Abstract
Human mating and reproductive behaviour can vary depending on various mechanisms, including the local sex ratio. Previous research shows that as sex ratios become female-biased, women from economically deprived areas are less likely to delay reproductive opportunities to wait for a high-investing mate but instead begin their reproductive careers sooner. Here, we show that the local sex ratio also has an impact on female fertility schedules. At young ages, a female-biased ratio is associated with higher birth rates in the poorest areas, whereas the opposite is true for the richest areas. At older ages, a female-biased ratio is associated with higher birth rates in the richest, but not the poorest areas. These patterns suggest that female–female competition encourages poorer women to adopt a fast life-history strategy and give birth early, and richer women to adopt a slow life-history strategy and delay reproduction.
Keywords: sex ratio, socioeconomic status, life history, birth rate, reproductive scheduling, female competition
1. Introduction
Humans exhibit considerable within-species variation in reproductive behaviour. One well-established finding is that high-mortality, harsh environments favour early reproduction [1]. Yet, research on many species has demonstrated that the operational sex ratio (OSR) affects the relative importance of reproductive behaviours, such as mate choice and sexual competition, which can have a knock-on effect to actual fertility scheduling. OSR is the ratio of sexually active males to sexually receptive females [2], and in both animal and human populations the scarcer sex becomes in demand and has greater influence on subsequent behaviour [3,4].
In humans, male-biased populations have been associated with higher crime rates, perhaps indicating increased male–male competition [5]. Furthermore, where there is a male-biased ratio, women's mate preferences become more selective resulting in the limited marital success of low socioeconomic status men [6]. By contrast, societies with female-biased ratios favour male-mating preferences. For example, female-biased populations across cultures tend to have higher marital instability as demonstrated by increased divorce rates and lower remarriage rates [7]. In addition, female-biased OSRs increased non-marital reproduction amongst females living in deprived areas as they failed to attract high-investing spouses and began to reproduce at younger ages [8]. There is also evidence that a female-biased ratio lowers women's expectations of the opposite sex. This has been shown experimentally where women's expectations of the amount of money men will spend on them when dating is lowered when there are fewer men available [9].
In this study, we aim to shift the discussion from mate market dynamics to fertility scheduling by examining the effect of OSRs on birth rates between neighbourhoods in England. Birth rates can serve as another important indicator of intrasexual competition, as females may adjust their reproductive timing based on the relative number of potential mates in their local environment. Furthermore, women's responses to female-biased OSRs are likely to differ based on their socioeconomic status and position on the life-history continuum.
Women of high socioeconomic status with slow life-history characteristics may respond to environmental stressors by moving further towards the slow end of the life-history continuum and delaying reproduction [10]. Conversely, low socioeconomic status women with fast life-history strategies may seek to have children sooner in response to the same environmental conditions. Therefore, low socioeconomic status women in female-biased neighbourhoods may begin reproducing earlier in a bid to maximize their fitness by strategically adopting fast life-history characteristics rather than delaying reproduction in the hope of attracting a high-investing mate and investing in offspring quality. We predict that (i) in the most deprived UK neighbourhoods, birth rates at young ages will be higher when the OSR is female biased and (ii) in the least deprived neighbourhoods, birth rates at older ages will be higher when the OSR is female biased.
2. Material and methods
We focused on England, where economic inequality is high relative to other developed nations and material conditions between neighbourhoods can vary markedly within the space of a few kilometres, particularly in urban areas. We conducted our analysis at ward level as wards are the primary unit of British administrative geography and the smallest area of geography for which the Office for National Statistics (ONS) releases data on birth rates.
There are a total of 7933 Census Area Statistical wards in England, which were used for the 2001 census outputs. As wards can vary in both geographical and population size we retained 5223 urban wards for analysis. We focused on urban wards because in rural areas wards can be sparsely populated which might result in less intrasexual competition. Furthermore, owing to greater distances in rural areas, individuals must travel to reach their workplaces or to socialize with friends and family; individuals may be more mobile and may leave their local area more frequently. Hence the sex ratio that affects their behaviour may not be because of the ward itself. Wards are defined as urban if they belong to a metropolitan area whose population is greater than 10 000 (mean population = 7984, s.d. = 4342) [11].
Data on live births, by ward and mother's age, were released as the rate of live births per 1000 female population in aggregate 3 year bands [12]. Data were taken for the years 2005–2007, and mothers' ages were aggregated into quintiles from 15–19 years to 35–39 years. Strict password protection arrangements prevent individuals being identified. Wards with fewer than five conceptions were suppressed to further protect individuals from identification, leaving 2876 wards.
We calculated the OSR for each ward as the ratio of males to females between 15 and 50 years of age living in a particular ward [13]. OSRs greater than one indicate a male bias. Population figures were taken from the Mid Year Population Estimate released by the ONS for 2007 (mean = 1.00, s.d. = 0.84) [14]. The range for OSRs in the most deprived 50 per cent of urban wards was 0.74–1.71 (s.d. = 0.97), and in the least deprived 50 per cent of urban wards 0.68–1.79 (s.d. = 1.10). These figures include individuals normally resident in a ward, including prisoners, boarding school students and armed forces personnel, meaning that some such wards with small populations were heavily male-biased (OSRs > 1.4). Exclusions of these wards from the analysis do not alter the statistical significance of the model.
To measure ward-level deprivation, we used the Index of Multiple Deprivation (IMD) for 2007 [15]. The IMD is a composite index of socioeconomic hardship that includes income, employment, health, education, housing and access to services. Higher scores indicate more-deprived areas; scores range from 1.44 to 79.18 (mean = 23.80, s.d. = 13.83).
Using R [16], we created generalized linear models fitted by the Laplace approximation [17] with OSR, IMD and age as fixed variables and birth rate as the response variable, treated as a proportion and analysed with logistic regression. We ran individual models for live births at each age category to determine the direction of effects of OSR, IMD and their interaction at separate birth rate quintiles.
3. Results
Up to the age of 29, increased deprivation was associated with a higher birth rate, whereas after the age of 29, decreased deprivation was associated with a higher birth rate; these results suggest that poorer women gave birth at young ages, whereas wealthier women delayed reproduction until they were older. In general, female-biased OSRs were associated with higher birth rates, although for two out of five age categories this effect was not significant. The interaction between OSR and IMD was significant at each age category accept for ages 25–29. Under the age of 25, female-biased OSRs were associated with higher birth rates in deprived areas, but lower birth rates in less-deprived areas. After the age of 29, this pattern reversed, and female-biased OSRs were associated with higher birth rates in less-deprived areas, and lower birth rates in more-deprived areas, supporting both predictions (table 1).
Table 1.
Parameter estimates with effect sizes for birth rate by age quintile. (Data presented from five generalized linear models.)
| parameter estimate | s.e. | p-value | partial η² | ||
|---|---|---|---|---|---|
| model 1: 15–19 birth rate | intercept | −4.879 | 0.083 | <0.001 | |
| OSR | −0.069 | 0.083 | n.s. | 0 | |
| IMD | 0.038 | 0.002 | <0.001 | 0.018 | |
| OSR × IMD | −0.006 | 0.002 | <0.05 | 0.006 | |
| model 2: 20–24 birth rate | intercept | −3.257 | 0.049 | <0.001 | |
| OSR | −0.179 | 0.049 | <0.001 | 0.011 | |
| IMD | 0.034 | 0.002 | <0.001 | 0.002 | |
| OSR × IMD | −0.020 | 0.002 | <0.001 | 0 | |
| model 3: 25–29 birth rate | intercept | −2.388 | 0.043 | <0.001 | |
| OSR | −0.502 | 0.043 | <0.001 | 0.028 | |
| IMD | 0.004 | 0.002 | <0.01 | 0.005 | |
| OSR × IMD | −0.002 | 0.002 | n.s. | 0.009 | |
| model 4: 30–34 birth rate | intercept | −2.226 | 0.042 | <0.001 | |
| OSR | −0.438 | 0.042 | <0.001 | 0.014 | |
| IMD | −0.024 | 0.002 | <0.001 | 0.018 | |
| OSR × IMD | 0.015 | 0.002 | <0.001 | 0.013 | |
| model 5: 35–39 birth rate | intercept | −3.265 | 0.054 | <0.001 | |
| OSR | −0.027 | 0.053 | n.s. | 0.003 | |
| IMD | −0.027 | 0.002 | <0.001 | 0.002 | |
| OSR × IMD | 0.018 | 0.002 | <0.001 | 0.001 |
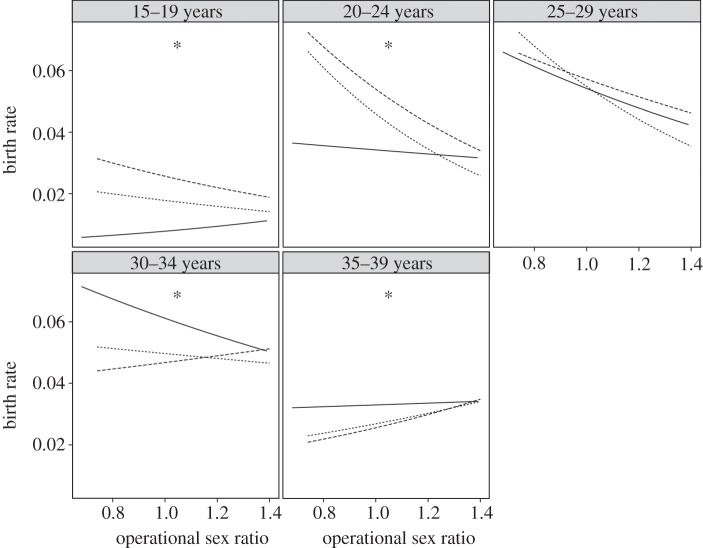
To display the shift in the direction of the interaction effect after age 25, we present the regression line for birth rates by age quintile plotted against OSR for socioeconomic status terciles in figure 1.
Figure 1.
Birth rates plotted against OSR. Logistic regression lines (solid line, high; dotted line, intermediate; dashed line, low) are presented for each socioeconomic status terciles, and the data points have been removed for clarity. Significant interactions are marked with an asterisk.
4. Discussion
We show that in addition to the well-established effect of deprivation, OSR also predicts the birth rate. Furthermore, female-biased OSRs predict early reproduction in the most deprived wards. This effect reverses at older ages, such that from the age of 30 it is in the least deprived wards where female-biased OSRs predict higher birth rates. Each variable only explained a small amount of the variance in overall birth rate, but this is not surprising given that the decision to have children is complex, with many different factors contributing to birth rates [18]. Furthermore, the effect size of OSR is at similar levels to effects of deprivation which is a well-established antecedent of age of first birth. These findings are consistent with Pedersen's [4] prediction that individuals will adjust their behaviour as a result of same sex competition induced by differences in the sex ratio. Our interpretation of the findings is that as the chance of attracting a high-investing partner is reduced in female-biased wards, perhaps owing to increased female–female competition, women from deprived areas calibrate towards the fast end of the life-history continuum. Thus, they begin to reproduce earlier, boosting early reproduction in these areas. By contrast, women from less-deprived areas with slow life-history characteristics adopt the opposite strategies in response to female-biased OSRs and delay reproduction [10]. We suggest, therefore, that the interaction between ward-level deprivation and OSR is vital to understand individual adjustments to fertility schedules. Given the geographical scale of wards, we must consider the psychological and biological reality of such neighbourhood divisions. Previous research has highlighted the importance that subjective experiences of local neighbourhoods play in predicting teenage pregnancy, even compared with environmental predictors such as ward IMD [19]. Wards are local ecologies which individuals can monitor for perceived environmental risks and perhaps the supply of one sex to the other which may be related to reproductive behaviour accounting for our results. Wards might be especially salient for those from the low end of the socioeconomic gradient owing to lower geographical mobility, as reduced car ownership and greater unemployment may mean individuals are less likely to travel beyond their local neighbourhoods, making the environment of their ward particularly important.
To our knowledge, this is the first time that the effect of the OSR on human birth rates has been shown in a national sample in a developed country at the level of small geographical areas, that is, wards. This study adds to the growing evidence that the ratio of sexually active and competing males to females explains various aspects of male and female behaviour. In principle, this study demonstrates that an oversupply of females within a local economically deprived environment leads young women to adjust their strategies for reproductive success by beginning their reproductive careers earlier and thus predicts an increase in the rate of teenage pregnancy within that environment.
Acknowledgements
We thank Dr Paul Morris, Dr Bridget Waller and Jerome Micheletta for feedback, and the ONS (www.ons.gov.uk) for making data available. We also thank the reviewers for their helpful comments.
References
- 1.Nettle D. 2011. Flexibility in reproductive timing in human females: integrating ultimate and proximate explanations. Phil. Trans. R. Soc. B 366, 357–365 10.1098/rstb.2010.0073 (doi:10.1098/rstb.2010.0073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kvarnemo C, Ahnesjö I. 1996. The dynamics of operational sex ratios and competition for mates. Trends Ecol. Evol. 11, 404–408 10.1016/0169-5347(96)10056-2 (doi:10.1016/0169-5347(96)10056-2) [DOI] [PubMed] [Google Scholar]
- 3.Jirotkul M. 1999. Operational sex ratio influences female preference and male–male competition in guppies. Anim. Behav. 58, 287–294 10.1006/anbe.1999.1149 (doi:10.1006/anbe.1999.1149) [DOI] [PubMed] [Google Scholar]
- 4.Pedersen FA. 1991. Secular trends in human sex ratios: their influence on individual and family behaviour. Hum. Nat. Int. Bios. 2, 271–291 10.1007/BF02692189 (doi:10.1007/BF02692189) [DOI] [PubMed] [Google Scholar]
- 5.Hudson VM, Den Boer A. 2002. A surplus of men, a deficit of peace: security and sex ratios in Asia's largest states. Int. Security. 26, 5–38 10.1162/016228802753696753 (doi:10.1162/016228802753696753) [DOI] [Google Scholar]
- 6.Pollet TV, Nettle D. 2008. Driving a hard bargain: sex ratio and male marriage success in a historical US population. Biol. Lett. 4, 31–33 10.1098/rsbl.2007.0543 (doi:10.1098/rsbl.2007.0543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trent K, South SJ. 1989. Structural determinants of the divorce rate: a cross-societal analysis. J. Marriage Fam. 51, 391–404 10.2307/352502 (doi:10.2307/352502) [DOI] [Google Scholar]
- 8.Staples R. 1985. Changes in black family structure: the conflict between family ideology and structural conditions. J. Marriage Fam. 47, 1005–1013 10.2307/352344 (doi:10.2307/352344) [DOI] [Google Scholar]
- 9.Griskevicius V, Tybur JM, Ackerman JM, Delton AM, Robertson TE, White AE. 2012. The financial consequences of too many men: sex ratio effects on saving, borrowing, and spending . J. Pers. Soc. Psychol. 102, 69. 10.1037/a0024761 (doi:10.1037/a0024761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griskevicius V, Delton AW, Robertson TE, Tybur JM. 2011. The environmental contingency of life history strategies: influences of mortality and socioeconomic status on reproductive timing. J. Pers. Soc. Psychol. 100, 241–254 10.1037/a/0024761 (doi:10.1037/a/0024761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ONS 2004. Rural and urban statistics: guidance notes. London, UK: Office of National Statistics [Google Scholar]
- 12.ONS 2011. Mid year population estimates by ward 2007. London, UK: Office of National Statistics [Google Scholar]
- 13.Lummaa V, Merilä J, Kausse A. 1998. Adaptive sex ratio variation in pre-industrial human (Homo sapiens) populations? Proc. R. Soc. Lond. B 265, 563–568 10.1098/rspb.1998.0331 (doi:10.1098/rspb.1998.0331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ONS 2012. Live Births by age and conceptions in 2006–2008 by ward. London, UK: Office of National Statistics [Google Scholar]
- 15.ONS 2007. Index of multiple deprivation by ward. London, UK: Office of National Statistics [Google Scholar]
- 16.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 17.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS. 2008. Generalized linear mixed models: a practical guide for ecology and evolution . Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 18.Hardy JB, Astone NM, Brooks-Gunn J, Shapiro S, Miller TL. 1998. Like mother, like child: intergenerational patterns of age at first birth and associations with childhood and adolescent characteristics and adult outcomes in the second generation. Dev. Psychol. 34, 1220–1232 10.1037/0012-1649.34.6.1220 (doi:10.1037/0012-1649.34.6.1220) [DOI] [PubMed] [Google Scholar]
- 19.Johns SE. 2011. Perceived environmental risk as a predictor of teenage motherhood in a British population. Health Place 17, 122–131 10.1016/j.healthplace.2010.09.006 (doi:10.1016/j.healthplace.2010.09.006) [DOI] [PubMed] [Google Scholar]