Abstract
In humans KCNQ2/3 heteromeric channels form an M-current that acts as a brake on neuronal excitability, with mutations causing a form of epilepsy. The M-current has been shown to be a key regulator of neuronal plasticity underlying associative memory and ethanol response in mammals. Previous work has shown that many of the molecules and plasticity mechanisms underlying changes in alcohol behaviour and addiction are shared with those of memory. We show that the single KCNQ channel in Drosophila (dKCNQ) when mutated show decrements in associative short- and long-term memory, with KCNQ function in the mushroom body α/βneurons being required for short-term memory. Ethanol disrupts memory in wildtype flies, but not in a KCNQ null mutant background suggesting KCNQ maybe a direct target of ethanol, the blockade of which interferes with the plasticity machinery required for memory formation. We show that as in humans, Drosophila display age-related memory impairment with the KCNQ mutant memory defect mimicking the effect of age on memory. Expression of KCNQ normally decreases in aging brains and KCNQ overexpression in the mushroom body neurons of KCNQ mutants restores age-related memory impairment. Therefore KCNQ is a central plasticity molecule that regulates age dependent memory impairment.
Introduction
KCNQ (Kv7) channels mediate a range of important physiological functions and are a hotspot of genetic diseases and therefore target for existing and novel drug treatments. In human cardiac muscle, KCNQ1 loss of function mutations result in the most common form of cardiac arrhythmia, Long QT syndrome, while gain of functions mutations cause Short QT and atrial fibrillation [1], [2]. KCNQ1 mutations also result in adult onset type II diabetes [3], [4]. In the nervous system KCNQ3 can heteromultimerise with either KCNQ2 or KCNQ5 subunits to form a channel that mediates a M-current, a current that is suppressed by muscarinic acetylcholine receptor activation. Because the M-current operates at resting membrane potential it is well poised to regulate membrane excitability so that when it is open it acts as a brake on action potential firing while if it is suppressed it increases neural activity and neurotransmitter release [5], [6]. These features and its broad neuronal expression allow KCNQ channels to have an important function in synaptic plasticity and memory, alcohol response and nociception [2], [7], [8]. KCNQ2 or KCNQ3 loss-of-function mutations result in a developmental form of epilepsy called Benign familial neonatal convulsions [2], [5]. KCNQ4 loss-of-function mutations are a common cause of autosomal dominant deafness and age-dependent hearing impairment [9], [10]. M-current inhibitors increase excitability of cholingeric neurons and have shown some promise as cognitive enhancers in models of dementia. Conversely, M-current openers are of great interest as anticonvulsants, analgesics and treatments of psychiatric diseases [2], [5]. In mice expression of human dominant negative KCNQ2 transgene in hippocampal neurons increases neural excitability and results in associative memory deficits [7].
Drosophila has a single KCNQ (dKCNQ) channel that is most highly expressed in the nervous system [11]–[13], but like mammalian KCNQ1 [2] is also expressed in the heart. dKCNQ encodes a slowly activating and deactivating Kv current that can be suppressed by muscarinic acetylcholine receptor agonists and hence is an M-current [12], [14]. dKCNQ has been shown to have important age-dependent cardiac function, with hearts from young dKCNQ loss-of-function mutant flies showing arrhythmias similar to those seen in aged wildtype flies, whose hearts shows age dependent reduction in dKCNQ expression [13]. dKCNQ has many features of the M-current including conserved acute block by low concentrations of ethanol and broad neuronal expression [15]. Furthermore, targeted expression of KCNQ-RNAi in Drosophila neurons increased neural excitability, while KCNQ overexpression decreased excitability in vivo. dKCNQ loss-of-function mutant flies increased ethanol sensitivity and tolerance with acute activation of dopaminergic neurons by heat-activated TRP channel or KCNQ-RNAi expression shown to produce ethanol hyperexcitability [15]. In this study we characterise the role of dKCNQ mutants on memory, showing that dKCNQ expression decreases in the brain with aging and is linked to age-dependent cognitive deficits.
Materials and Methods
Drosophila Stocks
The KCNQ deletion mutant contained an imprecise excision of the EP2074 element (KCNQ186) that removes all the 5′ and transmembrane regions of the channel and therefore is a null [13]. The KCNQ control was a precise excision of the element (KCNQ97) leaving the gene completely intact [13]. uas-KCNQ flies allowed Gal4 promoter driven overexpression of KCNQ [13] while uas-KCNQ-RNAi (Bloomington stock 27252) allowed Gal4 targeted knockdown of the channel. The KCNQ stocks were kind gifts of Dr Rolf Bodmer. Elav-Gal4, uas-mCD8-GFP and OK107-Gal4 [16] were gifts from Dr Leslie Griffith. OK107-Gal4, Gal80ts [17] was a gift from Dr Yi Zhong. c305a-Gal4 [18], MB247-Gal4 [19] and Amn(c316)-Gal4 [20] stocks were gifts of Dr Scott Waddell. Wildtype flies were Canton S w- (CSw-) from a stock previously maintained in the Waddell lab. All KCNQ mutant, Gal4 and uas lines were out crossed with the relevant CSw- line prior to behavioural analysis. All genotypes and all other crosses were raised on corn-meal agar medium at 22±2°C and 60±10% humidity under 12∶12 hr light-dark cycle.
Immunohistochemistry
Adult fly brains were dissected in HL3.1 (70 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 115 mM sucrose, 4 mM MgCl2 5 mM trehalose, 1.5 mM CaCl2, and 5 mM HEPES, pH 7.3) and isolated brains were fixed in 4% paraformaldehyde in HL3.1 for 30 min before being washed in HL3.1 [21]. The samples were permeabilised in HL3.1 with 0.1% triton X (HL3.1-Tx) for 1 hr, and then blocked for 1 hr in HL3.1-Tx with 0.1% BSA and 2% normal donkey serum (HL3.1-Tx-BSA-NDS). In order to the visualise the mushroom body and antennal lobe, brains were incubated with (1∶2000) rabbit anti-Drosophila DLG (PDZ1-2) a protein known to be highly expressed in these memory-related structures [22] overnight at 4°C in HL3.1-Tx-BSA-NDS. After washing three times in HL3.1-Tx for 20 min, the brains were incubated with anti-rabbit Alexa-648 conjugated secondary antibody (1∶400 in HL3.1-Tx-BSA-NDS) for 2 hr at room temperature. Finally the brains were washed three times HL3.1-Tx before being mounted in Vectorshield (Vector Laboratories). Samples were stored at 4°C in the dark until examination using a Leica TCS SP5 confocal microscope. The endogenous KCNQ expression pattern was determined by visualising membrane targeted GFP expressed using KCNQ-Gal4 reporter lines (KCNQNP3423-Gal4, uas-mCD8-GFP).
Olfactory Aversive Conditioning
All experiments were performed at 25°C and 70% humidity under red light using the olfactory aversive conditioning protocol [23]. Groups of ∼100 1–4 day old male and female flies received either 1 cycle of training during which they were exposed sequentially to one odour (conditioned stimulus, CS+; 3-octanol (1∶74) or 4-methylcyclohexanol (1∶57) diluted in mineral oil) for 1 min paired with electric 60 V DC shock (US) and then to a second odour (CS-; the reciprocal odour) for 1 min without electric shock separated by a 30 sec rest period when they were exposed to fresh air. Memory was measured after 1 (∼2 min memory) training session at the choice point of the T-maze. To measure STM, flies were trained with 1 training cycle were stored for 1 hr and then allowed to distribute in the T-maze. LTM was assessed by giving flies either 5 cycles of spaced training cycles separated by 15 min rest intervals and then storing the flies for 24 hr before distribution in the T-maze. A performance index (PI) was calculated as the number of flies that distributed in the CS- arm minus the flies in the CS+ arm, divided by the total number of flies. Therefore a PI of 1.0 would be equivalent of 100∶0 distribution where all the flies avoided the CS+ (perfect memory), while a 50∶50 distribution would give a PI of zero (no memory). To test the effect of ethanol on Drosophila learning, flies were kept in bottles containing instant media (Formula 4–24 (R); Carolina biological supply company, Burlington, NC, USA) containing 10% ethanol in water containing a small amount of blue dye (0.05% Bromophenol blue) ∼12 hr before testing. The controls were given the same water-blue dye solution but lacking ethanol. The blue dye was used to monitor whether the flies had actually drunk the ethanol solution; this was confirmed as all the flies had blue abdomens prior to the test. For OK107-Gal4, Gal80ts experiments [17] flies were raised at 18°C and then shifted to 30°C allowing KCNQ transgene expression 1–2 days prior and during behavioural testing. Olfactory acuity was quantified by exposing naive flies to the odour versus air in the T-maze during a 2 min test trial. The performance index was calculated by counting the number of flies avoiding odour divided by total number flies. Shock reactivity was quantified by placing grids in each arm of the T-maze, and applying shock via the grid in one arm of the maze during a 2 min test trial. The performance index was calculated by counting the number of flies avoiding shock divided by total number flies [24]. Ethanol avoidance was quantified by placing a solution of 40% ethanol in the odour cup of one arm of the T-maze during a 2 min test trial. The performance index was calculated by counting the number of flies avoiding 40% ethanol divided by total number flies. All statistical analysis for behavioural data were performed and plotted with Graphpad Prism software.
Quantitative RT-PCR
Age-matched flies were frozen in liquid nitrogen and decapitated by vortexing. Heads were collected and an equal number of heads from each genotype were homogenised. Trizol was added directly in to the homogenised heads and RNA was extracted according to manufacturer’s instructions (Invitrogen). RNA was DNAase treated (Ambion Inc) and reverse-transcribed (Retroscript, Ambion). KCNQ mRNA was measured using a TaqMan Kit(KCNQ - Dm01846741_g1) and was normalised to Rpl23 (Rpl23-Dm02151827_g1) mRNAas a control allowing standardisation between the samples for aging experiments. The cDNA concentration was measured using Roche’s Light Cycler system and using multiplexing on a Stratagene Mx3000P system (Stratagene). All statistical analysis of data were performed and plotted with Graphpad Prism software.
Results
KCNQ Signalling Regulates Mushroom Body Dependent Associative Memory
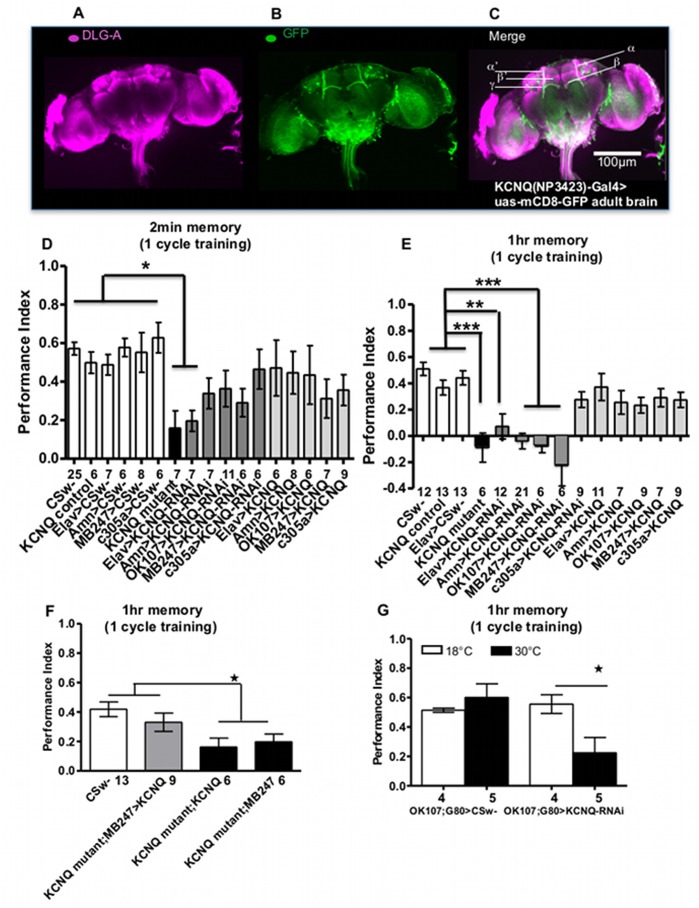
Adult flies were tested for associative memory using the olfactory aversive conditioning [23]. Anatomically, neurons critical for Drosophila memory include those of the mushroom body that are labelled by OK107-Gal4 [16]; these can be subdivided into α, β and γ neurons labelled by MB247-Gal4 (which expresses strongly in α/β neurons but only weakly in γ neurons, [19]) and α’/β’ neurons labelled by c305a-Gal4 [18]. The mushroom body-associated dorsal paired medial (DPM) neurons are also important for memory. They express the memory gene amnesiac (amn) and can be labelled by amn(c316)-Gal4 that expresses in the DPM [20]. KCNQ is broadly expressed in the brain [12], [15]. Using a Gal4 promoter enhancer trap within the KCNQ gene to drive GFP expression (Figure 1B), it appears KCNQ is expressed in the adult mushroom body α/β and surrounding neurons (Figure 1C), structures that are known to mediate memory formation and ethanol behaviour [24]–[26]. Compared with controls (Figure1D), the KCNQ mutant had reduced initial memory 2 min after training, as did flies with pan-neural KCNQ knockdown. KCNQ mutants and flies with pan-neural, DPM or mushroom body neuron deficient KCNQ completely lacked the ability to form short-term memory (STM) assessed 1 hr after training (Figure 1E).
Figure 1. KCNQ signalling is required in the mushroom body α and β neurons for short-term memory.
A.–C. Adult brains containing a Gal4 enhancer trap (KCNQNP3423) in the KCNQ gene locus revealed broad neuronal expression of KCNQ (labelled by membrane bound GFP in green (B)) especially in the fly memory structures of the mushroom body α and β neurons and surrounding neurons known to be visualised by DLG-A (Ruiz-Cañada et al., 2002) staining (in magenta (A), co-localised structures in white (C)). D. Initial (2 min) memory was reduced in the KCNQ mutant (black bar) and flies with reduced KCNQ levels (dark grey bars) in all neurons (Elav-Gal4, uas-KCNQ-RNAi) (p<0.05) compared with controls (CSw-, KCNQ control, and Gal4, +, white bars) but did not lead to any change in memory (p>0.05) between the remaining genotypes. E. KCNQ mutants and flies with reduced KCNQ in the mushroom body (OK107-Gal4 or MB247-Gal4, uas-KCNQ-RNAi), DPM (amn-Gal4, uas-KCNQ-RNAi) (p<0.001) or all (Elav-Gal4, p<0.01) neurons have a significant reduction in 1 hr STM compared to controls (CSw-, KCNQ control and Gal4, +), while KCNQ overexpression (light grey bars) had no effect (p>0.05) with these promoters. F. Mushroom body α/β neuron expression of the KCNQ transgene in the KCNQ mutant background (KCNQ mutant; MB247-Gal4, uas-KCNQ) rescued the KCNQ mutant memory deficit with its memory being greater (p<0.05) than KCNQ mutant with Gal4 or uas alone (KCNQ mutant; MB247-Gal4 and KCNQ mutant; uas-KCNQ) but statistically indistinguishable (p>0.05) from control (CSw- wildtype) levels. Data in D-F were analysed by 1-way ANOVA with Bonferroni post-hoc test. G. 1 hr memory was measured in OK107-Gal4, Gal80ts, uas-KCNQ-RNAi and OK107-Gal4, Gal80ts, CSw- control flies raised at 18°C throughout development and then tested at 18°C conditions that prevent transgene expression (white bars). These scores were compared to the 1 hr memory of the same genotypes raised at 18°C throughout development and then shifted to 30°C allowing KCNQ transgene expression 2 days prior and during behavioural testing (black bars). 2-way ANOVA indicates significant differences due to interaction between temperature and genotype (p = 0.0195). Post-hoc analysis showed OK107-Gal4, Gal80ts, uas-KCNQ-RNAi had less (p<0.05) memory at 30°C compared to flies at 18°C flies (∼100 flies per n). In this and all subsequent figures, error bars represent SEM with no asterisk p>0.05, *p<0.05, **p<0.01 and ***p<0.001. n is denoted by the number between the x axis and genotype names with experiments performed on multiple different days (∼100 flies were used per n, unless otherwise stated).
In order to map this STM phenotype further, we selectively knocked-down KCNQ in different parts of the mushroom body, finding that the α/β neurons that appear to express KCNQ (Figure 1C) were required for KCNQ’s role in STM (as opposed to α’/β’ neurons (Figure 1E) which do not seem to express KCNQ (Figure 1C)). KCNQ overexpression in any part of the mushroom body, DPM or all neurons did not change memory measured at 2 min or 1 hr (Figure 1D–E). We also found that expression of KCNQ in α/β neurons using MB247-Gal4 in a fly otherwise completely lacking KCNQ; rescued the KCNQ mutant STM defect to normal (Figure 1F). Acute reduction of KCNQ levels in the mushroom body was sufficient to decrease 1 hr memory compared with controls (Figure 1G), showing that KCNQ is required post developmentally to mediate physiological changes underlying memory. We then wished to determine the role of KCNQ in long-term memory (LTM) which is formed after spaced training and lasts about 7 days and is protein synthesis and CREB dependent [17], [27]. We found that the KCNQ mutant showed a drastic reduction in LTM (Figure 2A). No difference in ability of the flies to sense the odour (Figure S1A–B) or shock (Figure S1C) was observed between genotypes, showing that KCNQ mutants do not change peripheral sensory processing, but rather the memory defects are due to loss of KCNQ function in the mushroom body.
Figure 2. KCNQ signalling is required for long-term memory.
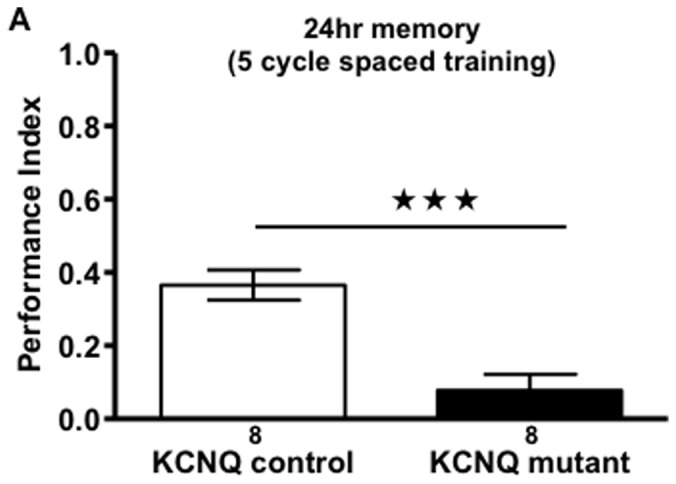
A. 5 cycles of spaced training produces 24 hr LTM in KCNQ control flies that is absent (p<0.001) in the KCNQ mutants. Data were analysed with unpaired t-test.
Drosophila Display Age Dependent Memory Deficits that are Rescued by Mushroom Body KCNQ Expression
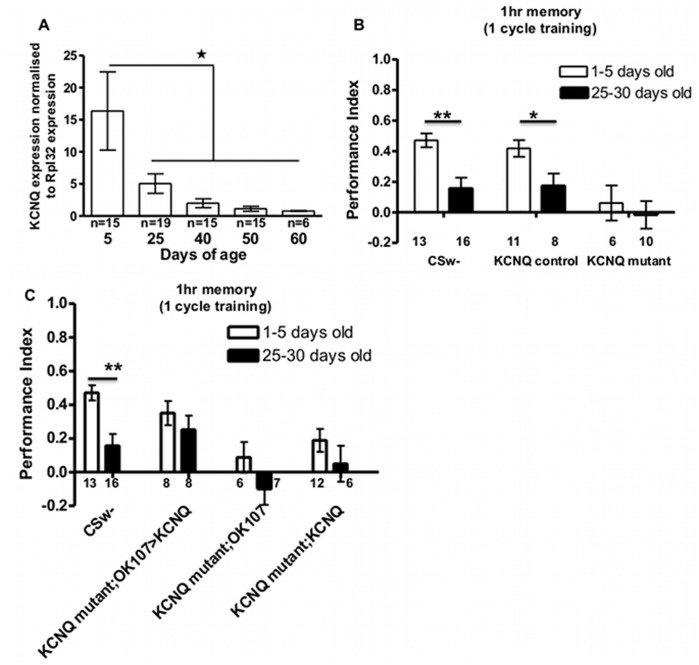
Fly associative memory is known to decrease with age [28] and KCNQ expression decreases in old fly hearts leading to age related cardiac impairments [13]. Therefore we decided to test whether or not KCNQ was involved in age dependent memory decline. We first determined KCNQ expression in heads over the lifespan of Drosophila (∼50 days, [28]) using quantitative RT-PCR. We found that by 25 days KCNQ expression had declined to about 10% of the level of 5 day olds (Figure 3A). Therefore, we tested STM of young (1–5 day old) as opposed to aged (25–30 day old) flies. Whereas control flies displayed an age dependent decrease in 1 hr STM (Figure 3B), KCNQ nulls were completely unable to form STM whether they were young or old. Previous experiments have implicated the amn DPM and mushroom body neurons in mediating the effect of age on 1 hr memory [28]–[30], with expression of a PKA transgene in mushroom body neurons restoring age-related memory impairment. We therefore overexpressed KCNQ in the mushroom body (Figure 3C) of KCNQ mutants and demonstrate rescue of age-dependent memory impairment. These experiments are consistent with decreases in KCNQ signalling being central forage dependent decrements in memory. This experiment also confirms that in young flies expression of KCNQ using mushroom body Gal4 lines (Figure 1F and 3C) in a fly otherwise completely lacking KCNQ rescues the KCNQ mutant STM defect to normal.
Figure 3. KCNQ mediates age-related memory impairment.
A. Quantitative RT-PCR data show a dramatic age dependent reduction (p<0.05) in KCNQ expression in adult brains (20 flies per n). B. 1 hr memory after 1 cycle training was compared between young (1–5 days old, white bars) and aged (25–30 days, black bars) adults. 2-way ANOVA indicates significant differences in memory due to age (p = 0.0013) and genotype (p = 0.0008). Post-hoc analysis revealed that memory becomes significantly impaired in aged as opposed to young CSw- wildtype (p<0.01) and KCNQ control (p<0.05) flies. KCNQ mutant flies had equally low (p>0.05) memory whether young or old. C. Overexpression of KCNQ in the mushroom body rescues memory impairment of young and old KCNQ mutant flies. 2-way ANOVA indicates significant differences in memory due to age (p<0.01) and genotype (p<0.001). Post-hoc analysis revealed that memory becomes significantly impaired in aged as opposed to young CSw- wildtype (p<0.01), while the memory of KCNQ mutant; OK107-Gal4, uas-KCNQ rescue flies stays similarly high (p>0.05) in young and old flies as opposed to KCNQ mutant with Gal4 or uas alone (KCNQ mutant; OK107-Gal4orKCNQ mutant; uas-KCNQ) whose memory was similarly low in young and old flies (p>0.05).
Ethanol Disrupts Memory an Effect Mimicked by KCNQ Mutation
Previous work has shown that many of the molecules and plasticity mechanisms underlying changes in ethanol behaviour and addiction are shared with those of associative memory with ethanol known to disrupt synaptic plasticity and memory in humans [29], [30]. Furthermore ethanol has been demonstrated to directly inhibit the M-current in dopaminergic neurons of the ventral tegmental area (VTA) a region of the brain important for ethanol reinforcement [26], [31]. Likewise it has been shown that dKCNQ shows conserved blockade by ethanol, with reduction in KCNQ function causing increased ethanol sensitivity and tolerance via changes in dopamine neurons [15]. Consequently we investigated whether or not ethanol disrupted fly memory. Wildtype flies were exposed to 10% ethanol solution for ∼12 hr and then immediately tested for 2 min memory (Figure 4A), although the flies did not appear sedated or intoxicated after the exposure or during the memory test, ethanol was found to reduce their memory. As KCNQ maybe a direct target of ethanol that is required for memory, we tested the KCNQ mutant and found that this resulted in a loss of the reduction in memory. This suggests that KCNQ is the plasticity molecule blocked by ethanol interfering with memory. No change in ethanol content was found between genotypes and was ∼10 mM at the time of the memory test (Figure 4B), this would be sufficient to block a significant proportion of neural KCNQ channels (IC50 = 19.8 mM, [15]).
Figure 4. Ethanol disrupts memory in wildtype flies an effect removed by the KCNQ mutation.
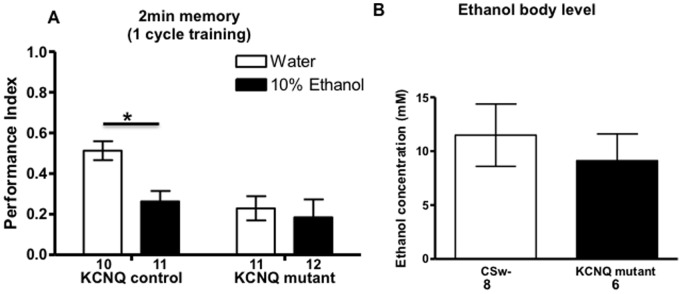
A. Flies received an overnight (∼12 hr) exposure to 10% ethanol and were tested for 2 min memory. 2-way ANOVA showed a significant effect due to genotype and ethanol (p<0.05). Post-hoc analysis showed that in KCNQ control ethanol caused a reduction (p<0.05) in memory compared to water. The reduction in memory was removed by the KCNQ mutation that had similarly low memory with or without ethanol (p>0.05). B. Ethanol content of KCNQ mutant and control (CSw- wildtype) flies exposed to 10% ethanol solution for ∼12 hr was similar (p>0.05, unpaired t-test, 20 flies per n).
Discussion
Drosophila KCNQ displays conserved electrophysiological and pharmacological properties with mammalian neuronal KCNQ2/3 channels, with both mediating a slowly activating and non-inactivating Kv current called a M-current due to its suppression by muscarinic acetylcholine receptors [12], [14]. In the hippocampus, expression of a dominant negative human KCNQ2 transgene was found to suppress the M-current [7]. This resulted in a decrease in the afterhyperpolarization (after a train of action potentials, the increase in Ca2+ activates K+ channels leading to a pronounced hyperpolarization) and caused deficits in associative memory. Kv channels have also been implicated in plasticity underlying fly memory [32]–[35] consistent with the conspicuous expression of Kv currents in mushroom body neurons [34], [36], [37]. We have extended these studies by showing dKCNQ has a role in immediate memory, STM and LTM without peripheral defects. Targeted reduction of KCNQ in the mushroom body α/β neurons was sufficient to reduce memory, while expressing KCNQ in the same neurons in a fly otherwise completely lacking KCNQ rescued the KCNQ mutant memory phenotype to normal (Figure 1F). These results show KCNQ is required in the mushroom body α/β neurons in order for the fly to form STM. We found that the KCNQ memory phenotype resulted from reduced KCNQ function in the adult mushroom body (Figure 1G), showing that the role of KCNQ in formation is an acute physiological one as opposed to a developmental one. Mushroom body and DPM neurons have been suggested to respond to acetylcholine [27], [38]–[42] and muscarinic acetylcholine receptor activation is known to suppress the dKCNQ current [12], [14]. Therefore cholinergic stimulation would be expected to close dKCNQ channels causing depolarization and increasing firing and/or release from the DPM and mushroom body neurons. This might be expected to result in induction of plasticity and strengthening of recurrent activity in the DPM-mushroom body loop that could potentially consolidate memory. In addition the DPM neurons are thought to be serotonergic with serotonin playing an important role in the DPM mediated memory [43], interestingly serotonin has be shown inhibit KCNQ currents in mammalian neurons [44], [45], it is not known if this is also true for KCNQ currents in Drosophila neurons.
The effect of KCNQ mutation on LTM maybe through disruption of appropriate changes in resting membrane potential of memory neurons, which are required to remove the Mg2+ block of Drosophila NMDA glutamate receptors that is necessary for LTM and CREB-dependent gene expression [46].
On the basis of our data, high ethanol levels would be expected to cause significant or complete KCNQ blockade [15], disrupting synaptic plasticity and memory formation, thereby leading to alcohol induced amnesia or blackout. Consistent with this proposition we found that ethanol disrupts fly memory, an effect that was removed in the KCNQ mutant background (Figure 4A). This suggests that KCNQ is a key molecule that ethanol interacts with in the plasticity machinery involved in memory. Given the conserved role of mammalian KCNQ in ethanol response and memory [15], we suggest that it is likely this will also be the case for mammals.
Fly memory is known to reduce with age [28], as does KCNQ expression and function in the heart [13]. We found that KCNQ brain expression dramatically decreases with age and this is accompanied by an age-dependent decrease in memory (Figure 3A). Young KCNQ mutant flies that have low levels of KCNQ comparable to the low levels of KCNQ in aged wildtype flies have comparably reduced levels of memory. As mushroom body neurons are known to be important for mediating changes in memory performance with age [28], [29] and mushroom body knockdown of KCNQ completely removed 1 hr memory (Figure 1E), we overexpressed a KCNQ transgene in the mushroom body (Figure 3C) of a fly otherwise completely lacking KCNQ and found that this restored age-related memory impairment with young and old flies having similarly high memory. In summary we show reduction in KCNQ function in mushroom body neurons mediates age-dependent cognitive decline.
In mammals, KCNQ specific modulators have been suggested to alleviate memory deficits associated with age related memory diseases such as Alzheimer’s disease [2]. It is not clear how KCNQ-mediated mechanisms may affect memory in aged animals. However, based on the contribution of KCNQ2/3 to hippocampal afterhyperpolarizations and memory [7], one candidate mechanism would involve reduced neuronal KCNQ, as this modulates afterhyperpolarization duration that is known to change with memory and in aged animals [47]–[49]. Recently, KCNQ channels have been implicated in age-dependent decrements in the memory of primates [50], suggesting that KCNQ function in cognitive impairments accompanying aging are likely conserved from flies to humans. Furthermore, PKA signalling has been implicated in age-related memory impairment in flies and mammals [28], [29], [50], [51] with the mammalian KCNQ channel open state being increased by PKA [50], [52], suggesting that the mushroom body neuron KCNQ-mediated memory and age-dependent memory defects maybe due to an interaction with PKA.
The genetic and experimental tractability of Drosophila combined with its ∼50 day lifespan and molecular conservation with human make it a convenient and powerful genetic model [28] to study further the age-dependent KCNQ cognitive deficits. We have shown that KCNQ neuronal function in memory and ethanol response are evolutionarily conserved with mammals, allowing further development of Drosophila models of KCNQ neuronal function and channelopathies to elucidate KCNQ signalling networks, mechanisms of aging and potential screening for new disease therapies.
Supporting Information
A. KCNQ channel mutants display normal olfactory acuity and shock reactivity. Experimental and control (CSw- wildtype or KCNQ control) flies similarly (p>0.05) avoided OCT A. and MCH B. odour used in the memory assay. C. Experimental and control (CSw- wildtype or KCNQ control) flies similarly (p>0.05) avoided the arm of the T-maze delivering 60 V DC electric shock. Data in A-C were analysed by 1-way ANOVA with Bonferroni post-hoc test.
(TIFF)
Acknowledgments
We thank Dr Rolf Bodmer, Leslie Griffith, Scott Waddell and Yi Zhong for flies. Additional stocks were from the Bloomington, Kyoto and Vienna stock centers. We are grateful to Dr Vivian Budnik for rabbit anti-Drosophila DLG (PDZ1-2). Confocal microscopy was performed in the Bristol University Wolfson bioimaging facility. We acknowledge Dr Jon Brown, Jules Hancox, Leslie Griffith, Neil Marrion, and Ralf Stanewsky for comments on the manuscript and Dr Stephen Henderson and Emma Robinson for advice on statistics.
Funding Statement
This work was supported by grants from the EU FP7 Marie-Curie (IRG200632), Royal Society (2008/R1), BBSRC (BB/G008973/1), and from a Wellcome PhD program (083361). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ashcroft FM (2006) From molecule to malady. Nature 440: 440–7. [DOI] [PubMed] [Google Scholar]
- 2. Wulff H, Castle NA, Pardo LA (2009) Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Disc 8: 982–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unoki H, Takahasi A, Kawaguchi T, Hara K, Horikoshi M, et al. (2008) SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 40: 1098–102. [DOI] [PubMed] [Google Scholar]
- 4. Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, et al. (2008) Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 40: 1092–1097. [DOI] [PubMed] [Google Scholar]
- 5. Delmas P, Brown DA (2005) Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6: 850–862. [DOI] [PubMed] [Google Scholar]
- 6. Huang H, Trussell LO (2011) KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci 4: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D (2005) Conditional transgenic suppression of M channel in mouse brain reveals functions in neuronal excitability, resonance and behaviour. Nat Neurosci 8: 51–60. [DOI] [PubMed] [Google Scholar]
- 8. Koyama S, Brodie MS, Appel SB (2007) Ethanol inhibition of M-current and ethanol induced direct excitation of Ventral Tegmental Area dopamine neurons. J Neurophysiol 97: 1977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El Amraoui A, et al. (1999) KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96: 437–446. [DOI] [PubMed] [Google Scholar]
- 10. Van Eyken E, Van Laer L, Fransen E, Topsakal V, Lemkens N, et al. (2006) KCNQ4: a gene for age-related hearing impairment? Human Mutation 27: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 11. Littleton JT, Ganetzky B (2000) Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron 26: 35–43. [DOI] [PubMed] [Google Scholar]
- 12. Wen H, Weiger TM, Ferguson TS, Shahidullah M, Scott SS, et al. (2005) A Drosophila KCNQ channel essential for early embryonic development. J Neurosci 25: 10147–10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, et al. (2007) KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci USA104: 3943–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cavaliere S, Hodge JJ (2011) Drosophila KCNQ channel displays evolutionarily conserved electrophysiology and pharmacology with mammalian KCNQ channels. PLoS One 6: e23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cavaliere S, Gillespie JM, Hodge JJ (2012) KCNQ channels show conserved ethanol block and function in ethanol behaviour. PLoS One 6: e23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, et al. (1996) Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science 274: 2104–2107. [DOI] [PubMed] [Google Scholar]
- 17. Shuai Y, Lu B, Hu Y, Wang L, Sun K, et al. (2010) Forgetting is regulated through Rac activity in Drosophila . Cell 140: 579–589. [DOI] [PubMed] [Google Scholar]
- 18. Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S (2007) Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 53: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zars T, Fischer M, Schulz R, Heisenberg M (2000) Localization of a short-term memory in Drosophila . Science 288: 672–675. [DOI] [PubMed] [Google Scholar]
- 20. Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG (2000) The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 103: 805–811. [DOI] [PubMed] [Google Scholar]
- 21. Hodge JJ, Mullasseril P, Griffith LC (2006) Activity-dependent gating of CaMKII autonomous activity by Drosophila CASK. Neuron 51: 327–337. [DOI] [PubMed] [Google Scholar]
- 22. Ruiz-Cañada C, Koh YH, Budnik V, Tejedor FJ (2002) DLG differentially localizes Shaker K+-channels in the central nervous system and retina of Drosophila . J Neurochem 82: 1490–1501. [DOI] [PubMed] [Google Scholar]
- 23. Tully T, Quinn WG (1985) Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 157: 263–277. [DOI] [PubMed] [Google Scholar]
- 24. Dura JM, Preat T, Tully T (1993) Identification of linotte, a new gene affecting learning and memory in Drosophila melanogaster . J Neurogenet 9: 1–14. [DOI] [PubMed] [Google Scholar]
- 25. King I, Tsai LTY, Pflanz R, Voigt A, Lee S, et al. (2011) Drosophila tao controls mushroom body development and ethanol-stimulated behaviour through par-1 . J Neurosci 31: 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U (2011) A Drosophila model of alcohol reward. Nat Neuro14: 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, et al. (2004) Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron 44: 521–533. [DOI] [PubMed] [Google Scholar]
- 28. Tamura T, Chiang AS, Ito N, Liu HP, Horiuchi J, et al. (2003) Aging specifically impairs amnesiac-dependent in Drosophila . Neuron 40: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 29. Yamazaki D, Horiuchi J, Miyashita T, Saitoe M (2010) Acute inhibition of PKA activity at old ages ameliorates age-related memory impairment in Drosophila . J Neurosci 30: 15573–15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tonoki A, Davis RL (2012) Aging impairs intermediate-term behavioural memory by disrupting the dorsal paired medial neuron memory trace. Proc Natl Acad Sci USA 109: 6319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spanagel R (2009) Alcoholism: A systems approach from molecular physiology to addictive behaviour. Physiol Rev 89: 649–705. [DOI] [PubMed] [Google Scholar]
- 32. Cowan TM, Siegel RW (1986) Drosophila mutations that alter ionic conduction disrupt acquisition and retention of a conditioned odor avoidance response. J Neurogenet 3: 187–201. [DOI] [PubMed] [Google Scholar]
- 33. Griffith LC, Wang J, Zhong Y, Wu F, Greenspan RJ (1994) Calcium/calmodulin dependent protein II and potassium channel subunit eag similarly affect plasticity in Drosophila . Proc Natl Acad Sci USA 91: 10044–10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gasque G, Labarca P, Reynaud E, Darszon A (2005) Shal and shaker differential contribution to the K+ currents in the Drosophila neurons. J Neurosci 25: 2348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gasque G, Labarca P, Delgado R, Darszon A (2006) Bridging behaviour and physiology: ion-channel perspective on mushroom body-dependent olfactory learning and memory in Drosophila . J Cell Physiol 209: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 36. Wright NJ, Zhong Y (1995) Characterization of K+ currents and the cAMP dependent modulation in cultured Drosophila mushroom body neurons identified by lacZ expression. J Neurosci 15: 1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rogero O, Hammerle B, Tejedor FJ (1997) Diverse expression and distribution of Shaker potassium channels during the development of the Drosophila nervous system. J Neurosci 17: 5108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gorczyca MG, Hall JC (1987) Immunohistochemical localization of choline acetyltransferase during development and in Chats mutants of Drosophila melanogaster . J Neurosci 7: 1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu D, Baird GS, Tsien RY, Davis RL (2003) Detection of calcium transients in Drosophila mushroom body neurons with camgaroo reporters. J Neurosci 23: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu H, O’Dowd DK (2006) Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ . J Neurosci26: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin JR, Rogers KL, Chagneau C, Brulet P (2007) In vivo bioluminescence imaging of Ca imaging in the brain of Drosophila . PLoS One 2: e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsydzik V, Wright NJ (2009) Dopamine modulation of the in vivo acetylcholine response in the Drosophila mushroom body. Dev Neurobiol69: 705–714. [DOI] [PubMed] [Google Scholar]
- 43. Lee PT, Lin HW, Change YH, Fu TF, Dubnau J, et al. (2011) Serotonin-mushroom body circuit modulating the formation of anesthesia-resistent memory in Drosophila . Proc Natl Acad Sci USA 108: 13794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hansen HH, Waroux O, Seutin V, Jentsch TJ, Aznar S, et al. (2008) Kv7 channels: interaction with dopaminergic and serotonergic neurotransmission in the CNS. J Physiol 586: 1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roepke TA, Smith AW, Ronnekleiv OK, Kelly MJ (2012) Serotonin 5-HT2C receptor-mediated inhibition of the M-current in hypothalamic POMC neurons. Am J Physiol Endocrinol Metab 302: E1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miyashita T, Oda Y, Horiuchi J, Yin JCP, Morimoto T, et al. (2012) Mg2+ block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron 74: 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tombaugh GC, Rowe WB, Rose GM (2005) The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci 25: 2609–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tzingounis A, Nicoll RA (2008) Contribution of KCNQ2 and KCNQ3 to the mediumband slow afterhyperpolarization currents. Proc Natl Acad Sci USA 105: 19974–19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaczorowski CC, Disterhoft JF (2009) Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem 16: 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang M, Gamo NJ, Yang Y, Jin LE, Xang XJ, et al. (2011) Neuronal basis of age-related working memory decline. Nature 476: 210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Dunman RS, et al. (2003) Dysregulation of protein kinase A signalling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron 40: 835–845. [DOI] [PubMed] [Google Scholar]
- 52. George MS, Abbott LF, Siegelbaum SA (2009) Hyperpolarization-activated HCN channels inhibit subthreshold EPSPs through voltage-dependent interactions with M-type K+ channels. Nat Neurosci 12: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. KCNQ channel mutants display normal olfactory acuity and shock reactivity. Experimental and control (CSw- wildtype or KCNQ control) flies similarly (p>0.05) avoided OCT A. and MCH B. odour used in the memory assay. C. Experimental and control (CSw- wildtype or KCNQ control) flies similarly (p>0.05) avoided the arm of the T-maze delivering 60 V DC electric shock. Data in A-C were analysed by 1-way ANOVA with Bonferroni post-hoc test.
(TIFF)