Abstract
Small hibernating rodents have greater maximum lifespans and hence appear to age more slowly than similar-sized non-hibernators. We tested for a direct effect of hibernation on somatic maintenance and ageing by measuring seasonal changes in relative telomere length (RTL) in the edible dormouse Glis glis. Average RTL in our population did not change significantly over the hibernation season, and a regression model explaining individual variation in post-hibernation RTL suggested a significant negative effect of the reduction in body mass over the inactive hibernation period (an index of time spent euthermic), supporting the idea that torpor slows ageing. Over the active season, RTL on average decreased in sub-adults but increased in adults, supporting previous findings of greater telomere shortening at younger ages. Telomere length increase might also have been associated with reproduction, which occurred only in adults. Our study reveals how seasonal changes in physiological state influence the progress of life-history traits, such as somatic maintenance and ageing, in a small hibernating rodent.
Keywords: hibernation, ageing, dormouse, torpor
1. Introduction
Small hibernating mammals have longer maximum recorded lifespans than their similar-sized non-hibernating counterparts [1,2]. This difference suggests that, because maximum lifespan is a good indicator of rates of ageing in survival [3], hibernators experience relatively slow rates of ageing. Evolutionary theories of ageing propose that rates of ageing are determined by the timing of a decline in selection pressure owing to extrinsic-caused mortalities [4]. Accordingly, there is a strong, nonlinear relationship between survival in the wild and rates of ageing [3,5]. This relationship can ultimately explain the relatively slow ageing of hibernating mammals because when maximum lifespan is plotted against annual survival rate, rather than body mass, hibernators do not differ from other mammals [1]. This suggests that a hibernation-specific mechanism is not required to explain the slower ageing of small hibernating mammals. Despite this, a small number of studies have found strong correlations between the use of daily torpor or hibernation and indices of rate of ageing [6–8], suggesting that torpor expression by endothermic animals might have a physiological effect on rates of ageing [9].
Telomeres are repeated sections of DNA that, along with associated proteins, ensure the integrity of the ends of chromosomes [10]. Telomere length dynamics have been correlated with rates of ageing and can predict future survival [11,12]. However, whether telomeres directly contribute to, or merely are affected by, the processes causing ageing is not known [13]. Telomeres shorten with age owing to incomplete DNA replication during mitosis, but the amount of shortening is largely dependent on accumulated oxidative damage to the triple guanine base pairs of the vertebrate telomere sequence [14]. Telomerase can increase telomere length, and is active in somatic tissues of small rodents [15], but its restorative ability is also diminished by oxidative stress [16]. To test for an effect of torpor use on rates of ageing, we measured changes in telomere length over the active and hibernation seasons in a small hibernating rodent (edible dormouse Glis glis, 120–220 g).
2. Material and methods
We estimated relative telomere length (RTL) by a quantitative PCR technique (see the electronic supplementary material) using DNA extracted from ear tissue punches taken at three time points: pre-hibernation (29 Sept., autumn 2011), post-hibernation (22 June, spring 2012) and post-active season (23 Aug., autumn 2012). The edible dormouse hibernates for more than eight months of the year, and samples were taken at the earliest and latest opportunities when all individuals in our study were active. In a preliminary study, we found that estimates of RTL from ear tissue and cells swabbed from inside the mouth were closely correlated (n = 5, R2 = 0.77). Samples were taken from 19 captive individuals living in outdoor enclosures located at 370 m a.s.l. near Vienna, Austria (48°10′ N, 16°20′ E). All dormice experienced similar ambient conditions and hibernated in burrows dug below the ground. At the beginning of the experiment, 12 individuals (5 f, 7 m) were juveniles born earlier in the same year and became sub-adults (i.e. still growing but capable of reproduction) after their first hibernation season, and eight were adults (3f, 5m) aged from 3 to 6 years. Both males and females lived together among several cages (6 × 4 × 3 m) and, therefore, had the opportunity to breed during the experiment. Hibernation duration was estimated from the dates of above-ground activity, which we determined weekly by checking nest-boxes and searching the enclosures. At these times, we captured and weighed all active animals, checked the reproductive status of females and measured testis length of males [17]. From the difference in estimated RTLs and the duration between sampling times, we calculated a daily rate of change in RTL for each individual over the hibernation and active seasons.
To analyse these data, we first tested for differences between age classes and seasons in the mean of daily change in RTL for the population. We then fitted linear models to explain variation in RTL among individuals at the end of each season. We simplified global models including probable explanatory variables on the basis of minimizing values of the second order Akaike's information criterion, which is a measure of goodness of fit penalized by the number of parameters [18]. We started with a global model explaining post-hibernation RTL (in spring 2012) that included: pre-hibernation RTL (in autumn 2011), age class (sub-adults or adults), sex, body mass, hibernation duration and the reduction in body mass over the inactive hibernation period. We started with a global model explaining post-active season RTL (in autumn 2012) that included: post-hibernation RTL (in spring 2012), body mass loss over the preceding winter, age class and sex.
3. Results
We found a significant interaction between the effects of season and age class on the mean daily rate of change in RTL for the population (figure 1; for individual data see the electronic supplementary material, table S1). Over the hibernation season, the mean daily change in RTL did not significantly differ from zero for either sub-adults (p = 0.10) or adults (p = 0.47). Whereas, over the active season, we found a significant rate of decrease in RTL for sub-adults (p < 0.01), but an increase in RTL for adults (p = 0.02). The rate of change in RTL over the active season differed significantly between sub-adults and adults (p < 0.001).
Figure 1.
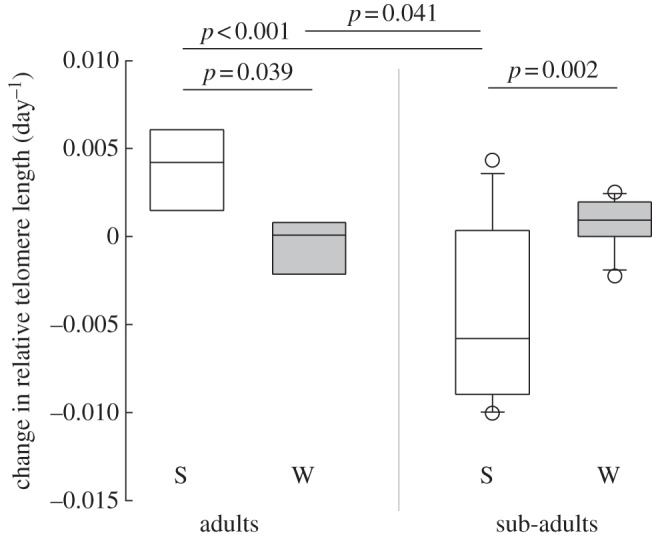
Change in relative telomere length per day in ear tissue of adult and sub-adult dormice (Glis glis) over the (S) summer active and (W) winter hibernation seasons. Horizontal lines show significant differences among groups (Tukey's HSD test).
The most parsimonious model explaining individual variation in post-hibernation RTL in spring 2012 included a strong negative effect of an individual's reduction in body mass over the hibernation duration, in addition to effects of age class, body mass and initial pre-hibernation RTL in autumn 2011 (table 1a; figure 2).
Table 1.
Estimated parameters of our best linear model explaining variation in relative telomere length (RTL) at (a) the end of the winter hibernation season (R2 = 0.65), and (b) the end of the active season (R2 = 0.43) among individual edible dormice, Glis glis.
| fixed effects | coefficient | s.e. | F value | p |
|---|---|---|---|---|
| (a) RTL at end of hibernation season (spring 2012) | ||||
| RTL in autumn 2011 | −0.93 | 0.42 | 4.88 | 0.044 |
| body mass loss (g) | −0.019 | 0.006 | 11.12 | 0.005 |
| age class (sub-adults) | 0.85 | 0.21 | 17.00 | 0.001 |
| body mass (g) | 0.013 | 0.004 | 11.31 | 0.005 |
| (b) RTL at end of active season (autumn 2012) | ||||
| age class (sub-adults) | −0.41 | 0.12 | 10.91 | 0.004 |
| RTL in spring 2012 | 0.52 | 0.18 | 8.25 | 0.011 |
Figure 2.
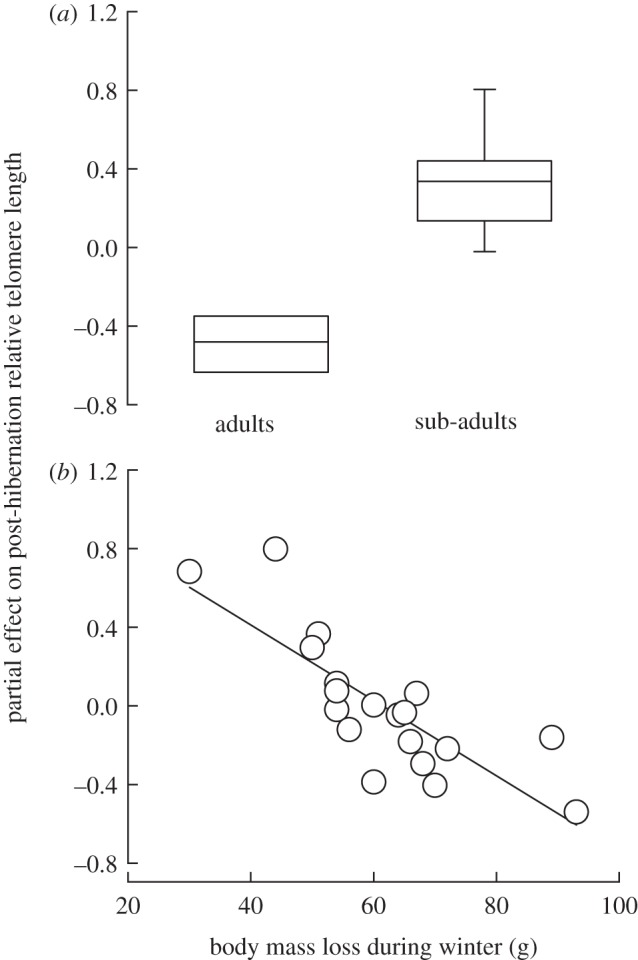
Partial residual plots of (a) age class and (b) body mass loss over the hibernation season (an index of torpor use: less body mass loss = greater torpor use) estimated from the best-fitting model, which also included significant effects of body mass and relative telomere length at the pre-hibernation sample time point (see table 1 for model parameters).
Individual variation in post-active season RTL in autumn 2012 was explained by a model including only the effects of age-class and post-hibernation RTL in the preceding spring 2012 (table 1b). When separate models were fitted to data for each sex, we found no effect within males of testis size on post-active season RTL in autumn 2012, but we did find a positive effect within females of reproduction on post-active season RTL in a model that also included effects of age class and RTL in spring.
4. Discussion
We found seasonal and age effects on changes in telomere length in a small hibernating dormouse, Glis glis. The mean RTL of our study population remained largely unchanged over the winter hibernation season, whereas over the active season, telomeres shortened in sub-adults, but lengthened in adults. At the individual level, variation in post-hibernation RTL was partly explained by the reduction in body mass over the period of inactivity, which we know is a positive linear function (p = 0.003) of time spent euthermic during hibernation in our captive population of edible dormouse [17]. These results for a hibernator, therefore, agree with our previous findings for daily torpor in the Djungarian hamster, Phodopus sungorus [8], that torpor use is positively related to the change in telomere length over winter.
We can only speculate on the mechanisms linking torpor use with telomere dynamics and possibly also ageing. Studies do not support a simple relationship between metabolic rate and telomeres [8] or ageing [19]. The production of reactive oxidative species is minimized by the very low metabolic rate during torpor but is invoked by the massive increase in metabolic rate during arousal from torpor [20]. Thus, animals losing more weight over hibernation, and hence arousing more frequently from torpor, might have suffered greater oxidative stress than other animals. Alternatively, both torpor use and telomere dynamics might be affected by other physiological changes associated with the winter season. For example, hibernation might be associated with a physiological state of enhanced somatic maintenance, which could affect both the loss and restoration of telomeres. Unlike in humans, many small rodents express telomerase activity in somatic tissues [15], although no data are available for Glis glis. Telomere lengthening has been reported in mice [21]. One study of a bat reported that telomerase activity was significantly greater during hibernation [22]. If telomere dynamics is indicative of oxidative stress and physiological condition, our study supports the hypothesis that hibernation enhances the maintenance of cellular homeostasis and, therefore, might slow the rate of ageing.
We also found strong effects of age class on telomere dynamics. In sub-adults, telomere length increased during hibernation and shortened during the active season. The spring following birth is the first breeding opportunity for edible dormice and many other animals born in a cold temperate climate. It is critical for their fitness to survive and maintain physiological function over the first winter season. A study of Djungarian hamsters (P. sungorus) found that exposure to a short-day photoperiod, which triggers a winter phenotype including the use of daily torpor, delayed ageing of the reproductive system relative to individuals kept under a long-day photoperiod [23]. Telomere length in juvenile finches is a predictor of future lifespan [24]. A positive effect of hibernation on telomere dynamics in young animals, therefore, might be associated with increased adult fitness.
Over the active season, we found that telomeres shortened in sub-adults but lengthened in adults. Telomeres generally shorten more rapidly in young animals than adults, perhaps as a consequence of growth and sexual development [24]. The sub-adult dormice in our study continued to grow through their first active season and a physiological cost of growth could account for their decrease in RTL. An age-class effect on change in RTL in females was confounded, however, by the fact that no sub-adults and all adult females reproduced in the active season. Yet an age-class effect was also apparent within males, irrespective of testis size, lending support to a negative effect of age rather than a positive effect of reproduction on telomere length.
Further studies are required to understand the broader ecological significance of torpor and hibernation in the context of changing environmental conditions [25]. Small mammals use torpor to cope with sporadic or seasonal reductions in food energy availability. We suggest that, more generally, torpor and hibernation are part of a physiological state enhancing survival and maintenance of physiological condition over times of unfavourable environmental conditions, analogous to the ‘waiting’ state of reproductive dormancy in Drosophila [26]. Telomere dynamics provides a unique method to examine such variation in rates of ageing over an organism's lifespan.
Acknowledgements
All procedures were in accordance with Austrian legislation and approved by the institutional ethics committee and the national authority according to § 8ff of Law for Animal Experiments, Tierversuchsgesetz—TVG (approval no. BMWF_68.205/0240-II/3b/2010). We thank Caroline Deimel, Anita Haiden and Peter Steiger for help with this project. The study was partly financially supported by the Austrian Science Fund (project P20534).
References
- 1.Turbill C, Bieber C, Ruf T. 2011. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B 278, 3355–3363 10.1098/rspb.2011.0190 (doi:10.1098/rspb.2011.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkinson GS, South JM. 2002. Life history, ecology and longevity in bats. Aging Cell 1, 124–131 10.1046/j.1474-9728.2002.00020.x (doi:10.1046/j.1474-9728.2002.00020.x) [DOI] [PubMed] [Google Scholar]
- 3.Ricklefs RE. 2010. Life-history connections to rates of aging in terrestrial vertebrates. Proc. Natl Acad. Sci. USA 107, 10 314–10 319 10.1073/pnas.1005862107 (doi:10.1073/pnas.1005862107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkwood TBL, Austad SN. 2000. Why do we age? Nature 408, 233–238 10.1038/35041682 (doi:10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 5.Turbill C, Ruf T. 2010. Senescence is more important in the natural lives of long- than short-lived mammals. PLoS ONE 5, e12019. 10.1371/journal.pone.0012019 (doi:10.1371/journal.pone.0012019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koizumi A, Tsukada M, Wada Y, Masuda H, Weindruch R. 1992. Mitotic activity in mice is supressed by energy restriction-induced torpor. J. Nutr. 122, 1446–1453 [DOI] [PubMed] [Google Scholar]
- 7.Lyman CP, O'Brien RC, Greene GC, Papafrangos ED. 1981. Hibernation and longevity in the Turkish hamster Mesocricetus brandi. Science 212, 668–670 10.1126/science.7221552 (doi:10.1126/science.7221552) [DOI] [PubMed] [Google Scholar]
- 8.Turbill C, Smith S, Deimel C, Ruf T. 2011. Daily torpor is associated with telomere length change over winter in Djungarian hamsters. Biol. Lett. 8, 304–307 10.1098/rsbl.2011.0758 (doi:10.1098/rsbl.2011.0758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walford RL, Spindler SR. 1997. The response to calorie restriction in mammals shows features also common to hibernation: a cross-adaptation hypothesis. J. Gerontol. 52A, B179–B183 10.1093/gerona/52A.4.B179 (doi:10.1093/gerona/52A.4.B179) [DOI] [PubMed] [Google Scholar]
- 10.Chan SRWL, Blackburn EH. 2004. Telomeres and telomerase. Phil. Trans. R. Soc. Lond. B 359, 109–122 10.1098/rstb.2003.1370 (doi:10.1098/rstb.2003.1370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165 10.1098/rspb.2009.0517 (doi:10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monaghan P. 2010. Telomeres and life histories: the long and the short of it. Ann. N Y Acad. Sci. 1206, 130–142 10.1111/j.1749-6632.2010.05705.x (doi:10.1111/j.1749-6632.2010.05705.x) [DOI] [PubMed] [Google Scholar]
- 13.Aubert G, Lansdorp PM. 2008. Telomeres and aging. Physiol. Rev. 88, 557–579 10.1152/physrev.00026.2007 (doi:10.1152/physrev.00026.2007) [DOI] [PubMed] [Google Scholar]
- 14.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 10.1016/S0968-0004(02)02110-2 (doi:10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 15.Seluanov A, Chen Z, Hine C, Sasahara THC, Ribeiro AACM, Catania KC, Presgraves DC, Gorbunova V. 2007. Telomerase activity coevolves with body mass not lifespan. Aging Cell 6, 45–52 10.1111/j.1474-9726.2006.00262.x (doi:10.1111/j.1474-9726.2006.00262.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cattan V, et al. 2008. Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Rad. Biol. Med. 44, 1592–1598 10.1016/j.freeradbiomed.2008.01.007 (doi:10.1016/j.freeradbiomed.2008.01.007) [DOI] [PubMed] [Google Scholar]
- 17.Bieber C, Ruf T. 2009. Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwiss. 96, 165–171 10.1007/s00114-008-0471-z (doi:10.1007/s00114-008-0471-z) [DOI] [PubMed] [Google Scholar]
- 18.Burnham KP, Anderson DR. 2002. Model selection and mulitmodel inference: a practical information-theoretical approach, 2nd ed New York, NY: Springer-Verlag [Google Scholar]
- 19.Speakman JR, et al. 2004. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3, 87–95 10.1111/j.1474-9728.2004.00097.x (doi:10.1111/j.1474-9728.2004.00097.x) [DOI] [PubMed] [Google Scholar]
- 20.Orr AL, Lohse LA, Drew KL, Hermes-Lima M. 2009. Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel. Comp. Biochem. Physiol. A 153, 213–221 10.1016/j.cbpa.2009.02.016 (doi:10.1016/j.cbpa.2009.02.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotrschal A, Ilmonen P, Penn DJ. 2007. Stress impacts telomere dynamics. Biol. Lett. 3, 128–130 10.1098/rsbl.2006.0594 (doi:10.1098/rsbl.2006.0594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, McAllan B, He G. 2011. Telomerase activity in the bats Hipposideros armiger and Rousettus leschenaultia. Biochemistry (Moscow) 76, 1017–1021 10.1134/s0006297911090057 (doi:10.1134/s0006297911090057) [DOI] [PubMed] [Google Scholar]
- 23.Place NJ, Tuthill CR, Schoomer EE, Tramontin AD, Zucker I. 2004. Short day lengths delay reproductive aging. Biol. Reprod. 71, 987–992 10.1095/biolreprod.104.029900 (doi:10.1095/biolreprod.104.029900) [DOI] [PubMed] [Google Scholar]
- 24.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA. 10.1073/pnas.1113306109 (doi:10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiser F, Brigham RM. 2012. The other functions of torpor. In Living in a seasonal world (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 109–121 Berlin, Germany: Springer [Google Scholar]
- 26.Tatar M, Chien SA, Priest NK. 2001. Negligible senescence during reproductive dormancy in Drosophila melanogaster. Am. Nat. 158, 248–258 10.1086/321320 (doi:10.1086/321320) [DOI] [PubMed] [Google Scholar]


