Abstract
The amount of salt-affected agricultural land is increasing globally, so new crop varieties are needed that can grow in salt-affected soils. Despite concerted effort to develop salt-tolerant cereal crops, few commercially viable salt-tolerant crops have been released. This is puzzling, given the number of naturally salt-tolerant grass species. To better understand why salt-tolerance occurs naturally but is difficult to breed into crop species, we take a novel, biodiversity-based approach to its study, examining the evolutionary lability of salt-tolerance across the grass family. We analyse the phylogenetic distribution of naturally salt-tolerant species on a phylogeny of 2684 grasses, and find that salt-tolerance has evolved over 70 times, in a wide range of grass lineages. These results are confirmed by repeating the analysis at genus level on a phylogeny of over 800 grass genera. While salt-tolerance evolves surprisingly often, we find that its evolution does not often give rise to a large clade of salt-tolerant species. These results suggest that salt-tolerance is an evolutionarily labile trait in grasses.
Keywords: halophyte, phylogeny, Poaceae
1. Introduction
Unprecedented growth in food production is needed to feed a global population likely to reach nine billion by the middle of this century [1,2]. One opportunity for attaining this growth is to increase crop yields in marginal environments, including salt-affected land and irrigated areas that are susceptible to increased salinity [2,3]. However, despite considerable effort there has been disappointingly little success in producing salt-tolerant cereal crop varieties—few have been released and their advantages over conventional varieties are relatively small [4–6]. The challenges faced in enhancing cereal crop salt-tolerance seem paradoxical, given that there are a large number of naturally salt-tolerant grasses (halophytes) [7].
One possible explanation for the difficulty in breeding salt-tolerant crops is that salt-tolerance is a genetically and physiologically complex trait [4]. If this complex trait requires specific genetic backgrounds or particular suites of physiological features to develop, we might expect it to be very difficult to increase salt-tolerance in lineages that lack those features. If this is the case, we might expect that salt-tolerance evolves rarely, with most halophytes being closely related, all arising from few origins of salt-tolerance [8]. Therefore, using phylogenetic analysis to ask whether the evolution of salt-tolerance is rare or common may contribute to our understanding of the challenges involved in developing salt-tolerant crops.
Although salt-tolerance is extensively researched, most studies focus on one or a small number of taxa, particularly crop or model species [9,10]. Few studies have taken a broader approach, asking what we can learn from considering the range of halophytes and the pattern of evolution of salt-tolerance. We use the distribution of halophytes on a phylogeny to examine the evolutionary lability of the trait across the grass family. We find that salt-tolerance has evolved a large number of times, in a wide variety of grass lineages, suggesting that the capacity to develop salt-tolerance is a feature of many genetic backgrounds. This result may have practical relevance to the development of salt-tolerant varieties for agriculture.
2. Material and methods
Using the definition of salt-tolerance outlined in the electronic supplementary material, we identified 350 halophytic grass species or subspecies by searching the published literature and an existing global halophyte database [7]. We analysed the distribution of halophytes on a molecular phylogeny of the grass family containing 2684 taxa, or approximately 20 per cent of described grass species [11], including 200 identified halophytes (see the electronic supplementary material, tables S1 and S2). We took two approaches to examining the evolutionary lability of salt-tolerance, estimating phylogenetic signal and reconstructing ancestral states (see the electronic supplementary material for details). To test whether the results were dependent of the phylogeny used, taxa included or definition of salt-tolerance used, we repeated our analyses on a different phylogeny containing all of the approximately 800 grass genera [12] and also repeated the analyses for only ‘euhalophyte’ species with greater salt-tolerance (see the electronic supplementary material for details).
3. Results
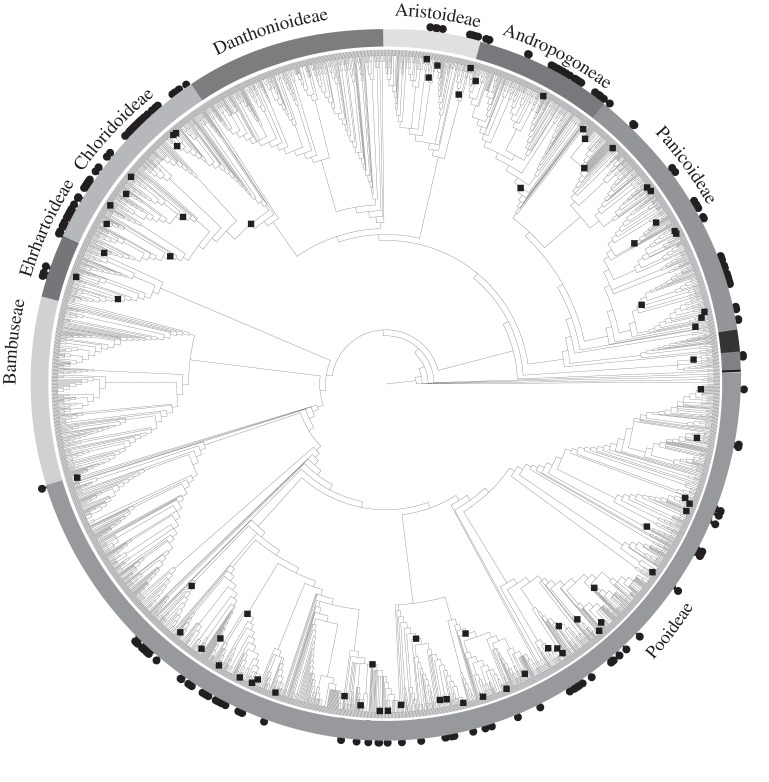
Salt-tolerance is phylogenetically non-random, suggesting that some clades are more likely than others to contain salt-tolerant species (see the electronic supplementary material for details). However, salt-tolerance is evolutionarily highly labile across much of the family (figure 1). Using maximum-likelihood reconstruction, we estimate that salt-tolerance has evolved 76 times (figure 1). The finding of a large number of origins was robust to the method of ancestral state reconstruction, with salt-tolerance estimated to have evolved over 100 times using unweighted parsimony. The pattern was also robust to the phylogeny used—we estimate 92 origins of salt-tolerance on the genus-level phylogeny (see the electronic supplementary material, figure S1). This pattern was also robust to the definition of salt-tolerance used: analysis of the distribution of euhalophytes on the genus-level phylogeny suggests that tolerance to high salinity has evolved at least 43 times in grasses (see the electronic supplementary material, figure S1). Sensitivity analyses also support a high number of origins (see the electronic supplementary material, figures S5 and S6). Some clades have more reconstructed origins of salt-tolerance than others, suggesting that some lineages are more likely to evolve salt-tolerance (or, possibly, that salt-tolerance is lost more frequently in some lineages). Furthermore, some lineages seem less likely to evolve salt-tolerance, for example there are no reported halophytes in the Bambuseae and Danthonioideae (figure 1).
Figure 1.
Distribution of 200 salt-tolerant species (circles at tips) on a phylogeny of grasses, modified from Edwards et al. [11]. We reconstructed 76 origins of salt-tolerance (squares on internal nodes) using the best-fitting likelihood model. Most origins occur close to the tips of the phylogeny, with a mean of 2.6 halophytes per origin.
Our analysis indicates that most origins of salt-tolerance in grasses occur near the tips of the species-level phylogeny, with most origins giving rise to few halophytes (figure 1). This ‘tippy’ pattern is unlikely to be due to incomplete taxon sampling, because most genera containing halophytes include only a small number (median = 1) and proportion (median = 0.25) of salt-tolerant species (see the electronic supplementary material, figure S2). In genera with one halophyte, unrecorded halophytic species could either cluster with the recorded halophytes (in which case the number of origins would remain the same) or would occur elsewhere in the genus (increasing the inferred number of origins). Only in genera with many closely related halophytes is there the potential for unrecorded salt-tolerant species to ‘fill in the gaps’ and decrease the inferred number of origins. Because most grass genera have only one or no halophytes, we expect that adding unrecorded halophytes would typically either increase or maintain the number of inferred origins of salt-tolerance. Therefore, the paucity of halophytes within these genera combined with their scattering on the phylogeny (see the electronic supplementary material, figure S1), supports many recent origins, each giving rise to few halophytes.
4. Discussion
Our analysis demonstrates that salt-tolerance has evolved many times in a wide variety of grass lineages. The exact number of origins is unknown, because the estimates vary with the method used or assumptions made, but all analyses suggest that the 200 halophytes included arose from more than 70 origins of salt-tolerance, scattered across much of the family. This observation implies that a wide variety of grass lineages have the capacity to adapt to saline conditions. Our observation of evolutionary lability is supported by studies finding intraspecific differentiation in salt-tolerance and associated physiological, anatomical, and biochemical characters in numerous grass species [13], adaptation which in some cases has occurred over short time-scales [14].
If salt-tolerance can evolve in a wide variety of lineages, then why is it so difficult to breed salt-tolerant crops? Salt-tolerance has evolved in lineages closely related to crop plants; for example the tribe Triticeae, which includes wheat, barley and rye, contains a number of halophytes such as tall wheatgrass (Thinopyrum spp.) [15]. Similarly, rice is closely related to the halophytic wild rice (Porteresia coarctata) [16]. One possible reason for the difficulty in breeding salt-tolerant crops is that some crops may have lost genetic variation for high salt-tolerance [6]. For example, recent work has demonstrated that some modern wheats lack sodium transporters that confer salt-tolerance in their wild relatives, and that tolerance can be improved by introducing these into crops [17]. However, there is genetic variation in crops for other traits associated with salt-tolerance [18].
Although salt-tolerance appears to be labile across the grass family, some lineages have more inferred origins of salt-tolerance than others (figure 1). This implies that there are heritable traits that influence the likelihood of developing (or retaining) salt-tolerance. For example, grass lineages with C4 photosynthesis have a greater rate of gain of salt-tolerance (L. Bromham & T. H. Bennett 2013, unpublished data), possibly because C4 increases water-use efficiency, limiting water stress and reducing ion uptake. Alternatively, phylogenetic signal may be linked to ecological preferences or biogeographic distribution. For example, C4 species are clustered in lineages from open and arid environments [11], which are more likely to contain saline soils. Similarly, there are some clades which have no reported salt-tolerant species, such as the Bambuseae (bamboos) and Danthonioideae. These lineages may have physiological limitations or biogeographic distributions that make it difficult for them to develop salt-tolerance or adapt to other characteristics of saline habitats. For example, bamboos are forest-adapted species, and saline soils do not tend to support the formation of closed forests. Characterizing the factors that influence the evolution of salt-tolerance might allow us to predict the potential to develop salt-tolerance in different lineages.
Unlike some other angiosperm lineages where there are large clades of halophytes arising from few origins of salt-tolerance (e.g. Chenopodacieae; [19]), most origins of salt-tolerance in grasses are close to the tips of the phylogeny. Most major groups of grasses contain some halophyte species, typically arising from a relatively large number of origins rather than clustering together (see the electronic supplementary material, figure S3), so that most salt-tolerant species have few close halophytic relatives. Given that salt-tolerance evolves frequently, why do we see relatively few deep origins (e.g. in the Chloridoideae) that give rise to large clades of salt-tolerant grasses?
There are a number of possible causes of this ‘tippy’ phylogenetic distribution, which are not mutually exclusive. First, a predominance of recent origins might occur because many lineages have responded similarly to a recent change in environmental conditions, or colonization of new habitats. For example, many grass lineages independently developed C4 photosynthesis as they moved from forests into more open, arid environments [11,20], so it may be that many grass lineages have relatively recently moved into saline environments. Second, a tippy pattern may be generated by a trait that is associated with an increased chance of extinction, so that lineages which evolve the trait are less likely to persist or speciate. The classic example is asexuality which arises often but is rarely shared by all members of large, deep clades, leading to suggestions that asexual species lack the genetic variation needed for long-term evolutionary persistence [21]. It has also been suggested that lineages in saline environments may have increased extinction rates [19]. Third, a highly labile trait could show a tippy pattern if it is often gained and lost. For example, colour-polymorphic bird species show a phylogenetically dispersed pattern, possibly because colour polymorphism is lost at a greater rate than it is gained [22]. If the tippy pattern is due to high trait lability, we would expect the trait to have low phylogenetic signal and a high inferred rate of reversal. While it is problematic to estimate trait reversals [23], our analyses suggest high rates of both gain and loss of salt-tolerance.
5. Conclusions
This study takes a broad, comparative approach to understanding the evolution of salt-tolerance in grasses, so there is a limit to the conclusions we can draw regarding the development of salt-tolerance in specific lineages. However, it is possible that future studies could build on this approach to identify specific backgrounds that promote the acquisition of salt-tolerance. This might allow us to better gauge the potential to develop salt-tolerance in major crop species both within and outside of the grass family, and to look at the pattern of plants adapting to harsh environments. This phylogenetic approach might also lead to identification of labile traits contributing to salt-tolerance, which could be targeted by breeding programmes.
Acknowledgements
We thank Colin Osborne, Yanis Bouchenak-Khelladi and their collaborators for phylogenies and discussion, and Rob Lanfear for methodological assistance. The project was supported by the Australian Research Council.
References
- 1.Godfray HCJ, et al. 2010. Food security: the challenge of feeding 9 billion people. Science 327, 812–818 10.1126/science.1185383 (doi:10.1126/science.1185383) [DOI] [PubMed] [Google Scholar]
- 2.Tester M, Langridge P. 2010. Breeding technologies to increase crop production in a changing world. Science 327, 818–822 10.1126/science.1183700 (doi:10.1126/science.1183700) [DOI] [PubMed] [Google Scholar]
- 3.Rozema J, Flowers T. 2008. Crops for a salinized world. Science 322, 1478–1480 10.1126/science.1168572 (doi:10.1126/science.1168572) [DOI] [PubMed] [Google Scholar]
- 4.Flowers TJ. 2004. Improving crop salt-tolerance. J. Exp. Biol 55, 307–319 [DOI] [PubMed] [Google Scholar]
- 5.Witcombe JR, Hollington PA, Howarth CJ, Reader S, Steele KA. 2008. Breeding for abiotic stresses for sustainable agriculture. Phil. Trans. R. Soc. B 363, 703–716 10.1098/rstb.2007.2179 (doi:10.1098/rstb.2007.2179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashraf M, Akram NA. 2009. Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol. Adv. 27, 744–752 10.1016/j.biotechadv.2009.05.026 (doi:10.1016/j.biotechadv.2009.05.026) [DOI] [PubMed] [Google Scholar]
- 7.Aronson JA. 1989. HALOPH: a data base of salt tolerant plants of the world. Tucson, AZ: Office of Arid Lands Studies [Google Scholar]
- 8.Donoghue MJ. 2008. A phylogenetic perspective on the distribution of plant diversity. Proc. Natl Acad. Sci. USA 105, 11 549–11 555 10.1073/pnas.0801962105 (doi:10.1073/pnas.0801962105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenn EP, Brown JJ, Blumwald E. 1999. Salt-tolerance and crop potential of halophytes. CRC Crit. Rev. Plant Sci. 18, 227–255 10.1016/S0735-2689(99)00388-3 (doi:10.1016/S0735-2689(99)00388-3) [DOI] [Google Scholar]
- 10.Flowers TJ, Galal HK, Bromham L. 2010. Evolution of halophytes: multiple origins of salt-tolerance in land plants. Funct. Plant Biol. 37, 604–612 10.1071/FP09269 (doi:10.1071/FP09269) [DOI] [Google Scholar]
- 11.Edwards EJ, Smith SA. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proc. Natl Acad. Sci. USA 107, 2532–2537 10.1073/pnas.0909672107 (doi:10.1073/pnas.0909672107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouchenak-Khelladi Y, Verboom GA, Savolainen V, Hodkinson TR. 2010. Biogeography of the grasses (Poaceae): a phylogenetic approach to reveal evolutionary history in geographical space and geological time. Bot. J. Linn. Soc. 162, 543–557 10.1111/j.1095-8339.2010.01041.x (doi:10.1111/j.1095-8339.2010.01041.x) [DOI] [Google Scholar]
- 13.Naz N, Hameed M, Ashraf M, Ahmad R, Arshad M. 2009. Eco-morphic variation for salt-tolerance in some grasses from Cholistan Desert, Pakistan. Pak. J. Bot. 41, 1707–1714 [Google Scholar]
- 14.Scott JW, Meyer SE, Merrill KR, Anderson VJ. 2010. Local population differentiation in Bromus tectorum L. in relation to habitat-specific selection regimes. Evol. Ecol. 24, 1061–1080 10.1007/s10682-010-9352-y (doi:10.1007/s10682-010-9352-y) [DOI] [Google Scholar]
- 15.Colmer TD, Flowers TJ, Munns R. 2006. Use of wild relatives to improve salt-tolerance in wheat. J. Exp. Biol. 57, 1059–1078 [DOI] [PubMed] [Google Scholar]
- 16.Flowers TJ, Flowers SA, Hajibagheri MA, Yeo AR. 1990. Salt tolerance in the halophytic wild rice, Porteresia coarctata. New Phytol. 114, 675–684 10.1111/j.1469-8137.1990.tb00439.x (doi:10.1111/j.1469-8137.1990.tb00439.x) [DOI] [Google Scholar]
- 17.Munns R, et al. 2012. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 30, 360–364 10.1038/nbt.2120 (doi:10.1038/nbt.2120) [DOI] [PubMed] [Google Scholar]
- 18.Ashraf M, Foolad MR. 2012. Crop breeding for salt-tolerance in the era of molecular markers and marker-assisted selection. Plant Breed. 132, 10–20 10.1111/pbr.12000 (doi:10.1111/pbr.12000) [DOI] [PubMed] [Google Scholar]
- 19.Kadereit G, Ackerly D, Pirie MD. 2012. A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proc. R. Soc. B 279, 3304–3311 10.1098/rspb.2012.0440 (doi:10.1098/rspb.2012.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osborne CP, Freckleton RP. 2009. Ecological selection pressures for C4 photosynthesis in the grasses. Proc. R. Soc. B 276, 1753–1760 10.1098/rspb.2008.1762 (doi:10.1098/rspb.2008.1762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwander T, Crespi BJ. 2008. Twigs on the tree of life? Neutral and selective models for integrating macroevolutionary patterns with microevolutionary processes in the analysis of asexuality. Mol. Ecol. 18, 28–42 10.1111/j.1365-294X.2008.03992.x (doi:10.1111/j.1365-294X.2008.03992.x) [DOI] [PubMed] [Google Scholar]
- 22.Hugall AF, Stuart-Fox D. 2012. Accelerated speciation in colour-polymorphic birds. Nature 485, 631–634 10.1038/nature11050 (doi:10.1038/nature11050) [DOI] [PubMed] [Google Scholar]
- 23.Christin PA, Freckleton RP. 2010. Can phylogenetics identify C4 origins and reversals? Trends Ecol. Evol. 25, 403–409 10.1016/j.tree.2010.04.007 (doi:10.1016/j.tree.2010.04.007) [DOI] [PubMed] [Google Scholar]