable of T helper type 1 polarization using OK-432 combined with prostaglandin E2. Cancer Sci. 2003;94:1091–1098. doi: 10.1111/j.1349-7006.2003.tb01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouas, R., Lewalle, P., Ouriaghli, F.E., Nowak, B., Duvillier, H., Martiat, P. Poly(I:C) used for human dendritic cell maturation preserves their ability to secondarily secrete bioactive IL-12. Int. Immunol. 2004;16:767–773. doi: 10.1093/intimm/dxh077. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, J. Heparin. N. Engl. J. Med. 1991;324:1365–1374. [Google Scholar]
- 21.Lindahl, U. What else can “Heparin” do? Haemostasis. 1999;29((Suppl. S1)):38–47. doi: 10.1159/000054111. [DOI] [PubMed] [Google Scholar]
- 22.Borghesi, L., Yamashita, A.Y., Kincade, P.W. Heparan sulfate proteoglycans mediate interleukin-7-dependent B lymphopoiesis. Blood. 1999;93:140–149. [PubMed] [Google Scholar]
- 23.Yang, L., Yang, Y.C. Heparin inhibits the expression of interleukin-11 and granulocyte macrophage colony-stimulating factor in primate bone marrow stromal fibroblasts through mRNA destabilization. Blood. 1995;86:2526–2533. [PubMed] [Google Scholar]
- 24.O'Sullivan, G.M., Boswell, C.M., Halliday, G.M. Langerhans cell migration is modulated by N-sulfated glucosamine moieties in heparin. Exp. Dermatol. 2000;9:25–33. doi: 10.1034/j.1600-0625.2000.009001025.x. [DOI] [PubMed] [Google Scholar]
- 25.Shikano, S., Bonkobara, M., Zukas, P.K., Ariizumi, K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- 26.Pietschmann, P., Stöckl, J., Draxler, S., Majdic, O., Knapp, W. Functional and phenotypic characteristics of dendritic cells generated in human plasma supplemented medium. Scand. J. Immunol. 2000;51:377–383. doi: 10.1046/j.1365-3083.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 27.Abbate, R., Gori, A.M., Modesti, P.A., Attanasio, M., Martini, F.C., Collela, A., Giusti, B., Cecioni, I., Neri Serneri, G.G. Heparin, monocytes and procoagulant activity. Haemostasis. 1990;20:98–100. doi: 10.1159/000216166. [DOI] [PubMed] [Google Scholar]
- 28.Adams, S., O,Naill, D.W., Bhardwaj, N. Recent advances in dendritic cell biology. J. Clin. Immunol. 2005;25:177–188. doi: 10.1007/s10875-005-4086-2. [DOI] [PubMed] [Google Scholar]
- 29.Verdijk, R.M., Mutis, T., Esendam, B., Kamp, J., Melief, C.J.M., Brand, A., Goulmy, E. Polyriboinosinic polyribocytidylic acid (Poly(I:C)) induces stable maturation of functionally active human dendritic cells. J. Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 30.Shortman, K., Lahoud, M.H., Caminschi, I. Improving vaccines by targeting antigens to dendritic cells. Exp. Mol. Med. 2009;41:61–66. doi: 10.3858/emm.2009.41.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]