Abstract
Since the discovery of echolocation in bats, the final phase of an attack on a flying insect, the ‘terminal buzz’, has proved enigmatic. During the buzz, bats increase information update rates by producing vocalizations up to 220 times s−1. The buzz's ubiquity in hawking and trawling bats implies its importance for hunting success. Superfast muscles, previously unknown in mammals, are responsible for the extreme vocalization rate. Some bats produce a second phase—buzz II—defined by a large drop in the fundamental frequency (F0) of their calls. By doing so, bats broaden their acoustic field of view and should thereby reduce the likelihood of insect escape. We make the case that the buzz was a critical adaptation for capturing night-flying insects, and suggest that the drop in F0 during buzz II requires novel, unidentified laryngeal mechanisms in order to counteract increasing muscle tension. Furthermore, we propose that buzz II represents a countermeasure against the evasive flight of eared prey in the evolutionary arms-race that saw the independent evolution of bat-detecting ears in various groups of night-flying insects.
Keywords: bats, echolocation, terminal buzz, superfast muscles, acoustic field of view
More than 1000 of the approximately 1200 bat species from Yangochiroptera and Yinpterochiroptera are laryngeal echolocators ([1]; figure 1). They produce high-frequency calls and use the returning echoes to orient themselves and estimate the position, size and shape of objects in their vicinity. The majority of these bats forage on airborne insects at night, which they capture in flight in open air (aerial hawking) and, in a few species, close to water surfaces (trawling). Indeed, powered flight and echolocation are considered to be key innovations that allowed bats to exploit this hitherto unrealized foraging niche [3,4]. Despite huge variation in echolocation call design (e.g. peak frequency varies from 9 to 212 kHz) between and within families of echolocating bats [1,4], the way call rate increases over the course of the aerial pursuit of prey appears highly conserved [4–6]. Laryngeal specializations that allow for production of calls of high fundamental frequencies (F0) and fast, smooth frequency modulation also appear similar across species [6,7].
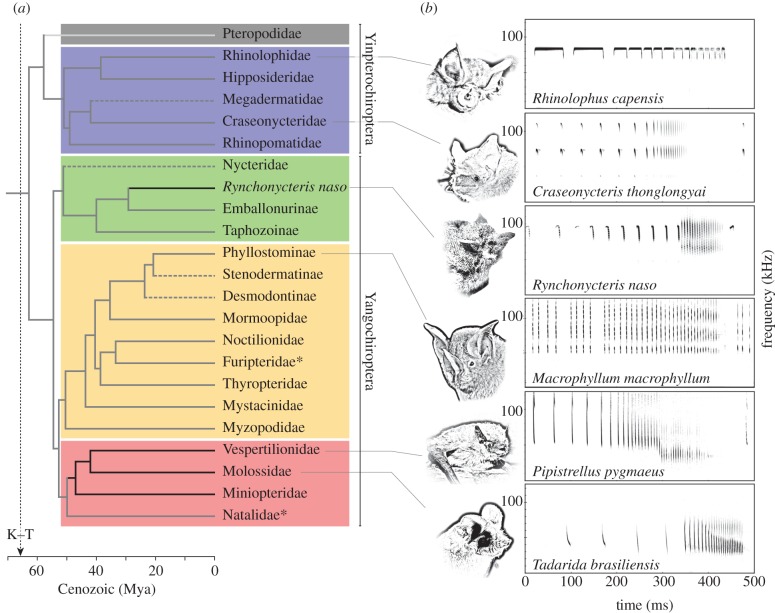
Figure 1.
(a) Molecular timescale and phylogenetic tree for Chiroptera (adapted from Miller-Butterworth et al. [2]). The light grey branch indicates the absence of laryngeal echolocation in Pteropodidae and dashed lines indicate no evidence for the buzz in these groups. Solid medium grey branches indicate the presence of buzz and black branches indicate the presence of buzz II. Asterisks indicate families for which there are no data on the presence/absence of the buzz and/or buzz II. Each coloured box comprises one of the four major lineages of echolocating bats (blue, Rhinolophoidea; green, Emballonuroidea; orange, Noctilionoidea; red, Vespertilionoidea). (b) Spectrograms of echolocation calls from attack sequences in six species that buzz. Buzz data complied from the published literature, excepting those for Myzopodidae, Thyropteridae and Rynchonycteris naso, which were provided by P. Racey, I. Geipel and S. Brinkløv, respectively.
Over the course of an attack, aerially hawking and water trawling bats increase call production rate while reducing call duration [3,4,8]. Attacks, successful or not, almost always end in a ‘terminal buzz’, where bats produce calls at rates above 100 calls s−1 and, in some species, up to and beyond 200 calls s−1 (figures 1 and 2) [3,6–9]. While the apparent function of the buzz is to quickly update the relative position of moving targets [4–6], how bats produce calls at such rates was until recently unknown. We now know that rare superfast muscles power the buzz [6]. For laryngeal echolocating bats, each call emitted is under active neuromuscular control [6,7]; we therefore assume that all bats that buzz possess superfast laryngeal muscles.
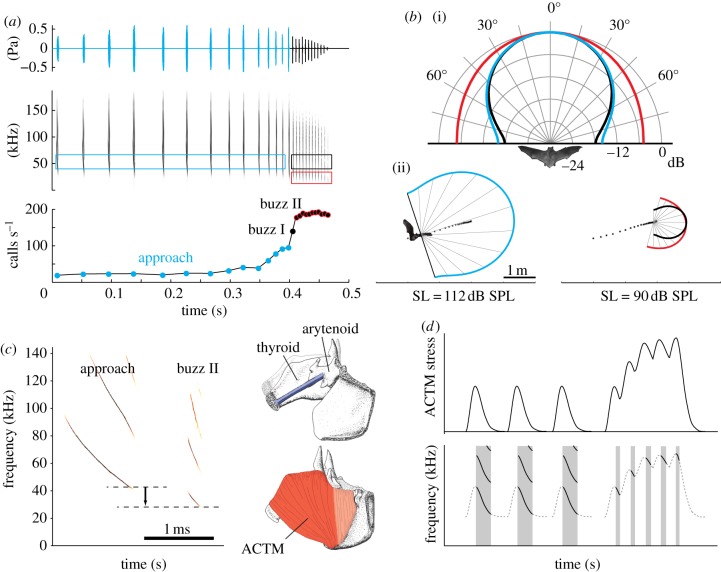
Figure 2.
(a) Echolocation parameters during prey pursuit in M. daubentonii. Oscillogram and spectrogram of the calls emitted and instantaneous call repetition rate. (b) Beam shape of echolocation calls emitted during prey pursuit by M. daubentonii. (i) The polar plot of the emitted beam shape measured at 55 kHz during the approach (blue) and buzz II (black) and at 27.5 kHz during buzz II (red). (ii) The estimated detection space during approach (left) and buzz II (right), illustrating how the beam is broadened when frequency is decreased (calculated using target strength of −40 dB and noise-limited hearing threshold of 20 dB). (c) High-resolution frequency–time representation of an approach call and buzz II call, showing frequency modulation and drop (horizontal dashed lines). Sounds are produced in the larynx (inset) and the fundamental frequency (F0) depends on the tension of vocal folds and membranes (blue). Contraction of the anterior cricothyroid muscle (ACTM) rotates the thyroid relative to the arytaenoid and increases vocal fold membrane length and tension. (d) Hypothetical buzz sequence where ACTM is the only muscle controlling vocal fold tension. As call rate reaches 160 calls s−1, muscle tension increases because relaxation is not complete, although the muscle still produces work. This increases tension in the fold and thus would increase F0 of the calls. Therefore the buzz II calls would exhibit a higher F0 than approach calls.
During the buzz, some bats (figures 1 and 2) produce a distinct second phase, buzz II, characterized by up to a one-octave drop in the F0 of their calls [3,5,10]. This drop in frequency has been argued to result from a physiological constraint on producing very short calls quickly (see Schmieder et al. [10] for review). However, the fastest buzzing bat, Craseonycteris thonglongyai (approx. 220 calls s−1), does not lower F0, suggesting otherwise (figure 1; [9]). An alternative, adaptive hypothesis proposes that bats reduce F0 to broaden their sonar beam. For a given emitter size (e.g. gape in mouth-emitting bats), the lower the frequency, the broader the sound beam [5,9]. Beam broadening has been demonstrated in the vespertilionids, Myotis daubentonii and Eptesicus serotinus (figure 2; [5]). In close quarters, even a small positional change on the part of the insect could put it outside the bat's acoustic field of view; hence, sonar beam broadening should improve bats' ability to track evasive prey as target distance decreases [5]. A drop in F0 and, presumably, a broad acoustic field of view during the buzz describe most species in Vespertilionidae and Molossidae [3,5,8], and the emballonurid, Rynchonycteris naso (figure 1). Buzz II is sometimes also observed during complicated landing manoeuvres, indicating its importance in motor control [11,12].
In this opinion piece, our goal is to provide a plausible functional explanation for the buzz and the drop in frequency that characterizes buzz II. We suggest an evolutionary scenario, where production of high repetition rate buzzes and thus superfast muscles were an adaptation for exploiting the niche of night-flying insects. Nocturnal insects from a variety of taxa then evolved ultrasound-sensitive ears and auditory-evoked evasive flight manoeuvres [4,13]. We hypothesize that buzz II beam broadening represents an evolutionary countermeasure selected for as a result of the unpredictable evasive flight manoeuvres of eared, night-flying insects. Beam broadening during the buzz would have improved a bat's ability to secure ever more evasive prey during the final phase of pursuit [5]. As we argue below, lowering F0 requires recruitment of an unknown laryngeal mechanism.
Laryngeally echolocating bats produce sound by airflow-induced oscillation of the specialized vocal membranes in the larynx (figures 1 and 2; [6,7]). These oscillations cause pressure changes in the vocal tract, radiated as sound. While observations of vocal fold oscillations have not yet been attempted in bats, in other mammals, the oscillation frequency and thus F0 of the sound wave is determined by fold tension [14]. Fold tension is under neural control and affected by rotation of the thyroid relative to the cricoid cartilages (figure 2). This rotation is primarily controlled by a contraction of hypertrophied cricothyroid muscles. Cricothyroid muscle contraction increases tension, increasing sound frequency [6,7]. Consequently, the frequency-modulated downwards sweep of bats' calls is produced as the cricothyroid muscles lengthen and relax.
The rate-limiting step for muscle speed in vertebrate synchronous striated muscle contractions—especially in superfast muscles—is relaxation [15]. If there is not enough time to relax, muscle tension will build up, and during relaxation in lengthening–shortening cycles, no net power will be produced [15]. We have found that bat laryngeal muscles can produce power at up to 180 cycles s−1, rates occurring during the buzz [6]. At these high cycling rates, however, we also observed that the superfast vocal muscles could not completely relax to baseline tension (figure 2). As muscle tension positively correlates to oscillation frequency of the vocal membranes and to sound [7], these results indicate that F0 would increase with call rate. Physiological constraints on muscle performance would therefore increase F0 (figure 2), rather than decrease F0 as previously suggested.
The only means of counteracting an increase in tension in the anterior cricothyroid muscle would be to recruit additional muscles with an antagonistic effect on vocal fold tension [14]. There are few data on the bats' laryngeal morphology and we hesitate to identify candidate muscles. However, in other mammals, an antagonistic effect can be achieved through an intrinsic (e.g. thyroarytenoid) or extrinsic (e.g. cricopharyngeus) laryngeal muscle. Vertical movement of the larynx may rotate the thyroid cartilage and thus also counteract tension [16].
Two scenarios for the evolution of laryngeal echolocation in bats are most parsimonious (see recent studies [1,4] for review). One suggests that laryngeal echolocation evolved once and was subsequently lost in the fruit- and nectar-feeding Pteropodidae (figure 1). The other scenario suggests that echolocation evolved twice, independently in Yangochiroptera and in Yinpterochiroptera. Regardless, approximately 55 Mya both groups hunted night-flying insects, insects that until then had enjoyed a relatively predator-free existence [2,4]. Today, more than a dozen taxa of insects have bat-detecting ears [11]. Eared or not, many display meandering or erratic flight, but dedicated evasive flight is linked exclusively to bat-sensitive ears in moths, lacewings and orthopterans as well as several other families of night-flying insects [13]. We assume that, before the existence of bats, most night-flying insects flew more directly than their bat-predated descendants do today [4].
As a result of the higher rate of information update, bats able to buzz should have had greater success in capturing airborne prey than those bats not so able [6]. We propose that in addition to powered flight and laryngeal echolocation, the buzz represents another important innovation that evolved in response to the challenge of capturing flying insects in the dark. Further, we hypothesize that buzz II evolved as a specific countermeasure against evasive prey, and improved the capture success of insects with bat-detecting ears. This hypothesis generates the testable prediction that, among morphologically similar bats, those without buzz II should eat a relatively smaller proportion of eared insects compared with those that do produce a buzz II. Genetics-based diet analysis across a broad range of species and locations would be an appropriate means of testing it.
The Vespertilionoidea is the most species-rich (approx. 500 species) of the four major bat lineages identified today, all of which originated ca 50 Mya (figure 1; [2]). Vespertilionids (approx. 400 species) and molossids (approx. 100 species) [1] are often portrayed as ‘advanced echolocators’ (see Jakobsen & Surlykke [5] for review). Their ability to lower F0, and thus presumably to broaden their beam during the buzz, supports this contention [5], and we speculate that buzz II may partially account for species-richness in Vespertilionoidea (figure 1). For example, buzz II may have allowed some species to out-compete others, and/or to subdivide foraging niches.
The ‘arms-race’ between bats and nocturnal insects is a textbook example of predator–prey interaction. There is little doubt that the ultrasound-sensitive ears found in many nocturnal insect families are an independently evolved defence against bats [4,13]. However, while a handful of bats use echolocation call frequencies outside the hearing ranges of moths and other eared insects, it is up for debate whether these calls are really a countermeasure against these insects' auditory-evoked defences [1,4,17]. While the use of passive hearing improves some gleaning bats' ability to capture prey, to date, only the very low intensity calls used by the moth-specializing vespertilionid bat, Barbastella barbastellus, have been convincingly demonstrated to be a bat countermeasure during aerial hawking attacks [17]. With respect to buzz II, the lowering of frequency and broadening of the beam can also be interpreted as an unambiguous adaptation to the sudden, unpredictable movements of eared prey. Therefore, we contend that buzz II was and is a countermeasure to insects with bat-detecting ears, a move on the part of bats that strengthens the metaphor of a predator–prey evolutionary arms-race.
Acknowledgements
We thank Brock Fenton, Hannah ter Hofstede, Jim Quinn and two anonymous reviewers for comments on the manuscript, and Signe Brinkløv, Inga Geipel and Paul Racey for sharing their unpublished data. Grants from the Danish Natural Sciences Research Council (FNU) supported this work.
References
- 1.Maltby A, Jones KE, Jones G. 2009. Understanding the evolutionary origin and diversification of bat echolocation calls. In Handbook of mammalian vocalization (ed. Brudzynski SM.), pp. 37–48 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 2.Miller-Butterworth CM, Murphy WJ, O'Brien SJ, Jacobs DS, Springer MS, Teeling EC. 2007. A family matter: conclusive resolution of the taxonomic position of the long-fingered bats, Miniopterus. Mol. Biol. Evol. 24, 1553–1561 10.1093/molbev/msm076 (doi:10.1093/molbev/msm076) [DOI] [PubMed] [Google Scholar]
- 3.Schnitzler H-U, Kalko EKV. 2001. Echolocation by insect-eating bats. BioScience 51, 557–569 10.1641/0006-3568(2001)051[0557:EBIEB]2.0.CO;2 (doi:10.1641/0006-3568(2001)051[0557:EBIEB]2.0.CO;2) [DOI] [Google Scholar]
- 4.Ratcliffe JM. 2009. Predator–prey interaction in an auditory world. In Cognitive ecology II (eds Dukas R, Ratcliffe JM.), pp. 201–225 Chicago, IL: University of Chicago [Google Scholar]
- 5.Jakobsen L, Surlykke A. 2010. Vespertilionid bats control the width of their biosonar sound beam dynamically during prey pursuit. Proc. Natl Acad. Sci. USA 107, 13 930–13 935 10.1073/pnas.1006630107 (doi:10.1073/pnas.1006630107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elemans CPH, Mead AF, Jakobsen L, Ratcliffe JM. 2011. Superfast muscles set maximum call rate in echolocating bats. Science 333, 1885–1888 10.1126/science.1207309 (doi:10.1126/science.1207309) [DOI] [PubMed] [Google Scholar]
- 7.Metzner W, Schuller G. 2009. Vocal control in echolocating bats. In Handbook of mammalian vocalization (ed. Brudzynski SM.), pp. 403–415 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 8.Simmons JA, Fenton MB, O'Farrell MJ. 1979. Echolocation and pursuit of prey by bats. Science 203, 16–21 10.1126/science.758674 (doi:10.1126/science.758674) [DOI] [PubMed] [Google Scholar]
- 9.Surlykke A, Miller LA, Mohl B, Andersen BB, Christensen-Dalsgaard J, Jorgensen MB. 1993. Echolocation in two very small bats from Thailand: Craseonycteris thonglongyai and Myotis siligorensis. Behav. Ecol. Sociobiol. 33, 1–12 10.1007/BF00164341 (doi:10.1007/BF00164341) [DOI] [Google Scholar]
- 10.Schmieder DA, Kingston T, Hashim R, Siemers BM. 2010. Breaking the trade-off: rainforest bats maximize bandwidth and repetition rate of echolocation calls as they approach prey. Biol. Lett. 6, 604–609 10.1098/rsbl.2010.0114 (doi:10.1098/rsbl.2010.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratcliffe JM, Dawson JW. 2003. Behavioural flexibility: the little brown bat, Myotis lucifugus, and the northern long-eared bat, M. septentrionalis, both glean and hawk prey. Anim. Behav. 66, 847–856 10.1006/anbe.2003.2297 (doi:10.1006/anbe.2003.2297) [DOI] [Google Scholar]
- 12.Melcon ML, Denzinger A, Schnitzler HU. 2007. Aerial hawking and landing: approach behaviour in Natterer's bats, Myotis nattereri (Kuhl 1818). J. Exp. Biol. 210, 4457–4464 10.1242/jeb.007435 (doi:10.1242/jeb.007435) [DOI] [PubMed] [Google Scholar]
- 13.Miller LA, Surlykke A. 2001. How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. BioScience 51, 571–582 10.1641/0006-3568(2001)051[0570:HSIDAA]2.0.CO;2 (doi:10.1641/0006-3568(2001)051[0570:HSIDAA]2.0.CO;2) [DOI] [Google Scholar]
- 14.Titze IR. 2000. Principles of voice production. Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- 15.Rome LC. 2006. Design and function of superfast muscles: new insights into the physiology of skeletal muscle. Annu. Rev. Physiol. 68, 193–221 10.1146/annurev.physiol.68.040104.105418 (doi:10.1146/annurev.physiol.68.040104.105418) [DOI] [PubMed] [Google Scholar]
- 16.Honda K. 1995. Laryngeal and extra-laryngeal mechanisms of F0 control. In Producing speech: contemporary issues (eds Bell-Berti F, Raphael LJ.), pp. 215–232 New York, NY: American Institute of Physics [Google Scholar]
- 17.Goerlitz HR, ter Hofstede HM, Zeale MR, Jones G, Holderied MW. 2010. An aerial-hawking bat uses stealth echolocation to counter moth hearing. Curr. Biol. 20, 1568–1572 10.1016/j.cub.2010.07.046 (doi:10.1016/j.cub.2010.07.046) [DOI] [PubMed] [Google Scholar]