Abstract
This study asked whether reductive traits in cave organisms evolve at a slower pace (suggesting neutral evolution under relaxed selection) than constructive changes, which are likely to evolve under directional selection. We investigated 11 subterranean and seven surface populations of Sundathelphusa freshwater crabs on Bohol Island, Philippines, and examined constructive traits associated with improved food finding in darkness (increased leg and setae length) and reductive traits (reduced cornea size and eyestalk length). All changes occurred rapidly, given that the age of the most recent common ancestor was estimated to be 722–271 ka based on three mitochondrial markers. In order to quantify the speed of character change, we correlated the degree of morphological change with genetic distances between surface and subterranean individuals. The temporal pattern of character change following the transition to subterranean life was indistinguishable for constructive and reductive traits, characterized by an immediate onset and rapid evolutionary change. We propose that the evolution of these reductive traits—just like constructive traits—is most likely driven by strong directional selection.
Keywords: trait reduction, regressive evolution, neutral evolution, relaxed selection, Sundathelphusa
1. Introduction
Cave animals are a promising model system for studying adaptation and ecological speciation in response to environmental gradients created by the presence or absence of light [1–4]. Many cave animals share two kinds of evolutionary modifications (‘troglomorphies’): characters that become functionless in darkness—most strikingly, the visual system and body pigmentation—are reduced (‘reductive traits’) [5], whereas ‘constructive traits’ are features that are related to improved non-visual orientation, navigation, food-finding and communication [6]. Typical constructive traits involve improved chemosensory capacities through elongated antennae, legs, fins, or setae [7,8].
It seems straightforward to assume that rapid evolutionary divergence in constructive characters is caused by strong, directional selection in the cave environment. Indeed, divergent selection may even promote ecological speciation [9]. Predictions regarding the speed of evolutionary reductive processes are less straightforward. In the classical literature on cave evolution, the view prevails that reductions are mainly due to the accumulation of neutral mutations under relaxed selection [10–12]. If this was true, reductive traits should be fixed at a much slower pace than constructive evolutionary changes (see the electronic supplementary material, figure S1). Alternatively, reductive traits could also be under directional selection, if fitness advantages drive the reduction of expensive, dispensable traits [13,14] in the often resource-limited cave environment. For example, directional selection has been proposed for the amphipod Gammarus minus because eye reduction may allow improved processing of sensory input from the antennae in the brain [7]. Antagonistic pleiotropic effects have been suggested to affect eye reduction in the cave fish Astyanax mexicanus, where increasing the number of taste buds is developmentally correlated with eye reduction [6].
To date, no comprehensive study has compared the timing and degree of both constructive and reductive processes in closely related taxa that have repeatedly colonized caves at different points in time. A major obstacle (even for well-established model systems such as A. mexicanus) is the identification of the surface-living source population and, therefore, the timing of evolutionary change in different cave populations. Most studies can only provide broad time estimates for the most recent common ancestor of surface and subterranean forms [15–17], and hence cannot correlate the degree of morphological change with the time spent in the cave environment.
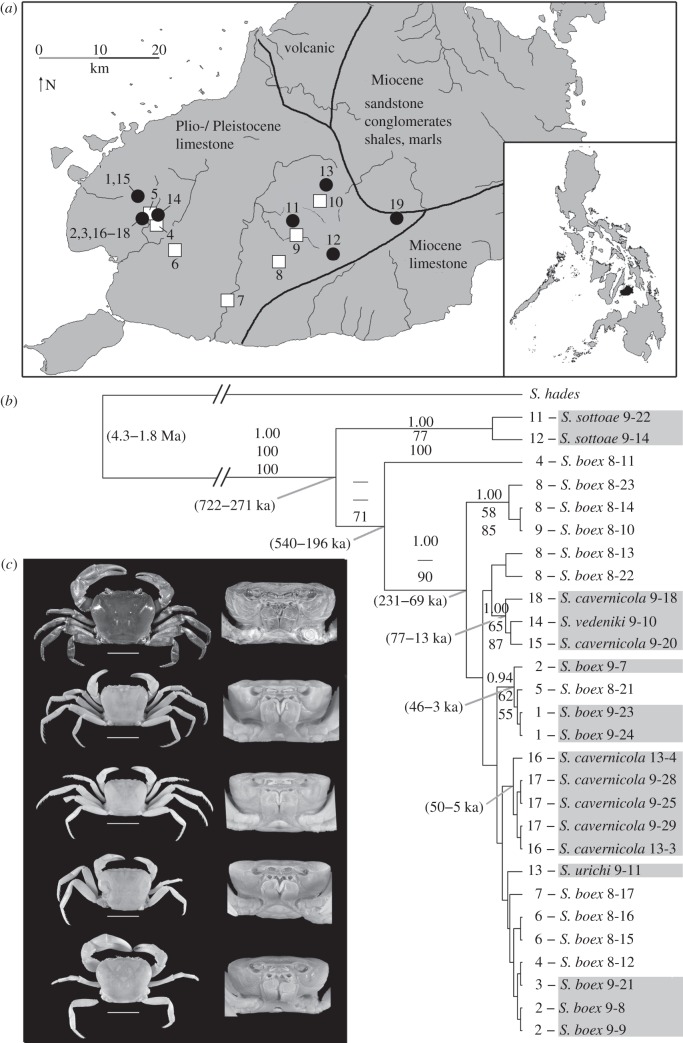
Here, we investigate adaptations to the subterranean environment in gecarcinucid freshwater crabs on Bohol Island, Philippines. Crabs of the genus Sundathelphusa inhabit both surface streams and caves and show varying degrees of troglomorphism within a small area of less than 40 × 40 km (figure 1a), which led to the recognition of five species ([18]; figure 1c). Bohol Island emerged in the Plio- or Pleistocene (5.1–0.01 Mya) [19] indicating that its freshwater fauna is young. We sought to infer the onset and relative pace of constructive and reductive evolutionary change in cave forms of varying age and predicted an early onset in the evolution of constructive traits, whereas different evolutionary theories (see above) provide conflicting predictions for the timing of reductive processes.
Figure 1.
(a) Map of Bohol Island, Philippines with subterranean (black circles) and surface (white boxes) sampling sites. Geological formations are adopted from [19]. (b) Maximum clade credibility tree of the Bayesian analysis. Values on the branches are posterior probabilities, and bootstrap values of ML and MP analyses. Values at nodes represent 95% credibility intervals of divergence time estimates. Grey shading, subterranean taxa. (c) Different degrees of morphological adaptation to darkness. From top to bottom: Sundathelphusa boex; Sundathelphusa vedeniki; Sundathelphusa urichi; Sundathelphusa sottoae; Sundathelphusa cavernicola. Scale bars, 2 cm; frontal view not to scale.
2. Material and methods
Samples for the molecular genetic analyses (n = 38; 19 sites) are listed in the electronic supplementary material, table S1. Phylogenetic and age estimation was conducted with BEAST v. 1.6.1 [20] on a concatenated dataset of mitochondrial Cytb (408 bp), 12S rRNA (293 bp) and 16S rRNA (526 bp) sequences (EMBL accession no. HE999758–HE999850), excluding site 19 (see the electronic supplementary material). For the 16S rRNA, we applied a mean (±s.d.) substitution rate of 2.03 ± 0.38% [21], and for the 12S rRNA and Cytb a mean of 2.0 per cent per million years with a broad s.d. of 2.0. For details on sample size, PCR conditions, phylogenetic analyses and divergence time estimation please refer to the electronic supplementary material.
For morphometric analyses of cave adaptation, we investigated n = 18 surface crabs from eight sites and n = 34 subterranean crabs from 12 caves (see the electronic supplementary material, table S2). We measured carapace height, width and length dextrally, and conducted a principal component analysis on these traits and extracted PC1 as a proxy of body size. Measurements of leg length, mean length of the setae on the dactylus of the second peraeopod, cornea area and eyestalk length were subjected to a general linear model with PC1 as covariate. The corresponding residuals were extracted as size corrected values.
To discriminate between slow, gradual trait reduction (i.e. relaxed selection; see the electronic supplementary material, figure S1), and rapid, immediate change of character states, we fitted a linear and a logarithmic curve onto trait residuals (y) of the subterranean populations as a function of the minimum uncorrected pairwise distance of the Cytb sequences to the closest surface relative (x) and compared both models with a likelihood ratio test. We tested for slope heterogeneity between traits using ANCOVA on log-transformed data (constructive characters with change of sign; see the electronic supplementary material). The results of this analysis could be biased if pre-adapted surface populations were overrepresented in the sample or if the closest surface relatives of the genetically most distant populations escaped sampling. We therefore tested the robustness of the analyses by analysing reduced datasets, excluding surface sites and/or those cave sites that were genetically most distant to the surface (greater than 4% pairwise distance).
3. Results
The age of the most recent common ancestor of the freshwater crabs of Bohol is compatible with the geological record and dates to the Pleistocene, with a 95% highest posterior density interval of 722–271 ka (figure 1b). The resulting substitution rate of Cytb was determined as 3.55–8.11% per Ma. Minimum uncorrected Cytb pairwise distances of cave crabs to their closest surface-dwelling relative ranged from 0 to 7.9 per cent (figure 2).
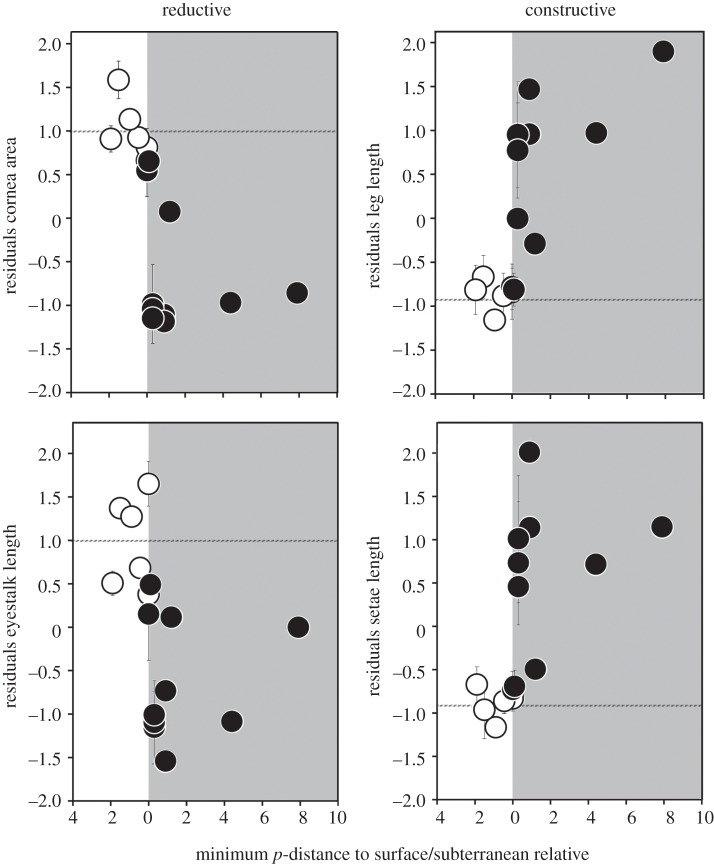
Figure 2.
Z-transformed residuals of morphological character states (corrected for body size) as a function of the minimum p-distances to the closest surface (black circles) or subterranean (white circles) relative (mean ± s.d. for each sampling site; horizontal line: mean value of surface sites).
Using Cytb pairwise distances as a proxy for the age of the respective cave populations revealed that the evolution of troglomorphic characters was fast for both reductive (cornea size, eyestalk length) and constructive (leg length, setae length) traits (figure 2). The onset of morphological changes appears to be immediate and there was no difference between constructive and reductive traits; indeed, character change was better explained by a logarithmic than by a linear function for all four traits (p < 0.001; ΔAIC 1.01–3.45; electronic supplementary material, figure S2).
The test for slope heterogeneity detected no significant difference in slopes between the four different traits (see the electronic supplementary material, table S3), suggesting that all troglomorphic traits evolved at a comparable speed. This result was not affected by the exclusion of surface sites or genetically distant cave populations.
4. Discussion
Using a comparative approach, we provide the first empirical test of whether eye reduction in cave organisms occurs at a slower pace (suggesting neutral evolution under relaxed selection) than ‘constructive’ changes that clearly evolve under directional selection [3]. The pace of character change after the shift from surface to cave-dwelling was virtually the same for constructive and reductive traits, with a rapid initial change. Also, the onset of reductive changes did not lag behind that of constructive changes. We propose that—for both kinds of modifications—this pattern is caused by strong, directional selection. This is in line with evo-devo studies on other cave organisms proposing that eye reduction is not simply caused by accumulation of selectively neutral mutations, but must be subject to selection [6,13,22].
Recent work on the underlying genetic and developmental mechanisms of eye reduction has been conducted in A. mexicanus [4], Asellus aquaticus [23] and G. minus [24]. These studies show that only few and homologous genes are involved in the eye reduction of vertebrates [4] and crustaceans [23,24]. More importantly, in A. mexicanus mutations only affect lens apoptosis, whereas all other genes involved in eye development seem to stay intact [4], which would be unlikely in the case of relaxed selection. Also, some genes involved in eye development (like sonic hedgehog) have an increased expression, and are not simply shut down [4]. In contrast to eye reduction, loss of pigmentation in Astyanax was proposed to be caused by neutral mutations, as the involved genes are both up- and downregulated, and thus not driven in one direction via selection [13]. This might also apply to cave crabs and should be addressed in the future based on fresh material.
Future studies should also investigate the proximate and ultimate factors responsible for the rapid evolutionary change reported here, considering both ecological factors and the developmental genetic basis of the observed morphological changes. Our study, however, is the first of its kind to show that eye reduction in a subterranean organism evolves at the same rapid pace as constructive changes, and this is most likely a direct effect of altered selective regimes in the subterranean environment.
Acknowledgements
We thank Peter K. L. Ng for his help and valuable discussion, and two anonymous reviewers for their constructive criticism. Financial support came from the National University of Singapore (grant no. R-154-000-519-720, R-154-000-465-133, R-154-000-476-112) and National Research Foundation and Economic Development Board (SPORE, COY-15-EWI-RCFSA/N197-1).
References
- 1.Porter ML, Crandall KA. 2003. Lost along the way: the significance of evolution in reverse. Trends Ecol. Evol. 18, 541–547 10.1016/S0169-5347(03)00244-1 (doi:10.1016/S0169-5347(03)00244-1) [DOI] [Google Scholar]
- 2.Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, Coss RG, Donohue K, Foster SA. 2009. Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496 10.1016/j.tree.2009.03.010 (doi:10.1016/j.tree.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 3.Borowsky R. 2010. The evolutionary genetics of cave fishes: convergence, adaptation and pleiotropy. In Biology of subterranean fishes. (eds Trajano E, Bichuette ME, Kapoor BG.), pp. 115–139 Boca Raton, FL: CRC Press/Taylor and Francis [Google Scholar]
- 4.Jeffery WR, Strickler AG. 2010. Development as an evolutionary process in Astyanax cavefish. In Biology of subterranean fishes. (eds Trajano E, Bichuette ME, Kapoor BG.), pp. 141–168 Boca Raton, FL: CRC Press/Taylor and Francis [Google Scholar]
- 5.Romero A, Green SM. 2005. The end of regressive evolution: examining and interpreting the evidence from cave fish. J. Fish. Biol. 67, 3–32 10.1111/j.0022-1112.2005.00776.x (doi:10.1111/j.0022-1112.2005.00776.x) [DOI] [Google Scholar]
- 6.Jeffery WR. 2005. Adaptive evolution of eye degeneration in the Mexican blind cavefish. J. Hered. 96, 185–196 10.1093/jhered/esi028 (doi:10.1093/jhered/esi028) [DOI] [PubMed] [Google Scholar]
- 7.Jones R, Culver DC. 1989. Evidence for selection on sensory structures in a cave population of Gammarus minus Say (Amphipoda). Evolution 43, 688–693 10.2307/2409074 (doi:10.2307/2409074) [DOI] [PubMed] [Google Scholar]
- 8.Mejía-Ortíz LM, Hartnoll RG. 2006. A new use for useless eyes in cave crustaceans. Crustaceana 79, 593–600 10.1163/156854006777584313 (doi:10.1163/156854006777584313) [DOI] [Google Scholar]
- 9.Riesch R, Plath M, Schlupp I. 2011. Speciation in caves: experimental evidence that permanent darkness promotes reproductive isolation. Biol. Lett. 7, 909–912 10.1098/rsbl.2011.0237 (doi:10.1098/rsbl.2011.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eigenmann CH. 1909. Cave vertebrates of America: a study in degenerative evolution. Washington, DC: Carnegie Institution [Google Scholar]
- 11.Culver DC. 1982. Cave life: evolution and ecology. Cambridge, MA: Harvard University Press [Google Scholar]
- 12.Wilkens H. 1988. Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces). Support for the neutral mutation theory. In Evolutionary biology (eds Hecht MK, Wallace B.), pp. 271–367 New York, NY: Plenum Publishing Corporation [Google Scholar]
- 13.Protas M, Conrad M, Gross JB, Tabin C, Borowsky R. 2007. Regressive evolution in the Mexican cave tetra, Astyanax mexicanus. Curr. Biol. 17, 452–454 10.1016/j.cub.2007.01.051 (doi:10.1016/j.cub.2007.01.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan S, Amos W, Laughlin SB. 2005. Captivity selects for smaller eyes. Curr. Biol. 15, R540–R542 10.1016/j.cub.2005.07.019 (doi:10.1016/j.cub.2005.07.019) [DOI] [PubMed] [Google Scholar]
- 15.Gross JB. 2012. The complex origin of Astyanax cavefish. BMC Evol. Biol. 12, 105. 10.1186/1471-2148-12-105 (doi:10.1186/1471-2148-12-105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribera I, Fresneda J, Bucur R, Izquierdo A, Vogler AP, Salgado JM, Cieslak A. 2010. Ancient origin of a Western Mediterranean radiation of subterranean beetles. BMC Evol. Biol. 10, 29. 10.1186/1471-2148-10-29 (doi:10.1186/1471-2148-10-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niemiller ML, Fitzpatrick BM, Miller BT. 2008. Recent divergence with gene flow in Tennessee cave salamanders (Plethodontidae: Gyrinophilus) inferred from gene genealogies. Mol. Ecol. 17, 2258–2275 10.1111/j.1365-294X.2008.03750.x (doi:10.1111/j.1365-294X.2008.03750.x) [DOI] [PubMed] [Google Scholar]
- 18.Ng PKL, Sket B. 1996. The freshwater crab fauna (Crustacea: Decapoda: Brachyura) of the Philippines. IV. On a collection of Parathelphusidae from Bohol. Proc. Biol. Soc. Wash. 109, 695–706 [Google Scholar]
- 19.Salomon J-N. 2011. Mysterious karst: the ‘Chocolate Hills’ of Bohol (Philippines). Acta Carsiol. 40, 429–444 [Google Scholar]
- 20.Drummond AJ, Rambaut A. 2007. BEAST, Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214. 10.1186/1471-2148-7-214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaus S, Schubart CD, Streit B, Pfenninger M. 2010. When Indian crabs were not yet Asian: evidence for Eocene proximity of India and Southeast Asia from freshwater crab biogeography. BMC Evol. Biol. 10, 287. 10.1186/1471-2148-10-287 (doi:10.1186/1471-2148-10-287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffery WR. 2009. Regressive evolution in Astyanax cavefish. Annu. Rev. Genet. 43, 25–47 10.1146/annurev-genet-102108-134216 (doi:10.1146/annurev-genet-102108-134216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Protas ME, Trontelj P, Patel NH. 2011. Genetic basis of eye and pigment loss in the cave crustacean, Asellus aquaticus. Proc. Natl Acad. Sci. USA 108, 5702–5707 10.1073/pnas.1013850108 (doi:10.1073/pnas.1013850108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aspiras AC, Prasad R, Fong DW, Carlini DB, Angelini DR. 2012. Parallel reduction in expression of the eye development gene hedgehog in separately derived cave populations of the amphipod Gammarus minus. J. Evol. Biol. 25, 995–1001 10.1111/j.1420-9101.2012.02481.x (doi:10.1111/j.1420-9101.2012.02481.x) [DOI] [PubMed] [Google Scholar]