Abstract
Background
Heparin-induced thrombocytopenia is an immune response mediated by anti-PF4/heparin antibody, which is clinically characterized by thrombocytopenia and thromboembolic events. In this study, a prospective and multi-center clinical investigation  determined the positive rate of anti-PF4/heparin antibody in maintenance hemodialysis patients in China,
determined the positive rate of anti-PF4/heparin antibody in maintenance hemodialysis patients in China,  identified the related risk factors, and
identified the related risk factors, and  further explored the effect of the anti-PF4/heparin antibody on bleeding, thromboembolic events, and risk of death in the patients.
further explored the effect of the anti-PF4/heparin antibody on bleeding, thromboembolic events, and risk of death in the patients.
Methods
The serum anti-PF4/heparin antibody was measured in 661 patients from nine hemodialysis centers, detected by IgG-specific ELISA and followed by confirmation with excess heparin. Risk factors of these patients were analyzed. Based on a two-year follow-up, the association between the anti-PF4/heparin antibody and bleeding, thromboembolic events, and risk of death in the patients was investigated.
Results
 The positivity rate of the anti-PF4/heparin antibody in maintenance hemodialysis patients was 5.6%. With diabetes as an independent risk factor, the positivity rate of the anti-PF4/heparin antibody decreased in the patients undergoing weekly dialyses ≥3 times.
The positivity rate of the anti-PF4/heparin antibody in maintenance hemodialysis patients was 5.6%. With diabetes as an independent risk factor, the positivity rate of the anti-PF4/heparin antibody decreased in the patients undergoing weekly dialyses ≥3 times.  The positivity rate of the anti-PF4/heparin antibody was not related to the occurrence of clinical thromboembolic events and was not a risk factor for death within two years in maintenance hemodialysis patients.
The positivity rate of the anti-PF4/heparin antibody was not related to the occurrence of clinical thromboembolic events and was not a risk factor for death within two years in maintenance hemodialysis patients.  Negativity for the anti-PF4/heparin antibody combined with a reduction of the platelet count or combined with the administration of antiplatelet drugs yielded a significant increase in bleeding events. However, the composite determination of the anti-PF4/heparin antibody and thrombocytopenia, as well as the administration of antiplatelet drugs, was not predictive for the risk of thromboembolic events in the maintenance hemodialysis patients.
Negativity for the anti-PF4/heparin antibody combined with a reduction of the platelet count or combined with the administration of antiplatelet drugs yielded a significant increase in bleeding events. However, the composite determination of the anti-PF4/heparin antibody and thrombocytopenia, as well as the administration of antiplatelet drugs, was not predictive for the risk of thromboembolic events in the maintenance hemodialysis patients.
Conclusions
A single detection of the anti-PF4/heparin antibody did not predict the occurrence of clinical bleeding, thromboembolic events, or risk of death in the maintenance hemodialysis patients.
Introduction
Hemodialysis is currently the major treatment method for end-stage renal disease (ESRD). Hemodialysis is a treatment model of extracorporeal circulation, and the heparin anticoagulants are its main anticoagulant drugs [1]. Heparin-induced thrombocytopenia (HIT) is one of the serious adverse effects of heparin, which often results in severe thrombotic diseases [2], [3]. The pathogenesis of HIT mainly involves the binding of heparin to platelet factor 4 (PF4) to form a heparin-PF4 complex that stimulates the body to produce anti-PF4/heparin antibodies and mediates an immune response, which leads to platelet activation and reduction and results in an elevated risk of thromboembolic disease [4]–[6].
Due to the long-term administration of heparin, hemodialysis patients have a high risk [7]of positivity for the anti-PF4/heparin antibody with a reported positivity rate of 1.2% – 10.3% for the anti-PF4/heparin antibody [8]–[12]; by contrast, other researchers determined that the positivity rate of the anti-PF4/heparin antibody is as high as 47% [13]. Several studies suggested that the anti-PF4/heparin antibody increases the occurrence of thrombotic events in maintenance hemodialysis (MHD) patients [14], [15], but these results were different. The main reason for the variations in the results from most of the investigations was the use of a single center and a small sample size, which was not sufficient to rule out the variations caused by the different hemodialysis centers. The positivity rate of the anti-PF4/heparin antibody in MHD patients from a large sample and from multi-center resources in China is currently not available. Thus, in the present study, we prospectively examined the anti-PF4/heparin antibody in 661 MHD patients from nine hemodialysis centers using a two-year follow-up period to ? determine the positivity rate of the anti-PF4/heparin antibody in the Chinese MHD patients; ? resolve its related risk factors; and ? explore the effect of the anti-PF4/heparin antibody on the occurrence of bleeding, thromboembolic events, and the risk of death in the MHD patients.
Methods
Recruitment of the patients and healthy controls
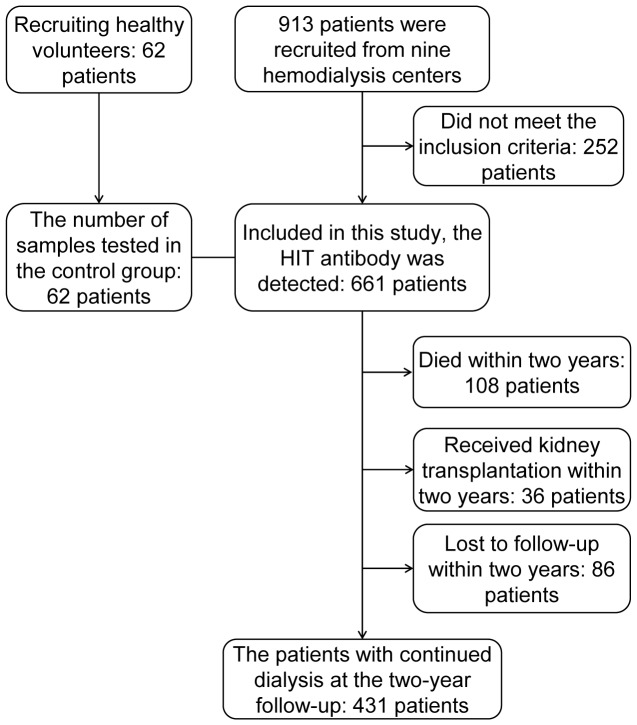
This study was approved by the Ethics Committee of the Chinese People’s Liberation Army (PLA) General Hospital and involved the hemodialysis centers of nine hospitals in three cities in northern China; 913 patients were recruited from December 2009 to January 2010. The patient inclusion criteria included the following: (1) maintenance hemodialysis for three months or longer; (2) age greater than 14 years, whether male or female; (3) use of heparin or low-molecular-weight heparin (LMWH) as an anticoagulant; and (4) provision of signed, informed consent. Consequently, 661 patients fulfilled these criteria and were included in this study. (Figure 1)
Figure 1. Diagram of MHD patients and healthy controls.
The diagram shows the numbers of MHD patients and healthy controls who met the criteria of inclusion in or exclusion from the study and the follow-up of the MHD patients.
Concurrently, 62 healthy subjects with ages ranging from 18 – 80 years were selected as the control group. Based on the standardized definition of health from on the SENIEUR protocol [16], the subjects who met all of the following criteria were included in the study: (1) serum liver enzyme level (both serum glutamate oxaloacetate transaminase, GOT, and serum glutamate pyruvate transaminase, GPT) <40 U/L, fasting blood glucose (FBG) <7.1 mmol/L; serum urea nitrogen (UN) 1.8 – 7.5 mmol/L, and serum creatinine (SCr) 50 – 133 µmol/L; (2) hemoglobin (Hb) 110 – 176 g/L, hematocrit (HCT) 0.40 – 0.52 for men and 0.37 – 0.47 for women; (3) normal routine urine test results; (4) absence of any clinical history of visceral (including heart, brain, lung, kidney, and liver) disease, malignancy, rheumatic disease, and chronic infectious disease; (5) no history of exposure to heparin drugs; (6) normal electrocardiogram, chest radiograph, echocardiography, and abdominal ultrasound; and (7) sound character, stable emotions, absence of mental disorders, teachability, and ability to adapt to the environment and to engage in appropriate social interactions and interpersonal relationships.
The heparin group was defined as patients who used heparin as a hemodialysis anticoagulant or to pre-rinse the dialyzer and had used heparin as a long-term central venous catheter anticoagulant. The LMWH group was defined as patients who used LMWH as the hemodialysis anticoagulant and had never been exposed to heparin.
Collecting the clinical data from the research subjects
The clinical data from the MHD patients were collected by the physicians in each hemodialysis center. The recorded contents included general information (i.e., age, sex, and dry weight), general clinical information (i.e., primary disease of renal failure, drug application, bleeding, and thromboembolic adverse events within the past three months), dialysis-related information (i.e., the dialysis duration in months, the number of weekly dialyses, and the type and amount of anticoagulant), and related laboratory tests (i.e., platelet count, routine blood test, routine urine test, and biochemical indicators). For the healthy controls, data regarding age, sex, weight, blood pressure, and laboratory indicators were determined and recorded. Written informed consent was obtained from each participant. Volunteers who did not meet the inclusion criteria or did not sign the informed consent were excluded from the study.
Definition of previous bleeding and thromboembolic events
Within three months prior to the collection of the clinical information, the clinical bleeding events included conjunctival hemorrhage, bleeding due to the puncture needle, gastrointestinal bleeding, skin ecchymosis, and cerebral hemorrhage. The thromboembolic events included peripheral venous thrombosis, peripheral arterial thrombosis, vascular access occlusion, pulmonary embolism, cerebral infarction, and myocardial infarction. All records of bleeding and thromboembolic events were based on the medical records, laboratory tests, and imaging studies.
Follow-up of the MHD patients
Two years after the detection of the anti-PF4/heparin antibody, follow-up was performed in the 661 patients in the study, of which 431 patients (65.20%) continued dialysis, 108 patients (16.34%) died, 36 patients (5.45%) received renal transplantation within two years, and 86 patients (13.01%) were lost to follow-up because they decided to discontinue the hemodialysis or their addresses were changed due to relocation. All of the above mentioned data were collected again from the 431 patients with continued dialysis and a complete two-year follow-up. The bleeding and/or thromboembolic events that occurred between two years from the first data collection and the time of follow-up were also recorded. For the patients who died within two years, the reasons for their bleeding, thromboembolic events, and deaths were ascertained from the medical records.
Collection of blood samples and detection of the serum anti-PF4/heparin antibody
For the 661 MHD patients included in this study, the predialysis blood samples were collected at various hemodialysis centers between December 2009 and January 2010, and the sera were isolated and transported in aliquots to the State Key Laboratory of Kidney Disease. The State Key Laboratory of Kidney Disease selected the volunteers for the healthy control group, collected their blood samples, and isolated and stored the sera in aliquots. All of the serum samples were stored at –80°C in the State Key Laboratory of Kidney Disease until the unified testing.
The serum anti-PF4/heparin antibody was detected using a heparin/PF4 ELISA kit (PF4 IgG™, HAT45G, GTI Diagnostics, Waukesha, WI, USA) [17]. The unified testing was conducted by certain technical staff members in strict accordance with the kit’s instruction guide. The positive control yielded OD405 nm >1.8, and the negative control yielded OD405 nm <0.3 with a difference between the duplicate wells of <20% [18]. To avoid false positives and improve the detection specificity [19], [20], the high-dose heparin confirmatory tests were performed for all of the samples with OD405 nm >0.4. The samples with OD405 nm >0.4 and an inhibition test result >50% were defined as positive for the anti-PF4/heparin antibody.
Data analysis
The chi-square test was used to compare the categorical variables. The t-test or Mann-Whitney U test was used for the continuous variables. Multivariate logistic regression was applied to analyze the correlation between positivity for the anti-PF4/heparin antibody and the risk factors of death in the MHD patients, as well as the correlation between positivity for the anti-PF4/heparin antibody and the occurrence of bleeding and thromboembolic events within two years. P value <0.05 was considered statistically significant. The statistical software SPSS version 17.0 was used in this study.
Results
General clinical data from the research subjects
In total, 62 healthy controls and 661 MHD patients met the inclusion criteria for this study, and their clinical data are presented in Table 1.
Table 1. MHD patients and healthy controls characteristics according to baseline status.
| MHD patients | healthy controls | |||
| Sex (female;n [%]) | 287 | (43%) | 39 | (63%) |
| Age (years;median [25th to 75th percentiles]) | 57 | (44–70) | 60 | (55–69) |
| Dry Weight(kg;median [25th to 75th percentiles]) | 62 | (54–71) | 65 | (55–70) |
| BMI (mean [SD]) | 22.6±4.0 | 24.3±3.0 | ||
| SBP(mmHg;median [25th to 75th percentiles]) | 140 | (130–155) | 130 | (120–135) |
| DBP(mmHg;median [25th to 75th percentiles]) | 80 | (70–90) | 78 | (70–80) |
| Hb(g/L; median [25th to 75th percentiles]) | 105 | (94–115) | 143 | (135–147) |
| Platelet count (10?9/L; mean [SD]) | 167±58 | 222±53 | ||
| Thrombocytopenia (n [%]) | 80 | (12%) | 0 | (0%) |
| SCr(µmol/L; mean [SD]) | 883±313 | 67±12 | ||
| ALB(g/L;median [25th to 75th percentiles]) | 40 | (37–42) | 46 | (44–48) |
| Diabetes(n [%]) | 127 | (19%) | 0 | (0%) |
| Bleeding events within the past three months (n [%]) | 83 | (13%) | 0 | (0%) |
| Thromboembolic events within the past three months (n [%]) | 66 | (10%) | 0 | (0%) |
| Dialysis duration (months ;n [%]) | ||||
| 3∼6 | 73 | (11%) | – | |
| 7∼12 | 69 | (10%) | – | |
| 13∼36 | 205 | (31%) | – | |
| 37∼60 | 149 | (23%) | – | |
| > = 60 | 165 | (25%) | – | |
| Number of weekly dialyses (n [%]) | ||||
| <3 | 209 | (32%) | – | |
| > = 3 | 452 | (68%) | – | |
| Dialysis membrane (n [%]) | ||||
| Non-synthetic membrane | 71 | (11%) | – | |
| synthetic membrane | 590 | (89%) | – | |
| Dialyzer (n [%]) | ||||
| Low-flux | 307 | (46%) | – | |
| Middle-flux | 238 | (36%) | – | |
| High-flux | 116 | (18%) | – | |
| Anticoagulant (n [%]) | ||||
| heparin | 578 | (87%) | – | |
| LMWH | 83 | (13%) | – | |
| Kt/v(;median [25th to 75th percentiles]) | 1.421 | (1.245–1.619) | – | |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; Hb:hemoglobin; SCr: serum creatinine; ALB: albumin.
The positivity rate of the serum anti-PF4/heparin antibody and its risk factors in the research subjects
The detection of the serum anti-PF4/heparin antibody in the 62 healthy subjects was negative. Among the 661 MHD patients, 37 subjects tested positive for the anti-PF4/heparin antibody (positivity rate of 5.6%), of which 33 individuals had a history of heparin exposure, and the remaining four cases had a history of exposure to LMWH only. Moreover, the 37 patients who tested positive for the anti-PF4/heparin antibody had no clinical diagnosis of HIT. The positivity rate of the anti-PF4/heparin antibody in the MHD patients did not significantly differ among the nine hemodialysis centers. The univariate analysis revealed that the positivity rate of the serum anti-PF4/heparin antibody was significantly increased in the MHD patients whose primary diseases were diabetes and elevated diastolic blood pressures (DBPs). No significant difference between the groups testing positive versus negative for the serum anti-PF4/heparin antibody was found in age, sex, dry weight, body mass index (BMI), systolic blood pressure (SBP), hemoglobin, platelet count and thrombocytopenia (defined as PLT <100×109/L), serum creatinine, albumin, bleeding events within the past three months, thromboembolic events within the past three months, dialysis duration in months, number of weekly dialyses, dialysis membrane, dialyzer, anticoagulant, and Kt/v (Table 2).
Table 2. Results of univariate analysis.
| Anti-PF4/heparin antibody | ||||||||
| Positive (n = 37) | Negative (n = 624) | P value | ||||||
| Age(years;mean [SD]) | 56±17 | 56±16 | 0.944 | |||||
| Sex (female;n [%]) | 15 | (41%) | 272 | (44%) | 0.737 | |||
| Dry Weight(kg;median [25th to 75th percentiles]) | 61.5 | (54–71) | 62.8 | (55–70) | 0.706 | |||
| BMI (mean [SD]) | 23.4±5.5 | 22.6±3.9 | 0.236 | |||||
| SBP(mmHg; mean [SD]) | 148±19 | 141±21 | 0.066 | |||||
| DBP(mmHg; mean [SD]) | 86±13 | 80±13 | 0.032 | |||||
| Hb(g/L; median [25th to 75th percentiles]) | 105 | (94–115) | 105 | (90–118) | 0.904 | |||
| Platelet count (10?9/L; mean [SD]) | 166±58 | 178±53 | 0.144 | |||||
| Thrombocytopenia (n [%]) | 4 | (11%) | 76 | (12%) | 0.804 | |||
| SCr(µmol/L; mean [SD]) | 885±314 | 838±279 | 0.493 | |||||
| ALB(g/L;median [25th to 75th percentiles]) | 40 | (37–42) | 40 | (38–42) | 0.811 | |||
| Diabetes(n [%]) | 13 | (35%) | 114 | (18%) | 0.017 | |||
| Bleeding events within the past three months (n [%]) | 4 | (11%) | 79 | (13%) | 0.941 | |||
| Thromboembolic events within the past three months (n [%]) | 3 | (8%) | 63 | (10%) | 0.913 | |||
| Dialysis duration (months ;n [%]) | ||||||||
| 3∼6 | 7 | (19%) | 66 | (11%) | 0.305 | |||
| 7∼12 | 6 | (16%) | 63 | (10%) | ||||
| 13∼36 | 11 | (30%) | 194 | (31%) | ||||
| 37∼60 | 6 | (16%) | 143 | (23%) | ||||
| > = 60 | 7 | (19%) | 158 | (25%) | ||||
| Number of weekly dialyses (> = 3 times; n [%]) | 20 | (54%) | 432 | (69%) | 0.095 | |||
| Dialysis membrane (synthetic membrane; n [%]) | 32 | (86%) | 558 | (89%) | 0.582 | |||
| Dialyzer (n [%]) | ||||||||
| Low-flux | 19 | (51%) | 288 | (46%) | 0.855 | |||
| Middle-flux | 12 | (33%) | 226 | (36%) | ||||
| High-flux | 6 | (16%) | 110 | (18%) | ||||
| Anticoagulant (heparin; n [%]) | 33 | (89%) | 545 | (87%) | 0.809 | |||
| Kt/v(;median [25th to 75th percentiles]) | 1.295 | (1.097–1.492) | 1.430 | (1.250–1.620) | 0.104 | |||
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; Hb:hemoglobin; SCr: serum creatinine; ALB: albumin.
The multivariate logistic regression analysis revealed that diabetes (OR = 4.405, 95% CI [1.573 – 12.334], P = 0.005) significantly increased the positivity rate of the serum anti-PF4/heparin antibody in the MHD patients, and the number of weekly dialyses (≥3 times) reduced the positivity rate of the serum anti-PF4/heparin antibody (OR = 0.324, 95% CI [0.114 – 0.925], P = 0.035). The positivity rate of the serum anti-PF4/heparin antibody was not significantly correlated with age, sex, body weight, BMI, SBP, DBP, hemoglobin, platelet count and thrombocytopenia, serum creatinine, albumin, bleeding events within the past three months, thromboembolic events within the past three months, the dialysis duration in months, the number of weekly dialyses, dialysis membrane, dialyzer, anticoagulant, and Kt/v (Table 3).
Table 3. Results of multivariate analysis.
| OR | 95% C.I. | P value | |
| Age | 1.002 | 0.969–1.037 | 0.888 |
| Sex (female]) | 0.877 | 0.411–1.870 | 0.733 |
| Dry Weight | 0.968 | 0.926–1.011 | 0.144 |
| BMI | 0.992 | 0.867–1.136 | 0.911 |
| SBP | 0.999 | 0.973–1.026 | 0.948 |
| DBP | 1.041 | 0.993–1.092 | 0.094 |
| Hb | 1.006 | 0.980–1.033 | 0.640 |
| Platelet count | 1.000 | 0.990–1.011 | 0.971 |
| Thrombocytopenia | 0.796 | 0.116–5.463 | 0.817 |
| SCr | 1.000 | 0.998–1.001 | 0.626 |
| ALB | 1.030 | 0.946–1.122 | 0.498 |
| Diabetes | 4.405 | 1.573–12.334 | 0.005 |
| Bleeding events within the past three months | 0.535 | 0.062–4.576 | 0.568 |
| Thromboembolic events within the past three months | 0.838 | 0.167–4.206 | 0.830 |
| Dialysis duration (months) | |||
| 7∼12 | 0.509 | 0.085–3.049 | 0.460 |
| 13∼36 | 0.452 | 0.111–1.850 | 0.270 |
| 37∼60 | 0.478 | 0.100–2.293 | 0.356 |
| > = 60 | 0.671 | 0.139–3.233 | 0.619 |
| Number of weekly dialyses (> = 3 times) | 0.324 | 0.114–0.925 | 0.035 |
| Dialysis membrane (synthetic membrane) | 1.204 | 0.088–16.418 | 0.889 |
| Anticoagulant (heparin) | 0.654 | 0.188–2.281 | 0.505 |
| Kt/v | 0.283 | 0.041–1.931 | 0.197 |
OR: odds ratio; CI: confidence interval; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressures; Hb:hemoglobin; SCr: serum creatinine; ALB: albumin.
The risk factors for death in the MHD patients
At the two-year follow-up, among the 661 MHD patients, there were 431 cases with continued dialysis, 108 cases of death, 36 cases of kidney transplant or peritoneal dialysis, and 86 cases lost to follow-up. The 37 patients who tested positive for the anti-PF4/heparin antibody included 21 cases with continued dialysis, six cases of death, two cases of kidney transplantation or peritoneal dialysis, and eight cases lost to follow-up. Among the 539 patients with complete follow-up data (431 cases with continued dialysis and 108 cases of death), the two-year cumulative mortality rate for the MHD patients was 20.0% with an average annual mortality rate of 10.6%. The multivariate logistic regression analysis revealed that advanced age and diabetes mellitus significantly increased the risk of death in the patients undergoing maintenance hemodialysis, whereas utilizing a synthetic membrane dialyzer significantly reduced the patient's risk of death. The death of the patients was not significantly associated with the sex, body weight, SBP, DBP, anti-PF4/heparin antibody, hemoglobin, platelet count and thrombocytopenia, serum creatinine, albumin, bleeding events within the past three months, thromboembolic events within the past three months, dialysis duration in months, number of weekly dialyses (≥3), dialyzer, anticoagulant, and Kt/v (P>0.05). (Table 4)
Table 4. The baseline-factor analysis between the patients with continued dialysis and death.
| Continued dialysis (n = 431) | Death (n = 108) | OR | 95% C.I. | P value | |||
| Age (years;median [25th to 75th percentiles]) | 55 | (27–77) | 67 | (43–85) | 1.069 | (1.039–1.099) | <0.001 |
| Sex (female;n [%]) | 201 | (47%) | 45 | (42%) | 0.564 | (0.292–1.089) | 0.088 |
| Dry Weight | 60.8 | (54–68) | 61.1 | (55–69) | 1.002 | (0.971–1.033) | 0.922 |
| BMI | 22.5±3.8 | 22.5±4.0 | 1.047 | (0.968–1.131) | 0.250 | ||
| SBP(mmHg; mean [SD]) | 141±21 | 142±22 | 1.003 | (0.988–1.019) | 0.681 | ||
| DBP(mmHg; mean [SD]) | 81±12 | 76±14 | 1.002 | (0.973–1.032) | 0.880 | ||
| Anti-PF4/heparin antibody(positive;n [%]) | 21 | (5%) | 6 | (6%) | 1.267 | (0.336–4.780) | 0.727 |
| Hb(g/L; median [25th to 75th percentiles]) | 107 | (96–116) | 102 | (91–115) | 0.994 | (0.977–1.011) | 0.506 |
| PLT(10?9/L; mean [SD]) | 167±57 | 163±59 | 0.998 | (0.992–1.005) | 0.607 | ||
| thrombocytopenia (n [%]) | 47 | (11%) | 13 | (12%) | 0.451 | (0.139–1.464) | 0.185 |
| SCr(µmol/L; mean [SD]) | 908 | (344–1392) | 705 | (208–1301) | 1.000 | (0.999–1.001) | 0.750 |
| ALB(g/L;median [25th to 75th percentiles]) | 40 | (32–45) | 37 | (29–45) | 0.966 | (0.911–1.025) | 0.253 |
| diabetes(n [%]) | 68 | (16%) | 41 | (38%) | 2.061 | (1.062–4.000) | 0.033 |
| Bleeding events within the past three months (n [%]) | 60 | (14%) | 14 | (13%) | 0.963 | (0.396–2.340) | 0.934 |
| Thromboembolic events within the past three months (n [%]) | 39 | (9%) | 17 | (16%) | 1.624 | (0.724–3.641) | 0.239 |
| Time on dialysis (months ;n [%]) | |||||||
| 3∼6 | 27 | (6%) | 13 | (12%) | 1.000 | – | – |
| 7∼12 | 50 | (12%) | 10 | (9%) | 0.835 | (0.189–3.683) | 0.812 |
| 13∼36 | 127 | (29%) | 41 | (38%) | 2.118 | (0.617–7.271) | 0.233 |
| 37∼60 | 104 | (24%) | 25 | (23%) | 1.321 | (0.365–4.779) | 0.671 |
| > = 60 | 123 | (29%) | 19 | (18%) | 1.175 | (0.309–4.472) | 0.813 |
| Number of weekly dialyses (> = 3 times; n [%]) | 281 | (65%) | 85 | (79%) | 1.792 | (0.805–3.991) | 0.153 |
| Dialysis membrane (synthetic membrane;n [%]) | 388 | (90%) | 96 | (89%) | 0.145 | (0.024–0.885) | 0.036 |
| Dialyzer (n [%]) | |||||||
| Low-flux | 199 | (46%) | 59 | (55%) | 1.000 | – | – |
| Middle-flux | 158 | (37%) | 32 | (30%) | 0.879 | (0.400–1.935) | 0.749 |
| High-flux | 74 | (17%) | 17 | (16%) | 1.130 | (0.453–2.818) | 0.794 |
| Anticoagulant (heparin; n [%]) | 381 | (88%) | 88 | (81%) | 0.693 | (0.323–1.485) | 0.345 |
| Kt/v(;median [25th to 75th percentiles]) | 1.430 | (1.266–1.640) | 1.462 | (1.230–1.599) | 0.681 | (0.285–1.623) | 0.386 |
OR: odds ratio; CI: confidence interval; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressures; Hb:hemoglobin; SCr: serum creatinine; ALB: albumin.
Prediction of bleeding and thromboembolic events in the MHD patients based on the serum anti-PF4/heparin antibody combined with either thrombocytopenia or the administration of antiplatelet drugs
Of the 431 patients with continued hemodialysis after two years of follow-up, 159 patients experienced new bleeding events within the two years with an incidence of 36.9% for the two-year cumulative bleeding events and an average annual incidence of 20.6%; 91 patients experienced new thromboembolic events with an incidence of 21.1% for the two-year cumulative thromboembolic events and an average annual incidence of 11.2%. According to the baselines of the anti-PF4/heparin antibody and platelet count or the anti-PF4/heparin antibody and antiplatelet drugs, the patients with continued MHD after two years of follow-up were divided into four groups: antibody negative + normal platelets, antibody negative + reduced platelets, antibody positive + normal platelets, and antibody positive + reduced platelets. In addition, there were four groups, as follows: antibody negative without antiplatelet drug administration, antibody negative with antiplatelet drug administration, antibody positive without antiplatelet drug administration, and antibody positive with antiplatelet drug administration. Respectively taking the group of antibody negative + normal platelets or the group of antibody negative without antiplatelet drug administration as the standard group (the OR value was set to 1), the risk of the occurrence of bleeding events and thromboembolic events during the two-year follow-up was analyzed for the patients in the other groups. The results revealed that the group of antibody negative + reduced platelets (OR = 1.890, 95% CI [1.014 – 3.522], P = 0.045) and the group of antibody negative with antiplatelet drug administration (OR = 1.586, 95% CI [1.034 – 2.432], P = 0.034) had a significantly increased risk of occurrence of bleeding events. The risks of bleeding events in the other groups were not significantly different from those in the standard groups. No significant difference was found in the risk of clinical thromboembolic events between the groups (Table 5).
Table 5. Analysis of clinical bleeding & thromboembolic events within the two years.
| Bleeding events within the two years | Thromboembolic events within the two years | |||||||||
| n/n, (percentage) | OR | 95% C.I. | P value | n/n, (percentage) | OR | 95% C.I. | P value | |||
| antibody negative + normal platelets | 130/365 | (36%) | 1.000 | — | 0.155 | 71/365 | (19%) | 1.000 | — | 0.220 |
| antibody negative + reduced platelets | 23/45 | (51%) | 1.890 | 1.014–3.522 | 0.045 | 13/45 | (29%) | 1.682 | 0.840–3.370 | 0.142 |
| antibody positive + normal platelets | 5/20 | (25%) | 0.603 | 0.214–1.696 | 0.337 | 7/20 | (35%) | 2.230 | 0.858–5.792 | 0.100 |
| antibody negative + reduced platelets | 1/1 | (100%) | — | — | >0.999 | 0/1 | (0%) | — | — | >0.999 |
| antibody negative without antiplatelet drug administration | 96/283 | (34%) | 1.000 | — | 0.153 | 59/283 | (21%) | 1.000 | — | 0.401 |
| antibody negative with antiplatelet drug administration | 57/127 | (45%) | 1.586 | 1.034–2.432 | 0.034 | 25/127 | (20%) | 0.931 | 0.552–1.570 | 0.787 |
| antibody positive without antiplatelet drug administration | 3/12 | (25%) | 0.649 | 0.172–2.454 | 0.524 | 3/12 | (25%) | 1.266 | 0.332–4.822 | 0.730 |
| antibody positive with antiplatelet drug administration | 3/9 | (33%) | 0.974 | 0.238–3.980 | 0.971 | 4/9 | (44%) | 3.037 | 0.791–11.666 | 0.106 |
Values are shown as the Number of bleeding (or thromboembolic) events/the number of the patients of the group, (percentage). OR: odds ratio; CI: confidence interval.
Discussion
In this study, we first investigated the serum anti-PF4/heparin antibody levels in 661 MHD patients from nine hemodialysis centers in three cities of northern China, in which 37 patients tested positive for the anti-PF4/heparin antibody, with a positivity rate of 5.6% being observed for the serum anti-PF4/heparin antibody. According to the diagnosis criteria[6], clinicians make a diagnosis of HIT when any of the following events occurs in association with the presence of “HIT antibodies” detected by in vitro assays: (1) an unexplained platelet count fall; (2) venous or arterial thrombosis; (3) skin lesions at heparin injection sites; or (4) acute systemic (anaphylactoid) reactions that occur after IV heparin bolus administration. Although some of the patients tested positive for the antibody also had thrombocytopenia, bleeding or thromboembolic events within the past three months, not a single patient was clinically diagnosed with HIT. The main reason for the low diagnosis rate is that the detection of anti-PF4/heparin antibodies is not a routine clinical test in China. In addition, few publications regarding HIT-related research on hemodialysis patients are available. Therefore, it is necessary to improve the clinical detection of anti-PF4/heparin antibodies in China.
Nearly a decade of research on HIT has revealed positivity rates of the anti-PF4/heparin antibody that are mostly within the range of 1.2% to 10.3% [8]–[12]; however, it has also been reported that the positivity rate of the anti-PF4/heparin antibody in maintenance dialysis patients is as high as 17.9% [21], and even up to 47% [13]. One of the reasons for the difference between the results of the individual reports is that most of the studies were based on a single-center resource; thus, the results are not representative. A study of 305 MHD patients from four centers in Japan revealed a 2.3% positivity rate of the anti-PF4/heparin antibody [11]. The CHOICE Cohort Study in the USA showed that the positivity rate of the anti-PF4/heparin antibody was 10.6% among 596 selected MHD patients [10]. Our present study demonstrated a serum anti-PF4/heparin antibody positivity rate of 5.6% among 661 MHD patients from nine centers in China, indicating that the positivity rate of the anti-PF4/heparin antibody might differ in MHD patients of various nationalities and ethnicities. However, the positivity of HIT antibody is also largely influenced by the kit and detection method used in a study [19]. The kit and detection method used in the previous studies were varied, and this variety can not be excluded from the reasons for the difference of anti-PF4/heparin antibody positivity rate in these studies. Currently, the major method of detection of anti-PF4/heparin antibodies is ELISA, in which the sensitivity can be as high as 80% – 100%. However, the specificity is low because of the simultaneous detection of non-specific antibodies [22], [23]. Studies have shown that only the IgG class of anti-PF4/heparin antibodies are platelet-activating and thus have the potential to cause heparin-induced thrombocytopenia [19], [24]–[26]. Therefore, in this study, we only detected the IgG anti-PF4/heparin antibody. Additionally, to improve the specificity of detection of the anti-PF4/heparin antibody, the high-dose heparin confirmatory tests were performed [19], [20], [27] to set the positive standard of OD405 nm >0.4 and the inhibition test >50% for the anti-PF4/heparin antibody. This step ensured the reliability of the results in the present study, and the negative result for the serum anti-PF4/heparin antibody using the same detection method in the 62 healthy controls also confirmed the accuracy of the method applied in our study.
Moreover, the frequency of antibody formation depends on a variety of factors, including the chain length of the heparin molecule and degree of sulfation, amount and frequency of heparin administration, clinical setting, and degree of nonimmune platelet activation [28]. The results of this study indicates that  the positivity rate of the anti-PF4/heparin antibody in MHD patients is not associated with their age, sex, and dialysis duration, which is similar to previous findings [13], [21].
the positivity rate of the anti-PF4/heparin antibody in MHD patients is not associated with their age, sex, and dialysis duration, which is similar to previous findings [13], [21].  The positivity rate of the anti-PF4/heparin antibody is unrelated to the occurrence of thrombocytopenia, which is also consistent with previously reported results [21].
The positivity rate of the anti-PF4/heparin antibody is unrelated to the occurrence of thrombocytopenia, which is also consistent with previously reported results [21].  The positivity rate of the anti-PF4/heparin antibody in MHD patients undergoing three or more dialyses per week was significantly lower than that in patients undergoing less than three dialyses per week. Although a high frequency of dialyses could lead to an increased dose and frequency of heparin use (as well as increase the levels of heparin exposure in the patients), whether long-term and high-frequency exposure to heparin can induce immune tolerance in patients remains to be studied.
The positivity rate of the anti-PF4/heparin antibody in MHD patients undergoing three or more dialyses per week was significantly lower than that in patients undergoing less than three dialyses per week. Although a high frequency of dialyses could lead to an increased dose and frequency of heparin use (as well as increase the levels of heparin exposure in the patients), whether long-term and high-frequency exposure to heparin can induce immune tolerance in patients remains to be studied.  Although the diabetic MHD patients in this study were significantly more likely than the non-diabetic patients to undergo more than three dialyses per week (97/127 cases, 76.4% vs 355/530 cases, 67.0%, respectively; P = 0.043), the positivity rate of the anti-PF4/heparin antibody was significantly increased in the diabetic patients with MHD, which differs from the conclusion of Diaz J et al.
[29] that diabetes does not increase the positivity rate of the anti-PF4/heparin antibody. The reason for the increased positivity rate of the anti-PF4/heparin antibody in the MHD patients remains unclear.
Although the diabetic MHD patients in this study were significantly more likely than the non-diabetic patients to undergo more than three dialyses per week (97/127 cases, 76.4% vs 355/530 cases, 67.0%, respectively; P = 0.043), the positivity rate of the anti-PF4/heparin antibody was significantly increased in the diabetic patients with MHD, which differs from the conclusion of Diaz J et al.
[29] that diabetes does not increase the positivity rate of the anti-PF4/heparin antibody. The reason for the increased positivity rate of the anti-PF4/heparin antibody in the MHD patients remains unclear.  The result of this study revealed no significant difference in the positivity rate of the HIT antibody between the patients using heparin and LMWH. Although it is widely accepted that the frequency of HIT is greater in non-hemodialysis populations treated with unfractionated heparin compared with LMWH [30], it has also been reported that no significant difference in the positivity rate of the antibody in MHD patients was found between these two types of heparin[11].In this study, the patients in the heparin group included the patients using heparin as the hemodialysis anticoagulant or to pre-rinse the dialyzer, as well as the patients using heparin for long-term anticoagulation of the central venous catheter; furthermore, the patients who used LMWH as the hemodialysis anticoagulant and had never been exposed to heparin were defined as the LMWH group. Because the patients in the heparin group were significantly more numerous than the patients in the LMWH group (578/83 cases), the difference in the number of cases between the groups may reflect the difference in the positivity rate of the anti-PF4/heparin antibody between the two anticoagulants.
The result of this study revealed no significant difference in the positivity rate of the HIT antibody between the patients using heparin and LMWH. Although it is widely accepted that the frequency of HIT is greater in non-hemodialysis populations treated with unfractionated heparin compared with LMWH [30], it has also been reported that no significant difference in the positivity rate of the antibody in MHD patients was found between these two types of heparin[11].In this study, the patients in the heparin group included the patients using heparin as the hemodialysis anticoagulant or to pre-rinse the dialyzer, as well as the patients using heparin for long-term anticoagulation of the central venous catheter; furthermore, the patients who used LMWH as the hemodialysis anticoagulant and had never been exposed to heparin were defined as the LMWH group. Because the patients in the heparin group were significantly more numerous than the patients in the LMWH group (578/83 cases), the difference in the number of cases between the groups may reflect the difference in the positivity rate of the anti-PF4/heparin antibody between the two anticoagulants.  It was confirmed that the unified detection of the PF4/heparin antibody demonstrated no significant differences in the positivity rate of the anti-PF4/heparin antibody between the patients from the different hemodialysis centers in the same region of the same country. This finding might be related to the similar qualities and methods of applications of the heparin drugs used by hemodialysis patients in the same region of the same country.
It was confirmed that the unified detection of the PF4/heparin antibody demonstrated no significant differences in the positivity rate of the anti-PF4/heparin antibody between the patients from the different hemodialysis centers in the same region of the same country. This finding might be related to the similar qualities and methods of applications of the heparin drugs used by hemodialysis patients in the same region of the same country.
The occurrence of HIT leads to platelet activation, thereby increasing the risk of occurrence of thromboembolic events in patients [31]–[34] and most likely increasing the mortality in MHD patients [9]. However, it has also been reported that an increase in anti-PF4/heparin antibody levels does not increase the incidence of thromboembolic events [8], [21] and the anti-PF4/heparin antibody had no effect on the overall mortality or cardiovascular events in the absence of thrombocytopenia[10]. Tsai YF et al. [34] reported that the anti-PF4/heparin antibody increases the risk of thromboembolic events, but they also determined that the anti-PF4/heparin antibody is not correlated with peripheral arterial disease (PAD) and coronary heart disease (CHD). In this study, a two-year follow-up was conducted for detection of the anti-PF4/heparin antibody in 661 MHD patients from nine centers, of which 431 patients (65.20%) were on continued dialysis and 108 patients (16.34%) died. The results further confirmed that the occurrence of thromboembolic events was not significantly associated with the anti-PF4/heparin antibody in the MHD patients, and testing positive for the anti-PF4/heparin antibody was not correlated with the two-year mortality of the patients. Further analysis of risk factors in the MHD patients who died revealed that advanced age and diabetes increased the risk of death, and dialysis with a synthetic membrane reduced the risk of death, which is consistent with findings of DOPPS [35]–[37]. However, no impact of the anti-PF4/heparin antibody on patient mortality was determined.
Based on the fundamental in vitro and in vivo experiments, antiplatelet drugs might prevent the occurrence of HIT [38], [39]; by contrast, several clinical trials have confirmed that antiplatelet drugs do not effectively suppress the occurrence of HIT [40], and antiplatelet drugs do not reduce the occurrence and degree of thrombocytopenia [41]. However, these studies were limited to case reports or research using a small sample size. Because the proportion of elderly patients and patients with diabetes and other cardiovascular complications (such as hypertension in MHD patients) has increased annually, the number of MHD patients taking antiplatelet drugs is also gradually increasing. In this study, the use of antiplatelet agents was an observational indicator, rather than an intervention. In the MHD patients who were using antiplatelet drugs, combined predictive effects were mainly observed for the presence of serum anti-PF4/heparin antibodies and the administration of antiplatelet drugs for bleeding and thromboembolic events. According to the baseline level of the anti-PF4/heparin antibody and antiplatelet drugs, the patients with continued MHD after the two years of follow-up were divided into four groups: antibody negative without antiplatelet drug administration, antibody negative with antiplatelet drug administration, antibody positive without antiplatelet drug administration, and antibody positive with antiplatelet drug administration. The results showed that the patients in the group of antibody negative with antiplatelet drug administration had a significantly increased risk for the occurrence of bleeding events during the two years of follow-up compared with the patients in the other three groups; whether the anti-PF4/heparin antibody was present and whether the patient was taking antiplatelet drugs had no significant effect on the occurrence of thromboembolic events during the two years of follow-up. In addition, the combined predictive effects of the serum anti-PF4/heparin antibody and the platelet count in MHD patients on the clinical bleeding and thromboembolic events were also observed. According to the baseline level of the anti-PF4/heparin antibody and platelet count, the patients with continued MHD after the two years of follow-up were divided into four groups: antibody negative + normal platelets, antibody negative + reduced platelets, antibody positive + normal platelets, and antibody positive + reduced platelets. The results showed that the patients in the antibody negative + reduced platelet group had a significantly increased incidence of bleeding events during the two-year follow-up compared with the patients in the other three groups, whereas neither the level of serum anti-PF4/heparin antibody nor the platelet count predicted the incidence of thromboembolic events. The pathogenesis of HIT by now are mainly regarded as HIT antibodies activate platelets intravascularly, causing the release of platelet microparticles and increased thrombin generation[42], [43]. Therefore, we consider that the activations of platelets by anti-PF4/heparin antibody, are likely to offset the risk of bleeding due to antiplatelet drugs and the decreased number of platelets. Although the majority of studies have reported an increased incidence of heparin-PF4 antibodies in patients with thromboembolic events, this has not been universal[8], [10], [13]. Therefore, we think that anti-PF4/heparin antibody or the activations of platelets are inadequate to cause thromboembolic events. Our data also showed that even considering the impact of the platelet count and antiplatelet drugs, the positive serum anti-PF4/heparin antibody can not predict the risk of occurrence of thromboembolic events in the MHD patients. In addition, the small number of patients tested positive for the serum anti-PF4/heparin antibody might potentially cause a deviation in the analysis.
The advantages of this study are as follows:  A standard detection method for the anti-PF4/heparin antibody was applied, which was verified by the healthy control.
A standard detection method for the anti-PF4/heparin antibody was applied, which was verified by the healthy control.  As a prospective and multi-center clinical cohort study with a large sample size, the present investigation should be more representative.
As a prospective and multi-center clinical cohort study with a large sample size, the present investigation should be more representative.  This study is the first multi-center investigation that addresses the anti-PF4/heparin antibody and its risk factors in Chinese MHD patients, at the level of clinical significance. The limitations of this study include the following:
This study is the first multi-center investigation that addresses the anti-PF4/heparin antibody and its risk factors in Chinese MHD patients, at the level of clinical significance. The limitations of this study include the following:  lack of dynamic monitoring and recording during the two years of follow-up (with the indicators being observed at multiple time points);
lack of dynamic monitoring and recording during the two years of follow-up (with the indicators being observed at multiple time points);  failure to redetect the serum anti-PF4/heparin antibody after the two years of observation;
failure to redetect the serum anti-PF4/heparin antibody after the two years of observation;  use of antiplatelet drugs as an observational indicator, rather than an intervention;
use of antiplatelet drugs as an observational indicator, rather than an intervention;  the smaller number of patients who tested positive for the serum anti-PF4/heparin antibody, which might have potentially caused a deviation in the analysis.
the smaller number of patients who tested positive for the serum anti-PF4/heparin antibody, which might have potentially caused a deviation in the analysis.
In summary, this study is the first to indicate that the positivity rate of the anti-PF4/heparin antibody in Chinese MHD patients was 5.6% using a prospective and multi-center clinical cohort study with a large sample size. We further verified that a single detection of anti-PF4/heparin antibody did not predict the occurrence of bleeding, thromboembolic events, and risk of death in the MHD patients. Finally, we demonstrated that the patients who tested negative for the anti-PF4/heparin antibody and who also exhibited a thrombocytopenia or underwent administration of antiplatelet drugs were at significantly increased risk for clinical bleeding.
Acknowledgments
We thank all the staff and participants of the hemodialysis centers of the nine hospitals for their important contributions.
Funding Statement
This work was supported by the grants from the Major State Basic Research Development Program of China (2013CB530800), http://www.973.gov.cn/AreaMana.aspx; National Natural Science Foundation of China (81270819), http://isis.nsfc.gov.cn; National Key Technology R&D Program (2011BAI10B00), http://kjzc.jhgl.org. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shen JI, Winkelmayer WC (2012) Use and Safety of Unfractionated Heparin for Anticoagulation During Maintenance Hemodialysis. American Journal of Kidney Diseases 60: 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aster RH (1999) Drug-induced immune thrombocytopenia: An overview of pathogenesis. Seminars in Hematology 36: 2–6. [PubMed] [Google Scholar]
- 3. Haase M, Bellomo I, Rocktaeschel J, Ziemer S, Kiesewetter H, et al. (2005) Use of fondaparinux (ARIXTRA((R))) in a dialysis patient with symptomatic heparin-induced thrombocytopaenia type II. Nephrology Dialysis Transplantation 20: 444–446. [DOI] [PubMed] [Google Scholar]
- 4. Warkentin TE (2004) An overview of the heparin-induced thrombocytopenia syndrome. Semin Thromb Hemost 30: 273–283. [DOI] [PubMed] [Google Scholar]
- 5. Hirsh J, Heddle N, Kelton JG (2004) Treatment of heparin-induced thrombocytopenia: a critical review. Arch Intern Med 164: 361–369. [DOI] [PubMed] [Google Scholar]
- 6. Warkentin TE, Greinacher A, Koster A, Lincoff AM (2008) Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 340S–380S. [DOI] [PubMed] [Google Scholar]
- 7. Kato S, Takahashi K, Ayabe K, Samad R, Fukaya E, et al. (2011) Heparin-induced thrombocytopenia: analysis of risk factors in medical inpatients. Br J Haematol 154: 373–377. [DOI] [PubMed] [Google Scholar]
- 8. O'Shea SI, Sands JJ, Nudo SA, Ortel TL (2002) Frequency of anti-heparin-platelet factor 4 antibodies in hemodialysis patients and correlation with recurrent vascular access thrombosis. Am J Hematol 69: 72–73. [DOI] [PubMed] [Google Scholar]
- 9. Pena de la Vega L, Miller RS, Benda MM, Grill DE, Johnson MG, et al. (2005) Association of heparin-dependent antibodies and adverse outcomes in hemodialysis patients: a population-based study. Mayo Clin Proc 80: 995–1000. [DOI] [PubMed] [Google Scholar]
- 10. Asmis LM, Segal JB, Plantinga LC, Fink NE, Kerman JS, et al. (2008) Heparin-induced antibodies and cardiovascular risk in patients on dialysis. Thromb Haemost 100: 498–504. [PubMed] [Google Scholar]
- 11. Matsuo T, Kobayashi H, Matsuo M, Wanaka K, Nakamoto H, et al. (2006) Frequency of anti-heparin-PF4 complex antibodies (HIT antibodies) in uremic patients on chronic intermittent hemodialysis. Pathophysiol Haemost Thromb 35: 445–450. [DOI] [PubMed] [Google Scholar]
- 12. Hutchison CA, Dasgupta I (2007) National survey of heparin-induced thrombocytopenia in the haemodialysis population of the UK population. Nephrol Dial Transplant 22: 1680–1684. [DOI] [PubMed] [Google Scholar]
- 13. Benjamin J, Moldavsky S, Lee J, Rubin R (2009) Prevalence of heparin-induced antibody in African-American hemodialysis patients—comparison to non-dialysis patients. Clin Nephrol 71: 263–266. [DOI] [PubMed] [Google Scholar]
- 14. Nakamoto H, Shimada Y, Kanno T, Wanaka K, Matsuo T, et al. (2005) Role of platelet factor 4-heparin complex antibody (HIT antibody) in the pathogenesis of thrombotic episodes in patients on hemodialysis. Hemodial Int 9 Suppl 1S2–7. [DOI] [PubMed] [Google Scholar]
- 15. O'Shea S I, Lawson JH, Reddan D, Murphy M, Ortel TL (2003) Hypercoagulable states and antithrombotic strategies in recurrent vascular access site thrombosis. J Vasc Surg 38: 541–548. [DOI] [PubMed] [Google Scholar]
- 16. Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, et al. (1984) Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev 28: 47–55. [DOI] [PubMed] [Google Scholar]
- 17. Warkentin TE, Sheppard JI, Moore JC, Kelton JG (2010) The use of well-characterized sera for the assessment of new diagnostic enzyme-immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost 8: 216–218. [DOI] [PubMed] [Google Scholar]
- 18. Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG (2008) Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost 6: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 19. McFarland J, Lochowicz A, Aster R, Chappell B, Curtis B (2012) Improving the specificity of the PF4 ELISA in diagnosing heparin-induced thrombocytopenia. Am J Hematol 87: 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aster RH (2010) Improving specificity in HIT testing. blood 116. [DOI] [PubMed]
- 21. Palomo I, Pereira J, Alarcon M, Diaz G, Hidalgo P, et al. (2005) Prevalence of heparin-induced antibodies in patients with chronic renal failure undergoing hemodialysis. J Clin Lab Anal 19: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arepally GM, Ortel TL (2006) Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med 355: 809–817. [DOI] [PubMed] [Google Scholar]
- 23. Warkentin TE, Sheppard JAI, Moore JC, Moore KM, Sigouin CS, et al. (2005) Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: How much class do we need? Journal of Laboratory and Clinical Medicine 146: 341–346. [DOI] [PubMed] [Google Scholar]
- 24. Greinacher A, Juhl D, Strobel U, Wessel A, Lubenow N, et al. (2007) Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J Thromb Haemost 5: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 25. Warkentin TE, Sheppard JA (2006) Testing for heparin-induced thrombocytopenia antibodies. Transfus Med Rev 20: 259–272. [DOI] [PubMed] [Google Scholar]
- 26. Pouplard C, Leroux D, Regina S, Rollin J, Gruel Y (2010) Effectiveness of a new immunoassay for the diagnosis of heparin-induced thrombocytopenia and improved specificity when detecting IgG antibodies. Thromb Haemost 103: 145–150. [DOI] [PubMed] [Google Scholar]
- 27. Whitlatch NL, Kong DF, Metjian AD, Arepally GM, Ortel TL (2010) Validation of the high-dose heparin confirmatory step for the diagnosis of heparin-induced thrombocytopenia. Blood 116: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davenport A (2009) Antibodies to heparin-platelet factor 4 complex: pathogenesis, epidemiology, and management of heparin-induced thrombocytopenia in hemodialysis. Am J Kidney Dis 54: 361–374. [DOI] [PubMed] [Google Scholar]
- 29. Diaz J, Prechel M, Emanuele M, Emanuele N, Walenga JM (2010) Profiling of heparin-induced thrombocytopenia antibody levels in patients with and without diabetes. Clin Appl Thromb Hemost 16: 121–125. [DOI] [PubMed] [Google Scholar]
- 30. Martel N, Lee J, Wells PS (2005) Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 106: 2710–2715. [DOI] [PubMed] [Google Scholar]
- 31. Matsuo T, Wanaka K (2008) Management of uremic patients with heparin-induced thrombocytopenia requiring hemodialysis. Clin Appl Thromb Hemost 14: 459–464. [DOI] [PubMed] [Google Scholar]
- 32. Ban-Hoefen M, Francis C (2009) Heparin induced thrombocytopenia and thrombosis in a tertiary care hospital. Thromb Res 124: 189–192. [DOI] [PubMed] [Google Scholar]
- 33. Yu A, Jacobson SH, Bygden A, Egberg N (2002) The presence of heparin-platelet factor 4 antibodies as a marker of hypercoagulability during hemodialysis. Clin Chem Lab Med 40: 21–26. [DOI] [PubMed] [Google Scholar]
- 34. Tsai YF, Chen CA, Kuo C, Lin KC (2012) Anti-PF4/heparin antibodies are associated with arteriovenous fistula thrombosis in non-diabetic hemodialysis patients. Clin Exp Nephrol 16: 300–305. [DOI] [PubMed] [Google Scholar]
- 35. Inaba M, Hayashino Y, Shoji T, Akiba T, Akizawa T, et al. (2012) Disappearance of association in diabetic patients on hemodialysis between anemia and mortality risk: the Japan dialysis outcomes and practice pattern study. Nephron Clin Pract 120: c91–c100. [DOI] [PubMed] [Google Scholar]
- 36. Canaud B, Tong L, Tentori F, Akiba T, Karaboyas A, et al. (2011) Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 6: 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chauveau P, Nguyen H, Combe C, Chene G, Azar R, et al. (2005) Dialyzer membrane permeability and survival in hemodialysis patients. Am J Kidney Dis 45: 565–571. [DOI] [PubMed] [Google Scholar]
- 38. Gruel Y, Lermusiaux P, Lang M, Darnige L, Rupin A, et al. (1991) Usefulness of antiplatelet drugs in the management of heparin-associated thrombocytopenia and thrombosis. Ann Vasc Surg 5: 552–555. [DOI] [PubMed] [Google Scholar]
- 39. Kappa JR, Fisher CA, Addonizio VP Jr (1989) Heparin-induced platelet activation: the role of thromboxane A2 synthesis and the extent of platelet granule release in two patients. J Vasc Surg 9: 574–579. [PubMed] [Google Scholar]
- 40.Greinacher A, Warkentin TE (2004) Treatment of heparin-induced thrombocytopenia: an overview. Heparin-induced thrombocytopenia, 3rd ed New York:Marcel Dekker: 335–370.
- 41. Bream-Rouwenhorst HR, Hobbs RA, Horwitz PA (2008) Thrombocytopenia in patients treated with heparin, combination antiplatelet therapy, and intra-aortic balloon pump counterpulsation. J Interv Cardiol 21: 350–356. [DOI] [PubMed] [Google Scholar]
- 42. Poncz M (2005) Mechanistic basis of heparin-induced thrombocytopenia. Semin Thorac Cardiovasc Surg 17: 73–79. [DOI] [PubMed] [Google Scholar]
- 43. Kelton JG (2005) The pathophysiology of heparin-induced thrombocytopenia: biological basis for treatment. Chest 127: 9S–20S. [DOI] [PubMed] [Google Scholar]