Abstract
Background
The past century has seen a significant contraction in the global extent of malaria transmission, resulting in over 50 countries being declared malaria free, and many regions of currently endemic countries eliminating the disease. Moreover, substantial reductions in transmission have been seen since 1900 in those areas that remain endemic today. Recent work showed that this malaria recession was unlikely to have been driven by climatic factors, and that control measures likely played a significant role. It has long been considered, however, that economic development, and particularly urbanization, has also been a causal factor. The urbanization process results in profound socio-economic and landscape changes that reduce malaria transmission, but the magnitude and extent of these effects on global endemicity reductions are poorly understood.
Methods
Global data at subnational spatial resolution on changes in malaria transmission intensity and urbanization trends over the past century were combined to examine the relationships seen over a range of spatial and temporal scales.
Results/Conclusions
A consistent pattern of increased urbanization coincident with decreasing malaria transmission and elimination over the past century was found. Whilst it remains challenging to untangle whether this increased urbanization resulted in decreased transmission, or that malaria reductions promoted development, the results point to a close relationship between the two, irrespective of national wealth. The continuing rapid urbanization in malaria-endemic regions suggests that such malaria declines are likely to continue, particularly catalyzed by increasing levels of direct malaria control.
Keywords: Urbanization, Global malaria endemicity, Plasmodium falciparum, Plasmodium vivax, Malaria elimination
Background
The range of malaria has contracted through a century of economic development and disease control [1-3]. During an era of renewed interest in elimination and eradication there is a need to better understand and quantify the forces behind this recession. A variety of direct control efforts were likely a major factor behind the global contraction in malaria transmission [2,3], although these gains were often coincident with rapid economic and social development and land use changes [4-6]. One major aspect of this development that has been shown to significantly impact malaria transmission is urbanization [7-9].
The process of urbanization includes physical landscape modification and transformation of environs through demand for resources and improved communications. Moreover, urbanization involves significant socio-economic change; generally improved health, housing and increased wealth [10,11]. These factors, common to urban areas, cause marked entomological, parasitological and behavioural effects that result in reduced malaria transmission both within the urban core and surrounding peri-urban areas [7,9,12]. A century of rapid urbanization has therefore likely had an impact on malaria transmission globally, but the size and importance of this impact has never been examined.
The recent construction of 20th Century time series of global urban extent data [13], and a contemporary malaria transmission map [14] that is comparable to historic data [15], enables a more detailed exploration of this relationship. Here, these datasets were utilized to examine for the first time the association at global, national and subnational scales between changes in malaria transmission and urban growth across the last century.
Methods
Data
Urbanization
HYDE 3.1 [13,16] is a dynamic modelling effort of long-term historical population growth, providing a consistent database of 20th-century population and urban distribution at 5-minute spatial resolution (equivalent to approximately 10 km at the Equator). Full details of dataset construction are provided in Goldwijk et al.[13,16]. In brief, urban-rural population numbers and fractions for each country and for each decade were obtained from a variety of sources, including the UN [17] and published databases and reviews [18]. Urban areas were then mapped by combining different spatial datasets [19-21] and contemporary urban population datasets, which were derived from existing databases [21,22]. Finally, urban population densities over time were estimated based on the fitting of asymmetric Gaussian probability density function models to the population data [13]. The range of input datasets, assumptions and modeling methods used in construction of the HYDE 3.1 datasets mean that significant uncertainties are inherent in the outputs, and these are likely higher in the mapping covering both the early 20th Century and lower income areas of the World, where data scarcity meant that more assumptions had to be made [13,16]. Nevertheless, at the broad spatial and temporal scales of analysis undertaken here, the effects of such assumptions and uncertainties on results are likely small. Figure 1 shows changes in urban population sizes across the malaria endemic world from 1900 to 2000. Additional file 1 shows the mapped urban areas for 1900 and 2000, while Additional file 2 maps the change in urban extent over the 100-year period, and Additional file 3 shows urban extents in 1900 and 2000 for Brazil.
Figure 1.
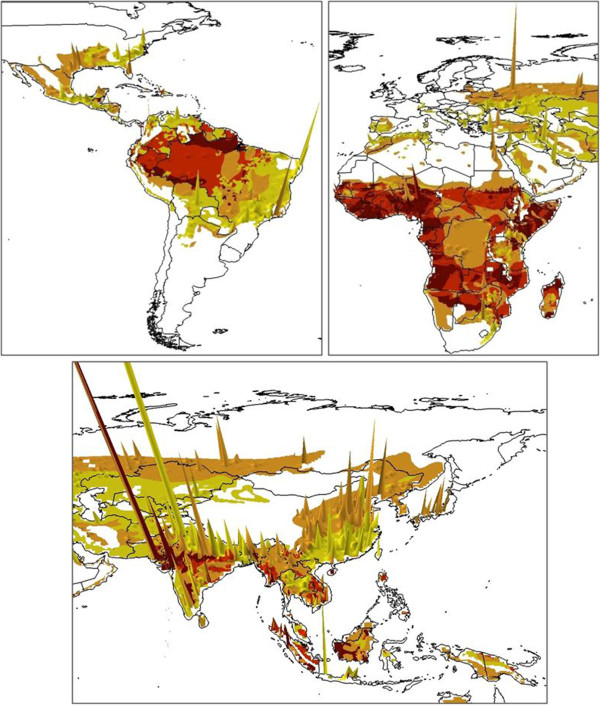
Maps showing global changes in urban population size 1900-2000 and change in malaria endemicity. The bar height is proportional to the size of urban population change. Areas that saw no change or an increase in endemicity are coloured dark red, those that saw a reduction by one endemicity class are in red, two classes in orange and three or more classes in yellow.
Malaria endemicity
The only global map of pre-intervention malaria endemicity dates from a 1968 study [15,23] in which a major synthesis of historical records, documents and maps of a variety of malariometric indices for all four Plasmodium species was used to map parasite rate (PR—the proportion of individuals with malaria parasites detectable in their peripheral blood) and stratified into four endemic classes associated with Plasmodium falciparum endemicity [24] (hypo-endemic, PR < 10%; meso-endemic, PR > 10% and <50%; hyperendemic, PR > 50% and < 75%; holo-endemic, PR > 75%). This map is the only reconstruction of historical malaria at its assumed historical peak around the start of the 20th Century and triangulates well with the plethora of national level malaria maps published throughout the last century [25]. The historical malaria endemicity map was scanned from the original publication, digitized on-screen and rasterized to a 5 × 5 km grid. The map for an area of Brazil is shown in Additional file 3(a) – the full map can be seen in Gething et al.[3].
The publication of an evidence-based map of contemporary malaria endemicity [14] and its conversion to classes that match the c.1900 map [3] described above, allows an audit of changes in the global epidemiology of malaria since the start of the 20th Century. With just two timepoints of malaria endemicity data, precise information on the timing and progression of changes is absent, placing a limitation on the conclusions that can be drawn from analyses. However, the datasets do provide a unique and valuable quantitative picture of the spatial changes in malaria epidemiology that have occurred over the last 100 years. The map of contemporary malaria endemicity was generated from a recently defined model of age-standardized P. falciparum parasite rate, PfPR2-10 [26]. Using a model-based geostatistical framework, the underlying value of PfPR2-10 at each location was modelled for the year 2007 as a transformation of a space-time Gaussian process (GP), with the number of P. falciparum-positive individuals in each survey modelled as a binomial variate given the unobserved age-standardized prevalence surface [14]. The GP was parameterized by a mean component and a space-time covariance function which was spatially anisotropic, used great-circle distance to incorporate the curvature of Earth, and included a periodic temporal component to capture seasonality. Bayesian inference was implemented using Markov chain Monte Carlo and direct simulation to generate posterior predictive samples of the 2007 annual mean prevalence surface and to assign each pixel to the endemicity class with the highest posterior probability of membership. This dataset for an area of Brazil is shown in Additional file 3(b). The classes matched those of the c.1900 endemicity map, enabling a class change dataset to be produced, which is shown in Figure 1 and Additional file 4.
In order to compare the observed changes in endemicity between these two time periods with levels of urbanization, it was necessary to translate both the historical and contemporary maps into approximate units of PfRC, the P. falciparum basic reproductive number under the levels of control that existed at the time. This enabled comparisons of the effect sizes of changes in endemicity, following previous studies [3], and was undertaken using a simple P. falciparum transmission model [27] to estimate a value of PfRC corresponding to the mid-value of each endemicity class. Using these conversion values, maps of PfRC were made corresponding to both historical and contemporary endemicity. These two maps were overlaid in a geographical information system (GIS) (ArcGIS 9.2, ESRI Inc, Redlands CA, USA), and the relative change in PfRC between the historic and contemporary map was calculated for each 5 × 5 km pixel. These relative changes were summarized as areas of increase, areas of no change, and areas of decrease of between zero and one, one and two and greater than two orders of magnitude (see Additional file 5).
Finally, the recent construction of an evidence-based map of the limits of Plasmodium vivax transmission [28] meant that analyses could be repeated to examine the similarity in results between the two parasite species. Additional file 6 shows the P. vivax transmission limits map, constructed using data on the presence of P. vivax infection and spatial information on climatic conditions that impede transmission (low ambient temperature and extremely arid environments) in order to delineate areas where transmission was unlikely to take place.
Analyses
The data analyses focused on statistical explorations of the link between urbanization and changes in malaria endemicity. They covered three areas, examining in each case different spatial scales and factors:
(i) National scale analyses of differences in urbanization between countries that eliminated malaria during the past century and those that remain endemic today. Here, urban area percentages between 1900 and 2000 for those countries that remain endemic today, and urban area percentages between 1900 and the date of elimination (Additional file 7) for those countries that achieved it were compared.
(ii) Within country analyses of subnational differences in urbanization trends between regions that underwent malaria elimination 1900-2007, and regions within the same country that remain endemic today. Here, countries for which >20% of their land area became malaria-free over the past century and for which >20% of their land area remains endemic today were identified, and differences in urban areas, populations and rates of change between them were examined.
(iii) Within country analyses of changes in transmission intensity over the past century and their relationship to urbanization trends. Here, per-country mean changes in transmission class in areas that are urban today, versus those that have remained rural, for malaria-endemic areas in 1900 were examined.
For (ii) and (iii), by undertaking analyses at the subnational scale and treating each country independently, the differences in wealth, governance and latitude between countries that are often confounding factors in assessing the drivers of long-term global changes in malaria endemicity are controlled for. For each set of analyses and each time period, the total urban areas and urban population counts were extracted using ArcGIS 9.3 (ArcGIS 9.2, ESRI Inc, Redlands CA, USA) and analysed using R2.10 [29].
Results
A consistent pattern of greater rates of urbanization in areas that either eliminated malaria or displayed the greatest transmission reductions was found. At the time of elimination certification of those countries that achieved malaria elimination, those areas that were originally malarious had significantly higher proportions (Mann-Whitney test: area, z = -3.207, p < 0.01; populations, z = -5.432, p < 0.01) of their populations living in urban areas and higher percentages of urban land area (Figure 2) than the contemporary situation in those areas of the world that remain endemic today. Moreover, the rate of urbanization from 1900 onwards was significantly larger in those countries that achieved elimination than those that remain endemic today (see Additional file 8). Those countries that achieved elimination exhibited significantly greater increases in the proportions of their populations (Mann-Whitney test: z = -1.985, p < 0.05) and land area (Mann-Whitney test: z = -3.232, p < 0.01) that became urban during the 20 years prior to elimination, compared to the recent rates of urbanization in currently endemic countries over the last 20 years. Such findings confirm the presence of a correlation between urbanization and the achievement of malaria elimination at national scales, but this relationship is likely to be intimately tied to wealth. Countries with greater wealth have greater malaria control resources at their disposal, and it is those wealthier countries that have succeeded in eliminating malaria, as confirmed by undertaking similar analyses based on average income (average income data from [30], Mann-Whitney test: z = -6.491, p < 0.01). Moreover, those higher income countries that succeeded in elimination are located in latitudinal regions of lower climatic and environmental receptivity to malaria transmission, making elimination likely an easier task with a fixed set of intervention tools. Therefore, untangling the effects of urbanization upon changes in malaria transmission required analysis independent of these economic or latitudinal biases, and these are described below.
Figure 2.
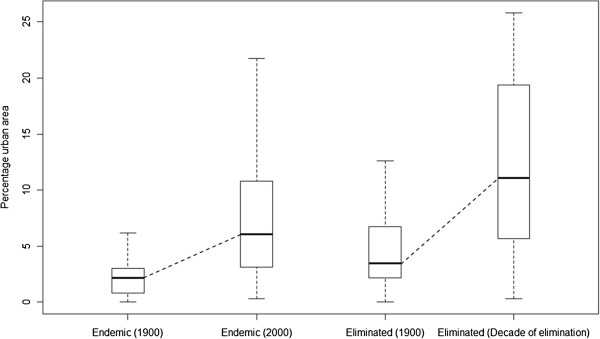
Box plots of the percentage urban area in 1900 and in 2000 for countries that are still endemic and in the decade that elimination was achieved for those countries that achieved elimination.
Twenty-nine countries were identified for which at least a fifth of their land area became malaria-free over the past century and for which at least a fifth of their land area remains endemic today. Over 75% of these countries had greater proportions of land area urbanized in the malaria-free areas, and showed a greater percentage increase in urban extent (Figure 3) than the areas that remain endemic today. Both of these differences are significant (Wilcoxen test: urban area: z = -2.505 p < 0.05, urban extent change: z = -2.001, p < 0.05). However, these relationships show regional variations, (Figure 3). Almost all countries analysed in the Americas (Figure 3a) and Asia (Figure 3c) showed greater urbanization in their malaria-free versus still-endemic areas. In the Africa and Arabian Peninsula region (Figure 3b), where both levels of urbanization and transmission reductions were smaller, the results were more mixed. Many of those falling below the one-to-one line (Figure 3a- 3c) are countries where extremely arid or mountainous conditions have influenced human settlement to occur principally in areas most suitable for malaria transmission (e.g., Saudi Arabia, Botswana, Swaziland). A full set of national level results can be found in the Additional file 9. In addition, the results of a repeat analysis for P. vivax yielding very similar results are shown in Additional file 10.
Figure 3.
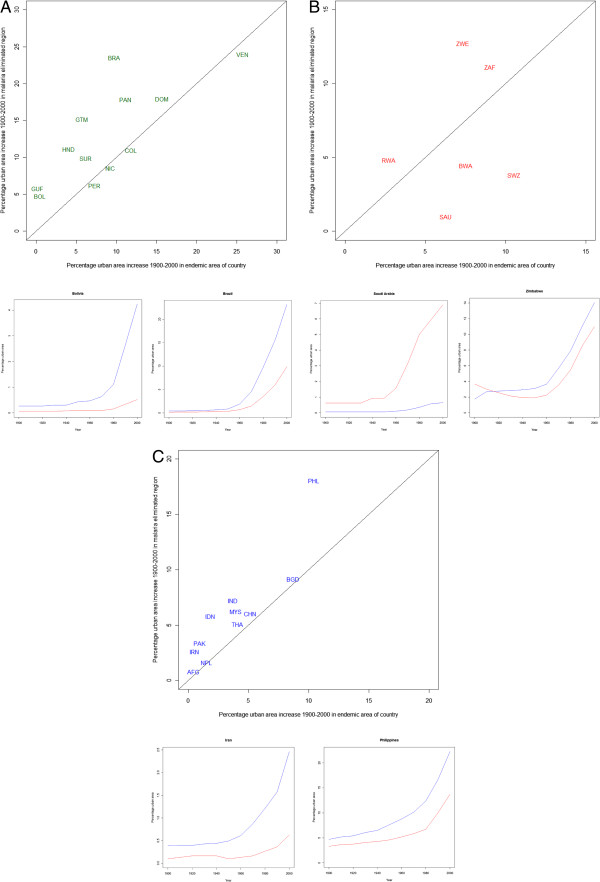
Plots showing urban area changes 1900-2000 between areas of countries that remain malaria endemic today, and those that have undergone elimination for (a) the Americas, (b) Africa plus Arabian peninsula and (c) Asia. In each case, scatterplots of urban area increases in endemic versus eliminated areas with one-to-one lines overlaid are shown at the top, and example plots of trends in urban area percentages between areas that eliminated malaria (blue) and that remain endemic (red) are shown at the bottom (the full set of these plots is provided in Additional file 9). The ISO country abbreviation for country name is used on the scatterplots ( http://www.iso.org/iso/english_country_names_and_code_elements).
Urban areas have been shown to exhibit lower levels of transmission than surrounding rural ones [8,9]. If the process of urbanization is a causal factor in malaria declines, a consistent pattern of greater transmission reduction in those areas that have undergone urbanization should be seen. Comparing mean changes in transmission class in areas that are urban today, versus those that have remained rural, for malaria-endemic areas in 1900, was possible in 158 countries. It shows that 82.3% underwent a greater transmission reduction in their urban areas than rural ones (Wilcoxen test: p < 0.001, see Additional file 10). Of the 28 countries that displayed a greater malaria reduction in rural areas, half of these were in sub-Saharan Africa, where the smallest levels of urbanization and reductions in transmission occurred. This confirms that greater transmission reductions occurred in areas that are urban today, but does not indicate if the largest declines were coincident with greater rates of urbanization.
Finally, examination was undertaken of whether the rate of urbanization was higher in regions of countries that saw the greatest reductions in PfRC. Sixty-three countries were identified that had two or more PfRC reduction classes, each covering at least 20% of their land area, and the rates of urbanization 1900-2000 in the areas exhibiting the smallest reduction in transmission were compared to those in the areas displaying the greatest reduction in transmission. Some 84.1% of countries displayed a greater urban increase in the areas that showed the greater PfRC reduction than those that showed the smallest transmission reductions (Wilcoxen test: p < 0.001, Figure 4). Further supporting analyses and more detail on these analyses are presented in Additional file 11.
Figure 4.
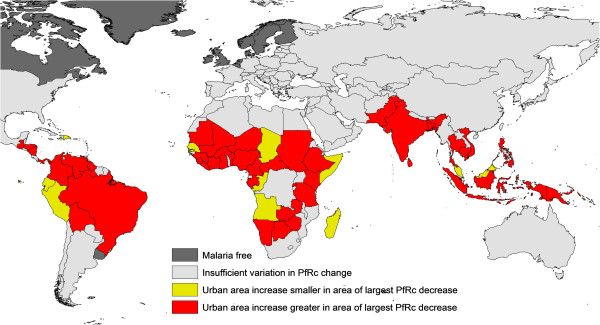
Plasmodium falciparum basic reproductive number (PfRC) changes and urbanization. Countries in red show a greater percentage increase in urban area over the past century in areas of the country with the greatest magnitude of PfRC decreases than areas that showed the smallest changes. Countries in yellow show the reverse. Countries in light grey had either insufficient variation in PfRC changes, or no transmission. Countries in dark grey have either always been malaria free, or only exhibited unstable transmission.
Discussion
The process of urbanization results in a variety of changes that reduce receptivity to malaria transmission [9-11]. Here, a clear picture of increased urbanization associated with greater malaria transmission reductions across countries and continents is documented for the first time. Whilst it remains challenging to untangle whether this urbanization resulted in decreased transmission, or that malaria reductions promoted development, a close relationship is evident, irrespective of national wealth and latitude.
Other local evidence [7,12,31,32] hints that changes commensurate with urbanization play a substantive role in driving malaria transmission declines. Whether measured by proportion of land area or population urbanized, the majority of nations that remain malaria endemic today exhibit substantially lower levels of urbanization compared to that at the time of elimination for those countries that have achieved it (Figure 2 and Additional file 8). Moreover, the magnitude of change in urban extent from 1900 is also correlated with malaria declines, with continental differences, notably sub-Saharan Africa showing lower levels of urbanization today, and smaller changes in urban extent and endemicity over the past century (Figure 3).
Recent malaria declines in sub-Saharan Africa point towards the success of interventions, however, in several cases the decline began before specific interventions were deployed, suggesting the contribution of alternative factors [33]. While malaria declines due to urbanization and its effects are likely to be more gradual than some of the sudden drops seen, it remains a possibility that the rapid urbanization ongoing in sub-Saharan Africa [34] is at least a contributory driver to these changes.
While over three-quarters of countries show decreasing transmission in areas that have undergone urbanization over the past century, a handful of countries go against this trend. A possible reason for this is the likely multi-factorial complexity behind both changes in transmission and human settlement dynamics, and the difficulty of attributing changes to a single cause. Recent analyses have indicated that vector-borne and parasitic diseases have systematically impacted economic growth [35], but more detailed studies of these types of relationships across disease types and ecozones are required to gain a fuller understanding. Almost all of the countries that show greater transmission reductions in rural areas are those where human settlement was constrained to the more malarious areas of the country, due to uninhabitable arid or mountainous areas elsewhere. Equally however, the reverse is true for some countries – i.e. uninhabitable areas with intense transmission forced human settlement in the lower transmission regions.
It is clear that various sources of uncertainty exist in the inputs and methodologies used in this study. Uncertainties are inherent in the urbanization datasets through the lack of data for many regions and time periods, and the assumptions made to fill these gaps [13]. The proposed levels of historical endemicity [15,23] are plausible when triangulated against other values reported from the pre-intervention era (for example, [26,36]), but the relatively crude categorization of all-cause malaria endemicity strata and the cartographic approach used preclude a more formal quantification of the global P. falciparum endemicity declines and their link to urbanization beyond the broad relationships presented here. Nevertheless, recent mapping of the limits of P. vivax transmission [28] and analyses of contemporary impacts of urbanization [8], indicate very similar effects and contractions as that seen for P. falciparum, with results repeated, where possible, for P. vivax showing almost identical results (Additional file 10), and transmission rarely more intense than meso-endemic [37,38].
Much of the low-income world, where malaria burden is highest and levels of urbanization are lowest, is set to undergo an urban and demographic transition in the coming decades [17,34], ultimately likely arriving at levels of urbanization similar to those exhibited by countries that eliminated malaria (Additional file 8). Significant efforts towards modelling future malaria scenarios have been completed or are underway, focused principally on the effects of a variety of interventions [39-41] or on climate change scenarios [42-45], but the impacts of urbanization are rarely considered [46]. Yet, if the past century of malaria declines are indicative, the study of its impacts should receive more attention as nations start to monitor their progress toward elimination [47]. There exist multiple data gaps and uncertainties in obtaining suitable data to build urbanization into scenario models, however. Firstly, simple consistent definitions of what constitutes an urban area in general are difficult to outline. Beyond this, multiple approaches to mapping urban extents in a consistent fashion have been attempted (e.g., [48-50]), but the spatial modelling of their future growth is lacking. Secondly, definitions and measures of urbanization that are relevant to understanding transmission patterns are poorly quantified, with only occasional attempts at empirical definitions made [8,9], and little consideration of the urban preferences of the dominant Anopheles species [51-53], or adaptation of them to urban environments [54,55]. Finally, the treatment of urban areas as single homogenous entities ignores the great variations in demographic, socio-economic and land uses within cities with, for example, urban agricultural practices often maintaining transmission within urban areas [56-58].
The quantification of a global recession in the range and intensity of malaria over the 20th century has allowed us to review the impact that urbanization has had on these declines, and gauge its importance as a driver of future changes in malaria epidemiology. Results highlight for the first time the consistent relationship between urbanization and malaria declines over the past century globally, and point towards continuing declines as urbanization permanently alters the receptivity of many areas to malaria transmission. The findings presented here suggest that these trends will likely continue to catalyze malaria declines on the path to the goal of a malaria-free future.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AJT conceived the analyses, developed the study design and conducted the analyses. PWG, DLS and SIH provided the malaria endemicity data and mathematical models for dataset conversions. All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Urban extents and malaria transmission mapped for 1900 and 2000. Description: Maps of urban extents in 1900 and 2000 overlaid onto mapped areas of where malaria was eliminated over the past century and where it remains endemic today.
Change in urban extent between 1900 and 2000. Description: Map of estimated changes in urban extent globally between 1900 and 2000.
Malaria endemicity classes and urban areas in Brazil. Description: Maps of malaria endemicity classes and urban areas in Brazil for 1900 and 2000.
Change in malaria endemicity class between 1900 and 2007. Description: Map of changes in malaria endemicity class between 1900 and 2007.
The magnitude of decrease in P. falciparum basic reproductive number 1900-2007. Description: Map of the magnitude of decrease in P. falciparum basic reproductive number for 1900-2007.
The global spatial limits of Plasmodium vivax malaria transmission in 2009. Description: Map of the global spatial limits of Plasmodium vivax malaria transmission in 2009.
Dates of malaria elimination for those countries that achieved it. Description: Table showing the estimated and official dates of malaria elimination for those countries that achieved it.
Urbanization and countries that have eliminated malaria. Description: Description and results of statistical analyses of urbanization levels between countries that achieved elimination and those that remain endemic today.
Urbanization and sub-national malaria elimination. Description: Description and results of per-country statistical analyses of urbanization levels between regions that became malaria free and those that remain endemic today.
Results using the contemporary limits of Plasmodium vivax transmission. Description: Description and results of analyses repeated using Plasmodium vivax transmission limits data.
Urbanization and changes in malaria transmission. Description: Description and results of per-country statistical analyses of changes in malaria transmission intensity and urbanization levels.
Contributor Information
Andrew J Tatem, Email: andy.tatem@gmail.com.
Peter W Gething, Email: peter.gething@zoo.ox.ac.uk.
David L Smith, Email: smitdave@gmail.com.
Simon I Hay, Email: simon.hay@zoo.ox.ac.uk.
Acknowledgements
AJT and DLS are supported by grants from the Bill and Melinda Gates Foundation (#49446, #1032350) and NIH/NIAID (#U19AI089674). All authors acknowledge funding support from the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health, USA. SIH is a Wellcome Trust Senior Research Fellow (#095066). PWG is a Medical Research Council (UK) Career Development Fellow (#K00669X) and acknowledges support from the Bill and Melinda Gates Foundation (#OPP1068048). This work forms part of the output of the AfriPop and AsiaPop projects ( http://www.afripop.org, http://www.asiapop.org), and part of the output of the Malaria Atlas Project (MAP, http://www.map.ox.ac.uk), principally funded by the Wellcome Trust, UK.
References
- Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64:85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Inf Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. Climate change and the global malaria recession. Nature. 2010;465:342–346. doi: 10.1038/nature09098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitron U. Malaria, agriculture, and development: lesson from past campaigns. Int J Health Services. 1987;17:295–326. doi: 10.2190/68UG-BAWQ-YXCT-HFKT. [DOI] [PubMed] [Google Scholar]
- Bruce-Chwatt L, De Zuluete J. The rise and fall of malaria in Europe. A histrico-epidemiological study. Oxford: Oxford University Press; 1980. [Google Scholar]
- Bradley G. A review of malaria control and eradication in the United States. Mosq News. 1966;26:462–470. [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Guerra CA, Moyes CL, Elyazar IR, Gething PW, Hay SI, Tatem AJ. The effects of urbanization on global Plasmodium vivax malaria transmission. Malar J. 2012;11:403. doi: 10.1186/1475-2875-11-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Guerra CA, Kabaria CW, Noor AM, Hay SI. Human population, urban settlement patterns and their impact on Plasmodium falciparum malaria endemicity. Malar J. 2008;7:218. doi: 10.1186/1475-2875-7-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C. Health and urban living. Science. 2008;319:766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Inf Dis. 2011;11:131–141. doi: 10.1016/S1473-3099(10)70223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- Goldewijk KK, Beusen A, Janssen P. Long-term dynamic modeling of global population and built-up area in a spatially explicit way: HYDE 3.1. The Holocene. 2010;20:565–573. doi: 10.1177/0959683609356587. [DOI] [Google Scholar]
- Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IRF, Brooker SJ, Smith DL, Moyeed RA, Snow RW. World malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko AJ, Semashko IN. In: Geography of malaria. A medico-geographic profile of an ancient disease [in Russian] Lebedew AW, editor. Moscow: Academy of Sciences USSR; 1968. pp. 25–146. [Google Scholar]
- Goldewijk KK. Three centuries of global population growth: a spatial referenced population (density) database for 1700-2000. Popul Environ. 2005;26:343–367. doi: 10.1007/s11111-005-3346-7. [DOI] [Google Scholar]
- United Nations Population Division. Book World population prospects, 2012 revision. New York: United Nations; 2012. World population prospects, 2012 revision. [Google Scholar]
- Lahnmeyer J. Populstat database. Growth of the population per country in a historical perspective, including their administrative divisions and towns. Book: Populstat database. Growth of the population per country in a historical perspective, including their administrative divisions and towns. 2004. http://www.populstat.info.
- Bartholome E, Belward AS. GLC2000: A new approach to global land cover mapping from earth observation data. Int J Rem Sens. 2005;26:1959–1977. doi: 10.1080/01431160412331291297. [DOI] [Google Scholar]
- Loveland TR, Reed BC, Brown JF, Ohlen DO, Zhu Z, Yang L, Merchant JW. Development of a global land cover characteristics database and IGBP DISCover from 1 km AVHRR data. Int J Rem Sens. 2000;21:1303–1330. doi: 10.1080/014311600210191. [DOI] [Google Scholar]
- LandScan. LandScan global population database, the 2004 revision. Oak Ridge: Oak Ridge National Laboratory; 2006. [Google Scholar]
- Demographia. Book Urban areas and urbanization, vol. 2013. 2006. http://www.demographia.com.
- Lysenko AJ, Beljaev AE. An analysis of the geographical distribution of Plasmodium ovale. Bull World Health Organ. 1969;40:383–394. [PMC free article] [PubMed] [Google Scholar]
- Metselaar D, Van Thiel PH. Classification of malaria. Trop Geogr Med. 1959;11:157–161. [Google Scholar]
- Mouchet J, Carnevale P, Coosemans M, Julvez J. Biodiversite´ du paludisme dans le monde. Montrouge, France: John Libbey Eurotext; 2004. [Google Scholar]
- Smith DL, Guerra CA, Snow RW, Hay SI. Standardizing estimates of the Plasmodium falciparum parasite rate. Malar J. 2007;6:131. doi: 10.1186/1475-2875-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, McKenzie FE, Snow RW, Hay SI. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IRF, Baird KJ, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org. [Google Scholar]
- Gapminder. http://www.gapminder.org/data/
- Trape JF, Zoulani A. Malaria and urbanisation in Central Africa: the example of Brazzaville. Part II. Results of entomological surveys and epidemiological analaysis. Trans Roy Soc Trop Med Hyg. 1987;81:34–42. doi: 10.1016/0035-9203(87)90472-x. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, McCall PJ, Lengeler C, Bates I, D'Alessandro U, Barnish G, Konradsen F, Klinkenberg E, Townson H, Trape JF, Hastings IM, Mutero C. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005;4:12. doi: 10.1186/1475-2875-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- United Nations Population Division. Book World urbanization prospects, 2011 revision. New York, USA: United Nations; 2011. World urbanization prospects, 2011 revision. [Google Scholar]
- Bonds MH, Dobson AP, Keenan DC. Disease ecology, biodiversity, and the latitudinal gradient in income. PLoS Biol. 2012;10:e1001456. doi: 10.1371/journal.pbio.1001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Dushoff J, Snow RW, Hay SI. The entomological innoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–539. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, Bousema T, Drakeley CJ, Ferguson NM, Basanez MG, Ghani AC. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7:e1000324. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LJ, Maude RJ, Pongtavornpinyo W, Saralamba S, Aguas R, Van Effelterre T, Day NPJ, White NJ. The role of simple mathematical models in malaria elimination strategy design. Malar J. 2009;8:212. doi: 10.1186/1475-2875-8-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Hay SI. Endemicity response timelines for Plasmodium falciparum elimination. Malar J. 2009;8:87. doi: 10.1186/1475-2875-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- Tanser FC, Sharp B, le Sueur D. Potential effect of climate change on malaria transmission in Africa. Lancet. 2003;362:1792–1798. doi: 10.1016/S0140-6736(03)14898-2. [DOI] [PubMed] [Google Scholar]
- Thomas CJ, Davies G, Dunn CE. Mixed picture for changes in stable malaria distribution with future climate in Africa. Trends Parasitol. 2004;20:216–220. doi: 10.1016/j.pt.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Beguin A, Hales S, Rocklov J, Astrom C, Louis V, Sauerborn R. The opposing effects of climate change and socio-economic development on the global distribution of malaria. Global Env Change. 2011;21:1209–1214. doi: 10.1016/j.gloenvcha.2011.06.001. [DOI] [Google Scholar]
- Hay SI, Tatem AJ, Guerra CA, Snow RW. Book Population at malaria risk in Africa: 2005, 2015 and 2030. London, UK: UK Government; 2006. Population at malaria risk in Africa: 2005, 2015 and 2030. [Google Scholar]
- The Global Health Group and the Malaria Atlas Project. Atlas of Malaria Eliminating Countries, 2011. San Francisco: The Global Health Group, Global Health Sciences, University of California; 2011. [Google Scholar]
- Balk DL, Deichmann U, Yetman G, Pozzi F, Hay SI, Nelson A. Determining global population distribution: methods, applications and data. Adv Parasitol. 2006;62:119–156. doi: 10.1016/S0065-308X(05)62004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Friedl MA, Potere D. Mapping global urban areas using MODIS 500-m data: New methods and datasets based on 'urban ecoregions'. Rem Sens Env. 2010;114:1733–1746. doi: 10.1016/j.rse.2010.03.003. [DOI] [Google Scholar]
- Linard C, Gilbert M, Snow RW, Noor AM, Tatem AJ. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS One. 2012;7:e31743. doi: 10.1371/journal.pone.0031743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IR, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Okara RM, Van Boeckel T, Godfray HC, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola T, Oduola A, Obansa J, Chukwurar N, Unyimadu J. Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. J Vector Borne Dis. 2007;44:241–244. [PubMed] [Google Scholar]
- Chinery WA. Effects of ecological changes on the malaria vectors Anopheles funestus and the Anopheles gambiae complex of mosquitoes in Accra, Ghana. J Trop Med Hyg. 1984;87:75–81. [PubMed] [Google Scholar]
- Dongus S, Nyika D, Kannady K, Mtasiwa D, Mshinda H, Gosoniu L, Drescher A, Ulrike F, Tanner M, Killeen G, Castro M. Urban agriculture and Anopheles habitats in Dar Es Salaam. Geospat Health. 2009;3:189–210. doi: 10.4081/gh.2009.220. [DOI] [PubMed] [Google Scholar]
- Stoler J, Weeks J, Getis A, Hill A. Distance threshold for the effect of urban agriculture on elevated self-reported malaria prevalence in Accra, Ghana. Am J Trop Med Hyg. 2009;80:547–554. [PMC free article] [PubMed] [Google Scholar]
- Baragatti M, Fournet F, Henry M-C, Assi S, Ouedraogo H, Rogier C, Salem G. Social and environmental malaria risk factors in urban areas of Ouagadougou, Burkina Faso. Malar J. 2009;8:13. doi: 10.1186/1475-2875-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Urban extents and malaria transmission mapped for 1900 and 2000. Description: Maps of urban extents in 1900 and 2000 overlaid onto mapped areas of where malaria was eliminated over the past century and where it remains endemic today.
Change in urban extent between 1900 and 2000. Description: Map of estimated changes in urban extent globally between 1900 and 2000.
Malaria endemicity classes and urban areas in Brazil. Description: Maps of malaria endemicity classes and urban areas in Brazil for 1900 and 2000.
Change in malaria endemicity class between 1900 and 2007. Description: Map of changes in malaria endemicity class between 1900 and 2007.
The magnitude of decrease in P. falciparum basic reproductive number 1900-2007. Description: Map of the magnitude of decrease in P. falciparum basic reproductive number for 1900-2007.
The global spatial limits of Plasmodium vivax malaria transmission in 2009. Description: Map of the global spatial limits of Plasmodium vivax malaria transmission in 2009.
Dates of malaria elimination for those countries that achieved it. Description: Table showing the estimated and official dates of malaria elimination for those countries that achieved it.
Urbanization and countries that have eliminated malaria. Description: Description and results of statistical analyses of urbanization levels between countries that achieved elimination and those that remain endemic today.
Urbanization and sub-national malaria elimination. Description: Description and results of per-country statistical analyses of urbanization levels between regions that became malaria free and those that remain endemic today.
Results using the contemporary limits of Plasmodium vivax transmission. Description: Description and results of analyses repeated using Plasmodium vivax transmission limits data.
Urbanization and changes in malaria transmission. Description: Description and results of per-country statistical analyses of changes in malaria transmission intensity and urbanization levels.


