Abstract
Anthropogenic noise has fundamentally changed the acoustics of terrestrial and aquatic environments, and there is growing empirical evidence that even a single noise exposure can affect behaviour in a variety of vertebrate organisms. Here, we use controlled experiments to investigate how the physiology of a marine invertebrate, the shore crab (Carcinus maenas), is affected by both single and repeated exposure to ship-noise playback. Crabs experiencing ship-noise playback consumed more oxygen, indicating a higher metabolic rate and potentially greater stress, than those exposed to ambient-noise playback. The response to single ship-noise playback was size-dependent, with heavier crabs showing a stronger response than lighter individuals. Repeated exposure to ambient-noise playback led to increased oxygen consumption (probably due to handling stress), whereas repeated exposure to ship-noise playback produced no change in physiological response; explanations include the possibility that crabs exhibited a maximal response on first exposure to ship-noise playback, or that they habituated or become tolerant to it. These results highlight that invertebrates, like vertebrates, may also be susceptible to the detrimental impacts of anthropogenic noise and demonstrate the tractability for more detailed investigations into the effects of this pervasive global pollutant.
Keywords: anthropogenic noise, habituation, tolerance, pollution, global change, interindividual variation
1. Introduction
Anthropogenic noise has substantially changed the acoustic environment both on land and underwater in the last century, and is now recognized as a major pollutant. Many different noise sources, including road and ship traffic, urban development, sonar and pile driving have been shown to have a negative impact on a wide variety of organisms [1–3]. However, empirical work has tended to focus on the overall response of cohorts of individuals, and experiments generally consider the consequences of a single noise exposure in isolation [4,5].
It is clear from other research fields that all members of a population are not necessarily affected in the same way by a given stimulus. For example, sex, dominance status, age and size can influence animal responses. In the context of environmental change, larger brook trout (Salvelinus fontinalis) were found to be more susceptible to increasing temperatures [6], whereas an inverse relationship between body size and metal sensitivity was seen among conspecific individuals of four macro-invertebrate species [7]. To date, potential intrapopulation differences in response to anthropogenic noise have received little consideration (but see [8]).
From a logistical perspective, it is understandable why noise-related experiments have often involved a single presentation of the relevant stimulus. However, organisms in most natural situations are likely to experience either chronic- or repeated-noise exposure, which might lead to changes in response through such processes as habituation, tolerance and sensitization [9]. Adjustments over time have been observed in relation to other pollutants [10,11], and thus it is important that this possibility is also tested with respect to anthropogenic noise [12,13].
Here, we use controlled experiments to explore how the physiology of different-sized shore crabs (Carcinus maenas) might be affected by single and repeated exposure to playback of ship noise, the most common source of anthropogenic noise in the aquatic environment [14]. Crustaceans have been shown to respond physiologically to other pollutants [15,16], they use underwater sound for a variety of reasons [17–19], and there is recent evidence that the behaviour of terrestrial crabs is affected by anthropogenic noise [4]. In response to noise, animals might be expected to mobilize energetic reserves and alter resource allocation in preparation for action (potentially as part of a more generalized stress response), one indicator of which would be enhanced metabolic rate and thus increased oxygen consumption [20]. We, therefore, hypothesized that the oxygen consumption of crabs would increase in response to ship-noise playback. Moreover, we predicted that the effect might be size-dependent, with larger crabs affected more strongly [6], and that repeated exposure would lead to a change in response, either an increased oxygen consumption as a consequence of sensitization, or a decrease arising from habituation or tolerance [9].
2. Material and methods
All crabs were collected from Newquay harbour (50°25′ N, 5°5′ W), transported to Bristol Aquarium by courier and held in polystyrene tanks. Full details of husbandry and all other methodology are available in the electronic supplementary material.
Both an ambient and a ship-noise recording were made at each of three UK ports, and playback tracks constructed in Audacity v. 1.3.13 (http://audacity.sourceforge.net/). Experimental tracks were played back using a similar setup to Purser & Radford [21]. Tracks were re-recorded 10 cm from the speaker in the experimental tank and modified (uniform amplification or attenuation) to generate a received level for that tank position of 108–111 dB r.m.s. re 1 µPa for ambient noise and 148–155 dB r.m.s. re 1 µPa for ship noise. Each track included 30 s fade in, 6½ min ambient or ship noise, and 30 s fade out, representative of a single ship pass.
For both experiments (single- and repeated-noise exposure), crabs were randomly allocated to one of the two sound treatments (ambient or ship noise); separate cohorts were used for each experiment. For each trial, the crab was placed in a 1 l airtight container completely filled with water, positioned 10 cm from the speaker in the experimental tank (see above). Playbacks lasted 15 min (time for two successive ship passes). The dissolved oxygen content of the water was measured at the start and end of the trial using an Oxyguard Handy Polaris oxygen meter. Animals were tested in counterbalanced blocks of six (ambient and ship noise from each of three harbours). In the single-exposure experiment, 36 crabs received one trial each (measurement errors reduced the sample size for analysis to 34); an independent-samples design was used to avoid potential carry-over effects. In the repeated-exposure experiment, individually marked crabs received the same playback trial eight times at 48 h intervals; 30 crabs were used on day one, but mortality resulted in a sample size of 22 for analysis. All animals from both experiments were weighed to the nearest 0.01 g (My Weigh iBalance 201).
Statistical analyses were conducted in R version 2.14.2 (The R Foundation). Data (uploaded to Dryad data repository (doi:10.5061/dryad.36f65) (Wale et al_data file)) fitted the assumptions of normality and heterogeneity of variance for parametric testing.
3. Results
Oxygen consumption in the single-exposure experiment was significantly affected by the interaction between crab mass and sound treatment (two-way ANOVA, interaction: F1,30 = 6.83, p = 0.014; mass: F1,30 = 29.20, p < 0.001; treatment: F1,30 = 34.34, p < 0.001). The greater consumption of oxygen in response to ship-noise playback—crabs experiencing this sound treatment consumed, on average, 67 per cent more oxygen than those exposed to ambient-noise playback—was most pronounced for heavier crabs (figure 1). The vast majority of crabs in both treatments remained stationary, and there were never any escape attempts.
Figure 1.
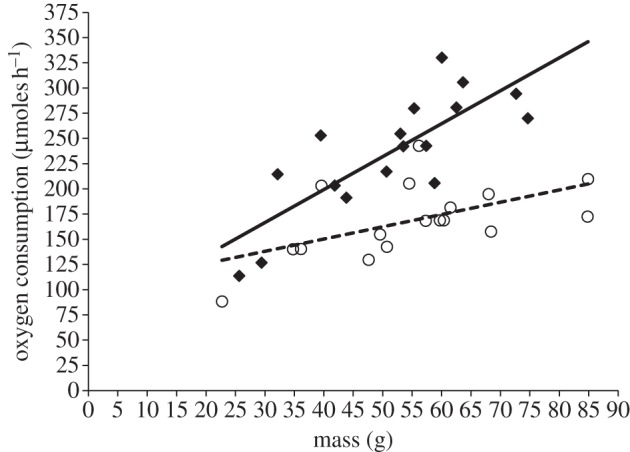
Oxygen consumption of shore crabs during single exposure to playback of either ambient noise (open circles, dotted line) or ship noise (filled diamonds, solid line). Shown are absolute values for individuals of different mass and least-squares regression lines; n = 17 individuals for each sound treatment.
In the repeated-exposure experiment, oxygen consumption was significantly affected by the interaction between day in the sequence and sound treatment (repeated-measures ANOVA, interaction: F7,160 = 2.18, p = 0.038; day in sequence: F7,160 = 0.85, p = 0.551; treatment: F1,160 = 171.99, p < 0.001). While animals repeatedly exposed to ambient-noise playback showed an increase in oxygen consumption across time, there was no significant change in those crabs repeatedly exposed to ship-noise playback (figure 2); crabs exposed to ship-noise playback always showed greater oxygen consumption than those experiencing ambient-noise playback (as per the single-exposure experiment). While larger crabs still consumed proportionately more oxygen than smaller crabs when exposed to ship-noise playback (mass: F1,84 = 4.60, p = 0.035), crabs of all sizes showed the same consistent oxygen consumption across time (interaction between day in sequence and mass: F1,84 = 0.34, p = 0.564).
Figure 2.
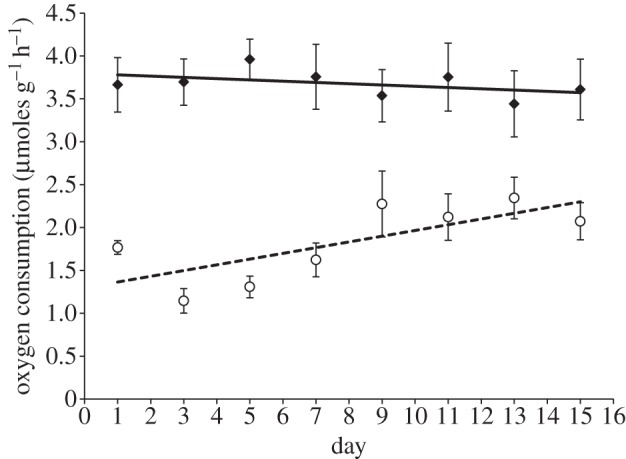
Oxygen consumption of shore crabs repeatedly exposed to playback of either ambient noise (open circles, dotted line) or ship noise (filled diamonds, solid line). Shown are mean ± s.e. mass-corrected values and least-squares regression lines; n = 11 individuals for each sound treatment.
4. Discussion
Crabs exposed to ship-noise playback consumed more oxygen than those experiencing playback of ambient harbour noise. Physiological impacts of noise have been previously demonstrated in a range of vertebrate species [22], but rarely in invertebrates (see [23] for an exception). Assessing how noise affects physiology, in addition to behaviour, is vital for a full understanding of both proximate and ultimate impacts on fitness [22].
The greater oxygen consumption in response to ship-noise playback is unlikely to have resulted from increased movement, because there was no discernible difference between sound treatments in the activity of animals. Increased oxygen consumption in static animals indicates a higher metabolic rate. If greater energy expenditure is not matched by an increased uptake of food, decreased growth and survival may result [24]. However, compensatory foraging may indirectly increase the risk of mortality through greater exposure to predatory threats [25]. Higher metabolic rate can also indicate increased cardiovascular activity arising from stress, and chronic stress can ultimately reduce fitness through detrimental effects on reproductive success and growth [22].
The increased oxygen consumption rate with each subsequent exposure to ambient-noise playback may result from repeated handling; holding tanks had similar noise profiles to the ambient-noise playback (see the electronic supplementary material). The lack of a similar positive relationship for individuals in the ship-noise treatment, which might, therefore, have been expected, could be explained in at least two ways. First, crabs may already show a maximum response on first exposure to ship-noise playback—as in the single-exposure experiment, oxygen consumption of individuals experiencing this treatment was higher than those exposed to ambient-noise playback; there may be no way to detect sensitization using this physiological response measure. Second, crabs might be habituating and/or becoming more tolerant to ship-noise playback; if their response to the playback lessened over time, this would counteract any increased oxygen consumption arising from repeated handling. Strong conclusions about habituation, tolerance and sensitization are, therefore, difficult, but further studies are clearly warranted and should also consider more frequent and/or longer exposures than here.
Previous work has indicated size-dependent differences in response to anthropogenic disturbances, such as rising temperatures and metal toxicity [6,7]. Our study suggests for the first time that there could be similar variation in response to noise; crabs differed in their response to single, but not repeated, noise exposure depending on their mass. One possibility is that bigger individuals are able to consume more oxygen proportionate to their body size when stressed; there may be size-related variation in the flexibility of crabs in their metabolic capacity. Consistent size-related differences in response could have impacts on population dynamics. In the current case, that might mean that larger individuals exposed to noise are less likely to survive; the smaller individuals that remain might be less likely to reproduce. For commercially important species, smaller-sized individuals are also less valuable.
In general, studies of anthropogenic noise have tended to focus on vertebrates [1–3]. The paucity of attention on invertebrates is not commensurate with their abundance and diversity (they make up 60% of marine species), their importance ecologically (as essential components of food webs) and economically (especially in light of changing fisheries) or their value in terms of new natural products [26,27]. Care is clearly needed when interpreting our results in a real-world context, both because tank playbacks cannot replicate natural sound fields perfectly (see the electronic supplementary material) and because crustaceans are likely to detect sounds, at least in part, using particle motion. However, our study highlights not only that invertebrates are potentially susceptible to the impacts of anthropogenic noise, but that they provide a tractable option for detailed investigations into the impacts of this pervasive global pollutant, which is likely to be complex.
Acknowledgements
We are grateful to the Bristol Aquarium for housing the study animals, to Sophie Holles and Irene Voellmy for the original sound recordings, to members of the Bristol Bioacoustics and Behavioural Ecology Group for thoughtful discussions, to Nick Roberts, Vincent Janik, Hansjoerg Kunc and an anonymous referee for comments on earlier manuscript versions, and to Defra for financial support.
References
- 1.Barber JR, Crooks KR, Fristrup KM. 2009. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189 10.1016/j.tree.2009.08.002 (doi:10.1016/j.tree.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 2.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427 10.1016/j.tree.2010.04.005 (doi:10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 3.Radford AN, Morley EL, Jones G. 2012. The effects of noise on biodiversity. Defra Report NO0235 See http://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=Move&Location=None&Completed=O&ProjectID=18136.
- 4.Chan AAY-H, Giraldo-Perez P, Smith S, Blumstein DT. 2010. Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461 10.1098/rsbl.2009.1081 (doi:10.1098/rsbl.2009.1081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin KE, Kunc HP. 2013. Experimentally increased noise levels change spatial and singing behaviour. Biol. Lett. 9, 20120771. 10.1098/rsbl.2012.0771 (doi:10.1098/rsbl.2012.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu CL, Letcher BH, Nislow KH. 2010. Size-dependent survival of brook trout Salvelinus fontinalis in summer: effects of water temperature and stream flow. J. Fish Biol. 76, 2342–2369 10.1111/j.1095-8649.2010.02619.x (doi:10.1111/j.1095-8649.2010.02619.x) [DOI] [PubMed] [Google Scholar]
- 7.Kiffney P, Clements W. 1996. Size-dependent response of macroinvertebrates to metals in experimental streams. Environ. Toxicol. Chem. 15, 1352–1356 10.1002/etc.5620150814 (doi:10.1002/etc.5620150814) [DOI] [Google Scholar]
- 8.Gross K, Pasinelli G, Kunc HP. 2010. Behavioral plasticity allows short-term adjustment to a novel environment. Am. Nat. 176, 456–464 10.1086/655428 (doi:10.1086/655428) [DOI] [PubMed] [Google Scholar]
- 9.Bejder L, Samuels A, Whitehead H, Finn H, Allen S. 2009. Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 395, 177–185 10.3354/meps07979 (doi:10.3354/meps07979) [DOI] [Google Scholar]
- 10.Piola RF, Johnston EL. 2009. Comparing differential tolerance of native and non-indigenous marine species to metal pollution using novel assay techniques. Environ. Poll. 157, 2853–2864 10.1016/j.envpol.2009.04.007 (doi:10.1016/j.envpol.2009.04.007) [DOI] [PubMed] [Google Scholar]
- 11.Whitehead A, Triant DA, Champlin D, Nacci D. 2010. Comparative transcriptomics implicates mechanisms of evolved pollution tolerance in a killifish population. Mol. Ecol. 19, 5186–5203 10.1111/j.1365-294X.2010.04829.x (doi:10.1111/j.1365-294X.2010.04829.x) [DOI] [PubMed] [Google Scholar]
- 12.Blickley JL, Word KR, Krakauer AH, Phillips JL, Sells SN, Taff CC, Wingfield JC, Patricelli GL. 2012. Experimental chronic noise is related to elevated fecal corticosteroid metabolites in lekking male greater sage-grouse (Centrocercus urophasianus). PLoS ONE 7, e50462. 10.1371/journal.pone.0050462 (doi:10.1371/journal.pone.0050462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halfwerk W, Bot S, Slabbekoorn H. 2012. Male great tit song perch selection in response to noise-dependent female feedback. Funct. Ecol. 26, 1339–1347 10.1111/j.1365-2435.2012.02018.x (doi:10.1111/j.1365-2435.2012.02018.x) [DOI] [Google Scholar]
- 14.Vasconcelos RO, Amorim MCP, Ladich F. 2007. Effects of ship noise on the detectability of communication signals in the Lusitanian toadfish. J. Exp. Biol. 210, 2104–2112 10.1242/jeb.004317 (doi:10.1242/jeb.004317) [DOI] [PubMed] [Google Scholar]
- 15.Bamber SD, Depledge MH. 1997. Evaluation of changes in the adaptive physiology of shore crabs (Carcinus maenas) as an indicator of pollution in estuarine environments. Mar. Biol. 129, 667–672 10.1007/s002270050209 (doi:10.1007/s002270050209) [DOI] [Google Scholar]
- 16.Metzger R, Sartoris FJ, Langenbuch M, Pörtner HO. 2007. Influence of elevated CO2 concentrations on thermal tolerance of the edible crab Cancer pagurus. J. Therm. Biol. 32, 144–151 10.1016/j.jtherbio.2007.01.010 (doi:10.1016/j.jtherbio.2007.01.010) [DOI] [Google Scholar]
- 17.Jeffs A, Tolimieri N, Montgomery JC. 2003. Crabs on cue for the coast: the use of underwater sound for orientation by pelagic crab stages. Mar. Freshwater Res. 54, 841–845 10.1071/MF03007 (doi:10.1071/MF03007) [DOI] [Google Scholar]
- 18.Stanley JA, Radford CA, Jeffs AG. 2010. Induction of settlement in crab megalopae by ambient underwater reef sound. Behav. Ecol. 21, 113–120 10.1093/beheco/arp159 (doi:10.1093/beheco/arp159) [DOI] [Google Scholar]
- 19.Simpson SD, Radford AN, Tickle EJ, Meekan MG, Jeffs AG. 2011. Adaptive avoidance of reef noise. PLoS ONE 6, e16625. 10.1371/journal.pone.0016625 (doi:10.1371/journal.pone.0016625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendelaar-Bonga SE. 1997. The stress response in fish. Physiol. Rev. 77, 591–625 [DOI] [PubMed] [Google Scholar]
- 21.Purser J, Radford AN. 2011. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PloS ONE 6, e17478. 10.1371/journal.pone.0017478 (doi:10.1371/journal.pone.0017478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kight CR, Swaddle JP. 2011. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 14, 1052–1061 10.1111/j.1461-0248.2011.01664.x (doi:10.1111/j.1461-0248.2011.01664.x) [DOI] [PubMed] [Google Scholar]
- 23.Regnault M, Lagardere J. 1983. Effects of ambient noise on the metabolic level of Crangon crangon (Decapoda, Natantia). Mar. Ecol. Prog. Ser. 11, 71–78 10.3354/meps011071 (doi:10.3354/meps011071) [DOI] [Google Scholar]
- 24.Bjerregaard P. 1991. Relationship between physiological condition and cadmium accumulation in Carcinus maenas (L.). Comp. Biochem. Physiol. A 99, 75–83 10.1016/0300-9629(91)90238-8 (doi:10.1016/0300-9629(91)90238-8) [DOI] [Google Scholar]
- 25.Lima SL, Dill LM. 1990. Behavioural decisions made under the risk of predation – a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 26.Ausubel J, Crist D, Waggoner P. 2010. First census of marine life 2010: highlights of a decade of discovery. Washington, DC: Census of Marine Life [Google Scholar]
- 27.Leal MC, Puga J, Serôdio J, Gomes NCM, Calado R. 2012. Trends in the discovery of new marine natural products from invertebrates over the last two decades—where and what are we bioprospecting? PloS ONE 7, e30580. 10.1371/journal.pone.0030580 (doi:10.1371/journal.pone.0030580) [DOI] [PMC free article] [PubMed] [Google Scholar]


