Abstract
The MADS family is an ancient and best-studied transcription factor and plays fundamental roles in almost every developmental process in plants. In the plant evolutionary history, the whole genome duplication (WGD) events are important not only to the plant species evolution, but to expansion of members of the gene families. Soybean as a model legume crop has experience three rounds of WGD events. Members of some MIKCC subfamilies, such as SOC, AGL6, SQUA, SVP, AGL17 and DEF/GLO, were expanded after soybean three rounds of WGD events. And some MIKCC subfamilies, MIKC* and type I MADS families had experienced faster birth-and-death evolution and their traces before the Glycine WGD event were not found. Transposed duplication played important roles in tandem arrangements among the members of different subfamilies. According to the expression profiles of type I and MIKC paralog pair genes, the fates of MIKC paralog gene pairs were subfunctionalization, and the fates of type I MADS paralog gene pairs were nonfunctionalization. 137 out of 163 MADS genes were close to 186 loci within 2 Mb genomic regions associated with seed-relative QTLs, among which 115 genes expressed during the seed development. Although MIKCC genes kept the important and conserved functions of the flower development, most MIKCC genes showed potentially essential roles in the seed development as well as the type I MADS.
Introduction
The MADS family, found in fungi [1], animals [2] and plants [3] [4], possesses a highly conserved N-terminal with a DNA-binding domain named MADS. Based on the phylogenetic analysis, MADS gene family is divided into two large lineages, type I and type II, which was created through a gene duplication occurred before the divergence of plants (and fungi) and animals [5]–[7].
In plant, the typical difference between type II MADS genes and type I MADS genes is that the plant type II, but not type I, has a K-domain [5], [6], [8]. The plant-special type II MADS is also named as MIKC MADS due to their four domains, MADS domain, I domain, K domain, and C domain [8], [9]. and except MADS-domain and K-box domain, I-domain and C-domain are not conserved although they have important functions for the MADS family [9], [10]. MIKC type can be further divided into MIKCC and MIKC* clade, both of which were present in a common ancestor of mosses and vascular plants, suggesting they are an ancestral kind of genes [11]. About 13 subfamilies compose of the MIKCC clade and most of them originate from ancestral seed plants and are often characterized by distinct sequence motifs in their C-terminal domains [5], [12]. And based on the phylogenic tree, AG-, AGL6-, AGL12-, DEF+GLO- (B), GGM13- (B(s)), STMADS11- and TM3-like genes very likely existed already in the most recent common ancestor of angiosperms and gymnosperms and AGL2-, AGL17-, and SQUA-like genes, existed at least already in the most recent common ancestor of monocots and eudicots [5]. MIKC* clade is characterized by an altered protein domain structure, probably evolved from an ancestral MIKCC gene by a duplication in the Keratin-like region and composed of S-clade, P-clade, lycophyte-clade and Bryophyte-clade [7], [13]. Heterogeneous type I MADS can be subgrouped into Mα, Mβ, and Mγ based on the sequence of the MADS domain and the presence of additional motifs [5], [14]–[16].
Since the plant MADS genes, AGAMOUS (AG) from Arabidopsis thaliana [3] and DEFICIENS (DEF) from Antirrhinum majus [4] are first discovered as regulators of floral organ identity, a lot of plant MIKCC genes have been isolated from various plant species and demonstrate their essential roles in almost all developmental processes in plants, such as the control of flower identity, root architecture, gametophyte development, fruit ripening, the regulation of flowering time ([17]–[20]). By contrast, less attention is paid for type I MADS genes. Recent studies indicate a key regulatory role for type I MADS genes in plant specifying female gametophyte, embryo, and endosperm development [20], [21]. MIKC* genes retained a conserved role in the gametophyte during land plant evolution [13]. And five Arabidopsis MIKC* genes (AGL30, AGL65, AGL66, AGL94, and AGL104) are expressed in pollen and regulate pollen development by repressing immature pollen genes and activating mature pollen genes [22], [23].
Extensive duplications in the angiosperms have resulted in the expansion of members of the gene families and gene diversifications. And duplications in plant MADS transcription factors have been studied to understand the origins and evolution of plant developmental mechanisms [24], [25], and the results demonstrated that duplicated genes either swapped roles, acquired novel roles or retained ancestral roles in different plant species [26]. The modern soybean genome has apparently undergone one whole-genome triplication (WGT) and two whole genome duplication (WGD) events (Legume WGD and Glycine WGD), and about 75% of genes have multiple paralogs [27], [28]. Among them, ∼50% of paralogous genes display expression subfunctionalization [29], which may contribute to phenotypic variation in polyploids [30]. In addition to WGD duplication, tandem duplication generates gene copies that are consecutive in the genome and is presumed to arise through unequal chromosomal crossing over [31] and may contribute to the expansion of some large gene families [32]. Dispersed duplicates are neither adjacent to each other in the genome nor within homeologous chromosome segments and may result from transposition events [31], [33]. Such distantly transposed duplications may occur by DNA-based or RNA-based mechanisms [34]. A total of 32,552 retrotransposons (Class I) and 6,029 DNA transposons (Class II) are found in the soybean genome [35]. And transposed duplication may play significant roles in shaping and reshaping of their host genomes regulating gene expression, altering gene function, and creating new genes [36], [37].
MADS families are identified and classified in many flowering plants such as Arabidopsis [14], petunia [38], tomato [39], poplar [40], rice [41], grapevine [42], maize and sorghum [43], cucumber [44]. But only genome-wide expression profiles of all the MADS genes have been reported in Arabidopsis and rice [14], [15], [41], and a comprehensive plant protein-protein interactome map of nearly all members of the Arabidopsis MADS family has been constructed to investigate the essential roles of Arabidopsis biological processes [45]. Expression profiles of MIKCC genes of conserved known biological functions are systematically analyzed in some plants [39], [42].These accomplishments benefit us to comprehensively understand the plant MADS family and their functions in plant development.
Soybean is one of the most important crop plants for seed protein and oil content, which are associated with the seed development, and genetic control of agronomic traits, such as seed constituents and yield, is inherited in a quantitative manner. Based on the soybase database (http://www.soybase.org) [46], about 2200 soybean quantitative trait loci (QTLs) were associated with about 134 soybean agronomic traits, among which about 1000 QTLs were relative to the seed or pod development. Furthermore, the soybean genome has been completely sequenced [47]. Therefore, QTLs can be mapped to the genome and provide the primary insight for understanding potential functions of co-localized genes in the soybean development.
The MADS family has obviously functional roles in the plant development. Soybean MADS genes and the characters of their evolution in the soybean genome evolution process were identified through the bioinformatic analysis. And based on the genome-wide expression patterns through in silico expressions and RT-qPCR, more attentions were paid to soybean MADS expressions in the seed development. In addition, co-localizations of MADS genes with QTLs relative to seed traits were investigated. According to our results, not only did type I MADS highly express in the seed development [21], [48], [49], but also MIKCC genes had important functions in the seed development, except the conserved and best-studied function of the flower development.
Results
Identification, Motif, Chromosome Location and Gene Structure of GmMADS Genes
In total, 163 MADS genes were obtained from soybean genome (G.max v1.0) through HMMER v3.0 based on the HMM model of SRF-type transcription factor (PF00319) and named as GmMADS1 through GmMADS163 (Table S1). These MADS genes can be phylogenetically classed into several subgroups as Arabidopsis MADS does [5] (Figure S1). These subgroups were type II (MIKCC (81 gnes) and MIKC* (7 genes)) and type I (Mα (36 genes), Mβ (14 genes) and Mγ (24 genes)). And the soybean MIKCC genes contained the MADS domains and K-box domains and were composed of 12 subfamilies: FLC (2 genes), SOC (TM3-like, 8 genes), AG (10 genes), AGL6 (6 genes), SEP (AGL2-like, 12 genes), SVP (STMADS11-like, 8 genes), AGL12 (2 genes), AGL15 (2 genes), AGL17 (8 genes), DEF/GLO (11 genes), SQUA (10 genes), and ABS (GGM13- (B(s))-like, 2 genes), and one ancient clade known in some flowering plant species, TM8 subfamily [5], was not found in the soybean. MIKC* has 2 subfamilies, MIKC*-S (4 genes) and –P (3 genes) according to Kwantes, et al. [13].
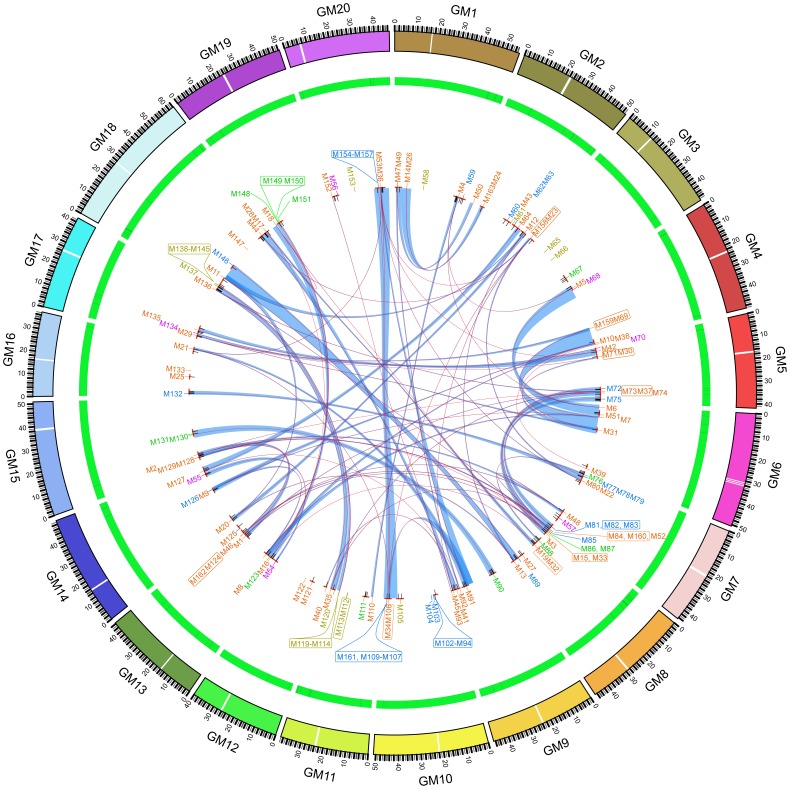
GmMADS genes were distributed on 20 chromosomes, especially in GM8 (18 MADS genes), GM10 (16 MADS genes) and GM18 (18 MADS genes) (Figure 1). And according to the characters of MADS gene distributions, the location of MIKC and Mγ genes were not biased in the chromosomes, but Mα genes mainly located in the end of GM10 through tandem or proximal duplicates, as well as Mβ genes in GM11 and GM18.
Figure 1. The soybean MADS gene family.
The gene names of MIKCc, MIKC*, Mα, Mβ and Mγ were abbreviated as M1 to M163 and were in orange, purple, blue, green and yellow, respectively, and short lines in corresponding color in the red blocks showed their locations in the soybean genome (Table S1). The short black lines in the green arcs showed the markers associated with non-seed QTLs and those in the red short blocks showed the markers associated with seed QTL (Table S4). The red blocks showed regions of QTLs relative to the seed traits according to the markers (Table S4). The light blue rainbows showed collinear relationships among the blocks containing MADS genes according to the MCScanX results (Table S3) and the red curves showed the paralogs. Twenty chromosomes (GM1-20) were in different colors and the size of the arc showed the size of chromosome (Mb). The figure was created through the software Circos (http://circos.ca/).
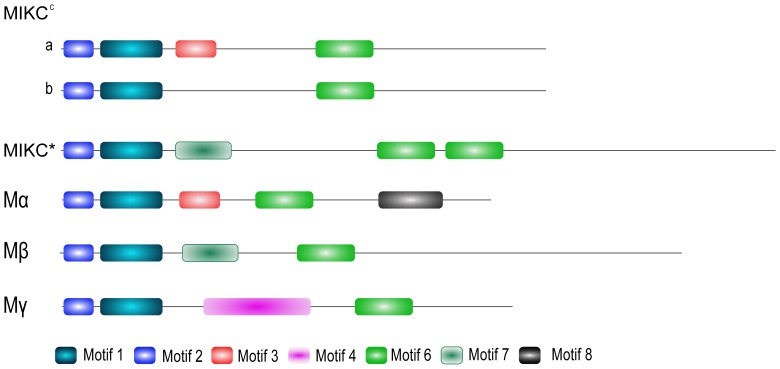
Based on the motif organization of 163 soybean MADS proteins, all soybean MADS proteins almost had three motifs, motif 1, 2 and 6 (Figure 2 and Figure S2). And motif 1 (29 aa, Table S2) and 2 (14 aa, Table S2) is localized in the MADS-box domain, while motif 6 (27 aa, Table S2) in the K-box domain. That indicated motif 1, 2 and 6 were very important to the function of the MADS family. Besides motif 1, 2 and 6, some subfamilies of MIKCC, MIKC*, Mα, Mβ and Mγ had their own typical motifs (Figure 2). Some members of subfamilies of MIKCC, such as AG (GmMADS1, 2, 3, 11, 13, 20, 39 and 74), SOC (GmMADS44, 45, 53, 80, 106, 135 and 158), SVP (GmMADS48-52, 124, 128 and 162), AGL17 (GmMADS46, 64, 92, 129 and 160), AGL6 (GmMADS22, 23 and 91), AGL15 (GmMADS43) and SQUA (GmMADS32), contained motif 3, which was localized in I-domain. In addition, most Mα members contained motif 3 and motif 8. Members of the MIKC* and Mβ family shared motif 7. Furthermore, MIKC* proteins had the double motif 6, whereas members of the Mγ family had motif 4.
Figure 2. Schematic diagrams of motif organizations.
MIKCC can be grouped into two type: a and b (Figure S2). The average length of members of each five families was as the length of a family. Motif 1 and 2 are equivalent to the MADS-box domain (PF00319), and motif 3 and motif 6 is the part of the I-domain and K-box domain (PF01486) for type II MADS proteins, respectively. Other motifs was unknown. The detailed information of soybean MADS motif organizations referred to Figure S2.
In term of the gene structure, the fist exon (about 180 bp) conservatively coded the MADS domain in the MADS genes with more than one exons (Table S1). 7 MIKC* genes had 9–11 exons, and covered about 4.7 kb in length in the genome. For the MIKCC genes, the number of exons were 4–9, and average number was about 7, and covered about average about 7.4 kb in length from 0.75 kb to 18.6 kb, and genome sequences of about 82.7% MIKCC genes were longer than 5 kb. About 44/75 type I MADS genes had only one exon and 12/75 type I MADS genes had more than 4 exons, and the average exon number of Mα, Mβ, and Mγ genes was 1.6, 2.4, and 2.1 respectively, and the average genome sequences were about 0.6, 1.2, and 0.8 kb respectively.
Different Expansion Patterns of Two Types of MADS within the Soybean Genome
To investigate GmMADS gene evolution in soybean, the syntenic relationships among G.max, M.truncatula, experiencing the WGT and Legume WGD events [50] and V.vinifera, experiencing the WGT event [51], were computed through MCScanX [52]. Based on our results, the blocks containing some MIKC genes, which belonged to SOC, AG, SQUA, AGL6, SEP, SVP, DEF/GLO and MIKC*, can be found the corresponding homologous blocks in M.truncatula and/or V.vinifera genome (Table S3). But for the type I genes, the inter-species syntenic relationships can hardly been found. That indicated gene orders of the blocks containing MIKC genes were more conserved than that containing type I MADS genes during the soybean evolution process.
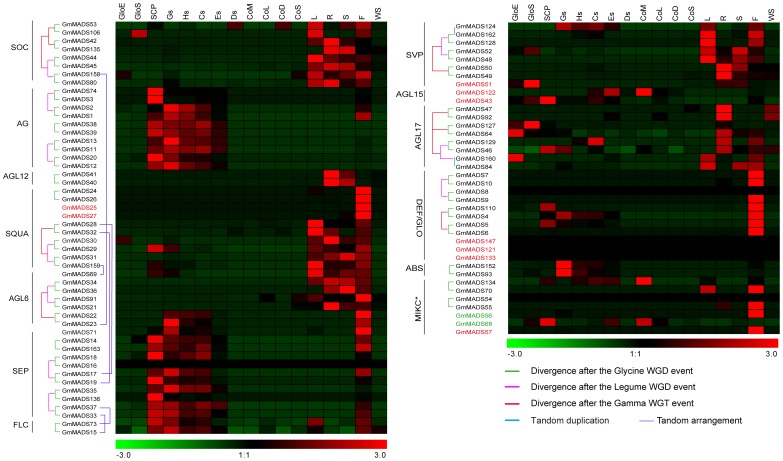
WGD events expanded the members of the MIKC family, and blocks containing about 85% (75/88) MIKC genes experienced WGD events (Figure 1, 3 and Table S3). Based on syntenic blocks, all the genes of subfamily AG (10 genes), AGL12 (2 genes), ABS (2 genes), FLC (2 genes), and SOC (8 genes) were originated from 3, 1, 1, 1, and 1 different ancestor sites before the Gamma WGT events respectively. In addition to the WGD duplication, other gene duplication patterns were found in some MIKCC subfamilies. In SQUA subfamily, six paralog genes (GmMADS28/32, 29/30, and 31/159) were the resultants of a common ancestor sites experiencing three WGD events, and one paralog gene pair (GmMADS24/26) were diverged after the Glycine WGD event, while GmMADS25 and GmMADS27 were the results of the transposed duplication (Table S3–4). Seven members of AGL17 subfamily were originated from an ancestor sites before the Gamma WGT events, and GmMADS84 was tandem connection to GmMADS160. In SVP subfamily, six paralog genes were derivates of a common ancestor, while GmMADS124 and 162 through the tandem duplication and a dispersed gene (GmMADS51) through the transposed duplication (Table S3–4). Eight DEF/GLO genes were originated from two different ancestor sites, and the transposable elements occurred at the up/dwonstrean of GmMADS121, 133, and 147, which were dispersed in the genome (Table S-3). The origins of 10/11 SEP genes were 3 different ancestor sites before the Gamma WGT events, and a transposed duplication member, GmMADS71, was proximal to GmMADS23 (AGL6) (Table S3–4). Three paralog gene pairs of the AGL6 subfamily GmMADS21/91, GmMADS22/23 and GmMADS34/36 were from the common ancestor site before three rounds of WGD events, but GmMADS69 located among two transposable elements had the tandem relationship with GmMADS159 (SQUA) (Table S3–4).
Figure 3. In silico expression profiles and the evolutional pattern of soybean MIKC genes.
The RNA-seq relative expression data of 17 tissues was used to re-construct expression patterns of MIKC genes. 3 samples from soybean seed compartments: GloE (Globular stage embryo proper), SCP (Early maturation seed coat parenchyma) and GloS (Globular stage suspensor); 10 soybean tissues samples: Gs (Globular Stage Seed), Hs (Heart Stage Seed), Cs (Cotyledon Stage Seed), Es (Early Maturation Stage Seed), Ds (Dry Seed), R (Root), S (Stem), L (Trifoliate leave), F (Floral bud), and WS (Whole seedling six days after imbibition); 4 soybean cotyledon development samples: CoM (Mid-maturation cotyledon), CoL (Late-maturation cotyledon), CoD (Dry seed) and CoS (Seedling cotyledon). The raw data was downloaded from the website http://seedgenenetwork.net/presentation. Gene names in red showed dispersed duplicate, in blue showed proximal duplicate, and in green its paralog genes were lost during evolution. The lines showed the blocks containing the corresponding MADS genes experienced the WGD events, and the evolution models of the blocks were displayed in Figure 1 and S5. The raw relative expressions of 163 MADS genes were in the Table S6.
Only AGL15 subfamily had not any paralog genes and was composed of two dispersed genes, GmMADS122 (GM12) and GmMADS43 (GM2), which were located among two transposable elements (Table S4). In the syntenic blocks, some MIKCC genes of different families showed the tandem relationship. For example, paralog SEP gene pairs GmMADS33/37 and GmMADS17/19 displayed tandem relationship with GmMADS15/73 (FLC subfamily) and GmMADS28/32 (SQUA subfamily) respectively (Figure 1). That may result from the transposable duplications (Table S4).
For MIKC* gene family, the members of MIKC*-S (GmMADS54/55 and GmMADS68) and –P (GmMADS70/134 and GmMADS56) experienced the same evolution processes. The paralog pair genes were diverged after the Glycine WGD event (Figure 1, 3 and Table S3). And GmMADS56 and GmMADS68 did not have the corresponding gene pairs in the soybean syntenic blocks, but their homologous blocks and collinear pairs were found in Medicago (two MIKC*genes, MtMADS52 and MtMADS23 respectively) (Table S3), suggesting that their collinear genes in their soybean syntenic blocks were not retentive after the Legume WGD events.
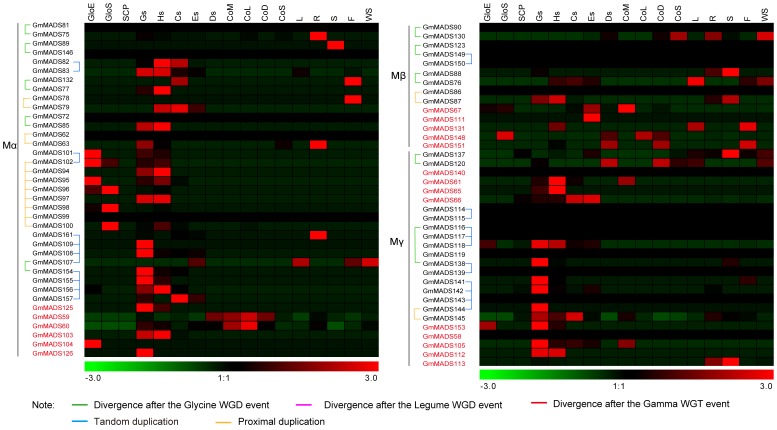
Multiple duplications made the number of the type I GmMADS expansion. 5 Mα, 3 Mβ and 2 Mγ paralog gene pairs were diverged only after the Glycine WGD event (Figure 1, 4 and Table S3). And 10 Mα, 1 Mβ and 8 Mγ genes were generated through tandem duplications, and 11 Mα, 2 Mβ and 1 Mγ genes were generated through proximal duplications, and 6 Mα, 9 Mβ and 15 Mγ genes were dispersed through the transposed duplication in the genome (Figure 1, 4 and Table S4). That suggested that type I genes had experienced a higher rate of birth-and-death evolution than type II genes.
Figure 4. In silico expression profiles and the evolutional pattern of soybean type I MADS genes.
Notes as Figure 3.
Soybean MADS Genes Co-localized with QTLs for the Seed-relative Features
Based on the soybean QTL database (http://www.soybase.org/search/qtllist.php), about 269 loci associated with 807 QTLs for 112 traits were found within 2-Mb genomic regions surrounding 148 soybean MADS genes (Figure 1 and Table S4). And 186 out of 269 loci were associated with 372 QTLs for 59 seed-relative traits, containing 295 QTLs relative to seed traits (constituent or size), 32 to pod maturity date, one to R3 beginning pod, one to R8 full maturity and 43 to the yield (Table S5). And 137 MADS genes (all members of Mα, Mβ and MIKC* family and 67 MIKCC genes and 12 Mγ genes) localized in 2-Mb genomic regions near to 186 loci associated with the seed-relative QTLs (Figure 1 and Table S5).
In addition, according to the in silico transcriptome (Figure 3, 4 and Table S6), transcriptions of 129 soybean MADS genes were detected in the seed tissues. However, 11/129 genes (GmMADS14, 26, 50, 65, 66, 69, 112, 113, 122, 153 and 159) were not co-localized with any QTLs, and 3/129 genes (GmMADS6, 18 and 110) were not with QTLs relative to the seed traits (Table S5). That indicated 115 MADS genes may be involved in the seed development.
Soybean MADS Genes Showing Highly Expression in the Seed Development through in silico Transcriptome
For the whole expression analyses of the soybean MADS family, the RPKM method was employed to correct biases in total gene exon size and to normalize for the total short read sequences obtained in 17 tissue libraries [53], [54]. And then relative RPKM values represented the relative expression of each MADS genes (Figure 3, 4 and Table S6). From the online database, 25 GmMADS genes (19 for type I and 6 for type II), were undetectable at the transcription level in all 17 tissues; 29 genes (22 for Mα, 1 for Mβ and 6 for Mγ, were detected only in seed tissues; 9 genes, (5 for MIKCc, 2 for MIKC*, and 2 for Mα, were detected only in non-seed tissues; 37 genes (34 for MIKC and 4 for type I), had no biased tissues and wide expression with fluctuant levels.
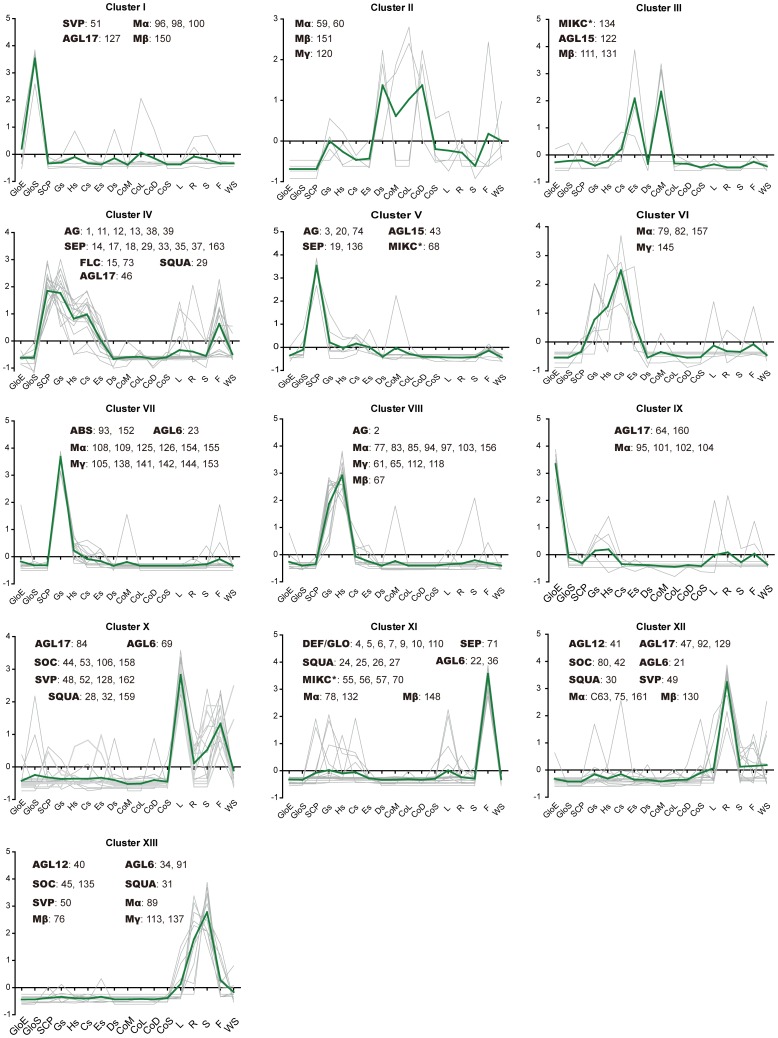
A hierarchical clustering analysis of transcription profiles in 17 tissues based on a Pearson correlation displayed 13 clusters for 138 GmMADS genes (Figure 5 and S3). Most of them (78 genes, 43 for type I and 35 for MIKC) highly expressed in the seed tissues and fell into Cluster I to IX. The genes in different clusters had their own abundant transcripts in different tissues, such as Cluster I and IX in suspensors and embryos at globular stage, respectively, both Cluster IV and V in seed coat parenchymas at the early stage of seed maturation, Cluster VII in seeds at the globular embryo stage, Cluster VIII in seeds at both the globular and heart embryo stages, both Cluster IV and Cluster VI at the seed developing stages, Cluster II in developing cotyledons or in dry seeds, and Cluster III in seeds at the early stage of seed maturation.
Figure 5. Expression cluster analysis based on in silico expression of 138 MADS genes.
The samples were designed as Figure 3. The gray line shows expression profiles of each genes, and the green line is the average expression and indicates the expression pattern of one cluster. For simplicity, gene names display the corresponding code numbers of every subfamily (Figure S1 and Table S6).
There were 36 GmMADS genes of Cluster X and XI highly expressed in flowers. And Cluster X genes also highly expressed in leaves. Cluster XII expressed mainly in roots, while Cluster XIII in both roots and stems (Figure 5 and S3).
By and large, the expression level of type I GmMADS genes was much lower than that of MIKC, and they were mainly in the seeds as the previous report in other species [49]. The function of MIKC genes is famous as regulators in the floral organ development, but our analysis showed that high expression of some MIKC genes were detected in roots, stems, leaves, and seeds besides flowers in soybean (Figure 5), indicating that soybean MIKC genes had extensive functions in developmental progresses.
Expression Profiles of GmMADS Genes by RT-qPCR
To confirm the expression profiles above, Real-time quantitative PCR (RT-qPCR) was employed to evaluate the transcripts of 96 genes in different tissues at different stages. Transcriptions of 83 MADS genes were consistent with the transcription profiles above (Figure S3 and S4) in most cases, but the expressions of GmMADS4, 48, 52, 55, 56, 57, 70, 93, 96, 112 and 126 were undetectable in all the 12 samples, and GmMADS8 and 16 can be detected in the flowers.
GmMADS Gene Expression Peaks in the Soybean Seeds
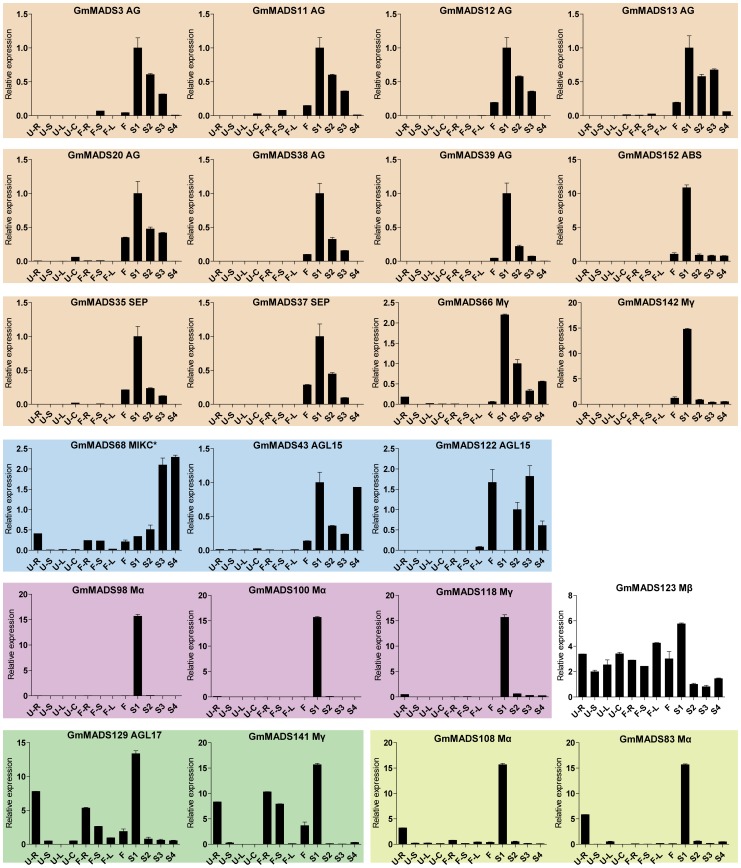
Some GmMADS genes strongly accumulated in the seed in different seed developmental stages (Figure 6). One expression tendency of them was that the transcription occurred at high level in the seed at the early stage, and then progressively down-regulated along with seed development; their transcripts were detected at very low level in the flowers and hardly in the dry seeds. Such a kind of genes included 7 AG genes (GmMADS3, 11, 12, 13, 20, 38, and 39), 2 SEP genes (GmMADS35 and 37), one ABS (GmMADS152) and 2 Mγ gene (GmMADS66 and 142). And 2 Mα genes (GmMADS98 and 100) and 1 Mγ (GmMADS118) strongly expressed only in the early seeds and barely in the flowers and other tissues. During the seed maturation process, AGL15 (GmMADS43 and 122) expressed in relatively high level and the transcriptions of GmMADS68 (MIKC*-S) increased with the seed maturation. Expression peaks of two Mα genes, GmMADS83 and 108, occurred both in seed at the early stage of the seed development and in the root at the seedling stage, and except the roots at the seedling stage, GmMADS129 (AGL17), and GmMADS141 (Mγ) strongly expressed in the roots at the flowering stage. The high expressions of GmMADS123 (Mβ) were not only in the early seeds, but in other tissues (Figure 6).
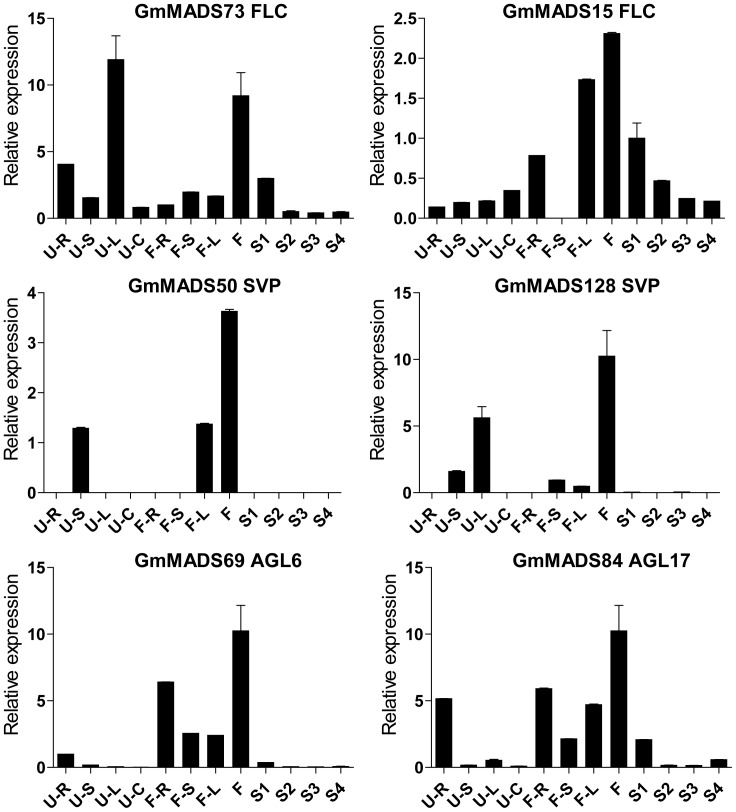
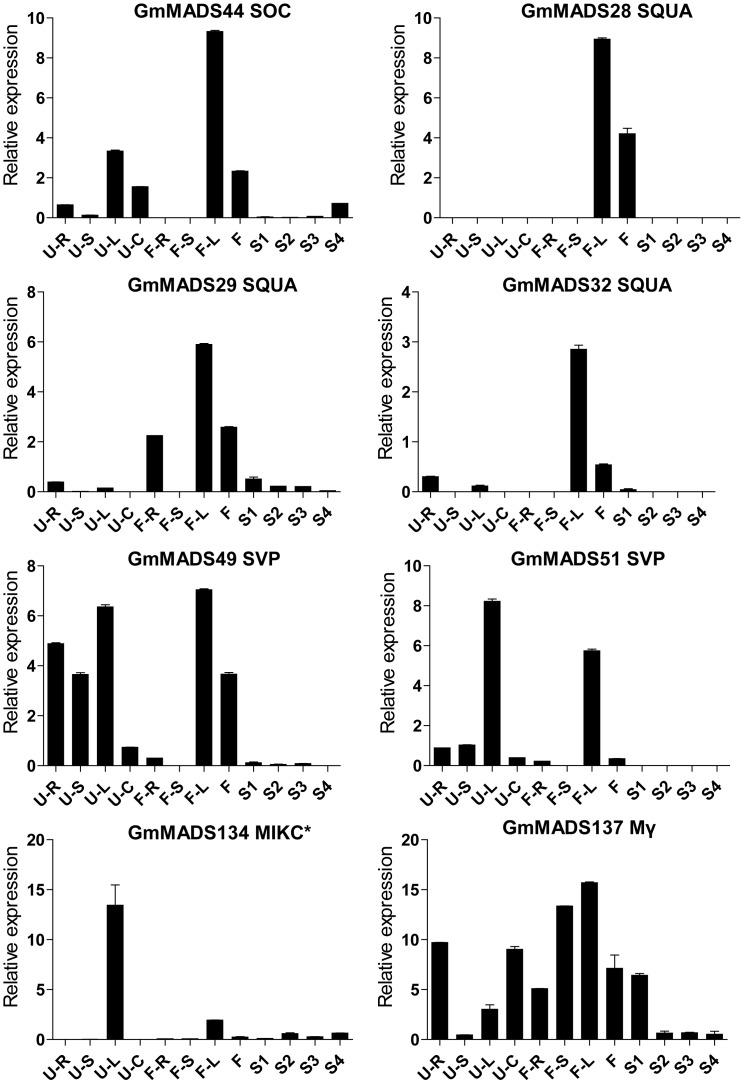
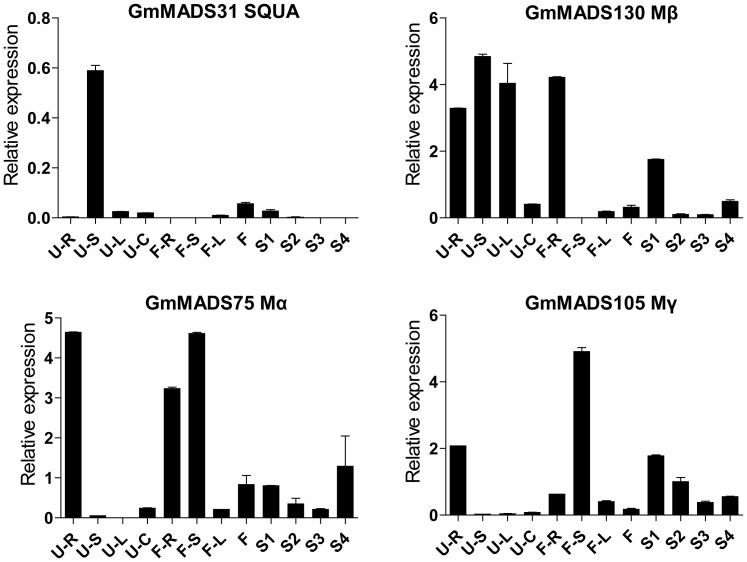
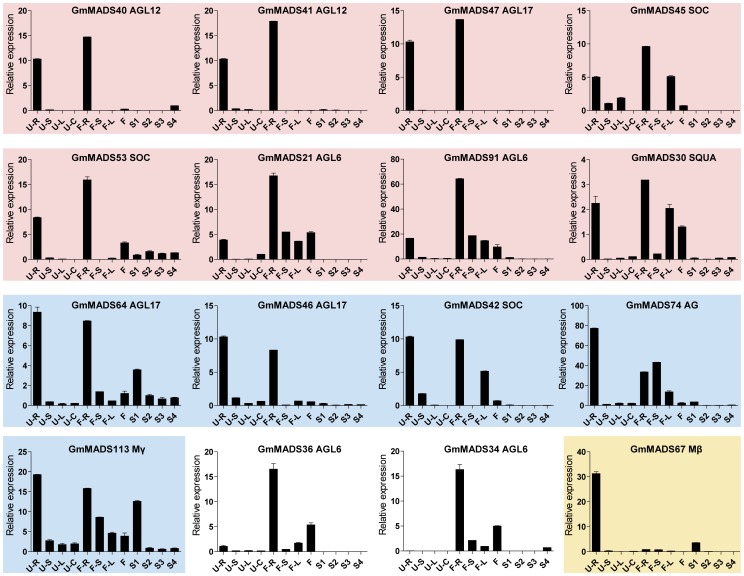
Figure 6. Expression patterns of GmMADS genes in the seeds by RT-qPCR.
4 samples at the seedling stage (the unifoliolates open fully): U-R (roots), U-S (stems), U-C (cotyledons) and U-U (leaves); 4 samples at the flowering stage: F-R (roots), F-S (stems), F-L (leaves) and F (flowers); 4 seed development samples: S1 (seeds at 7 days after flowering), S2 (seeds at 14 days after flowering), S3 (seeds at 21 days after flowering) and S4 (dry seeds). The similar expression profiles were in the similar color background. The bar is the average with standard deviation of the expression levels among three different replicates. The geometric means of GmSKIP16, GmUNKI and GmUNKII transcripts were used as the reference transcript. The values are means of three replicates, and each replicate represented a pool from at least five plants. Error bars represent SD.
GmMADS Gene Expression Peaks in the Soybean Flowers
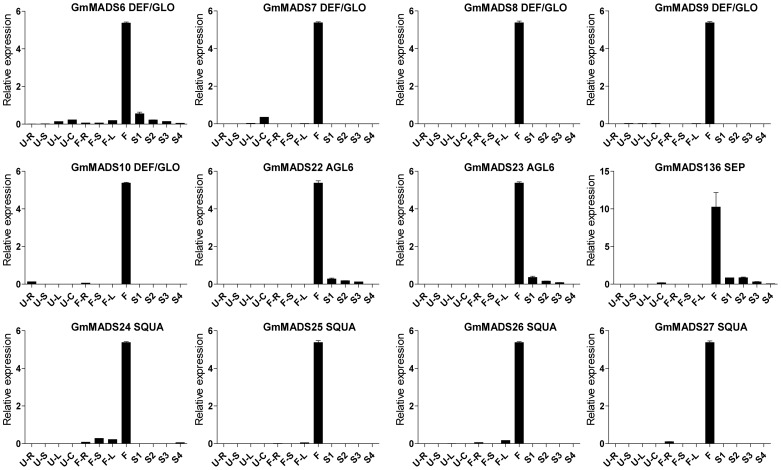
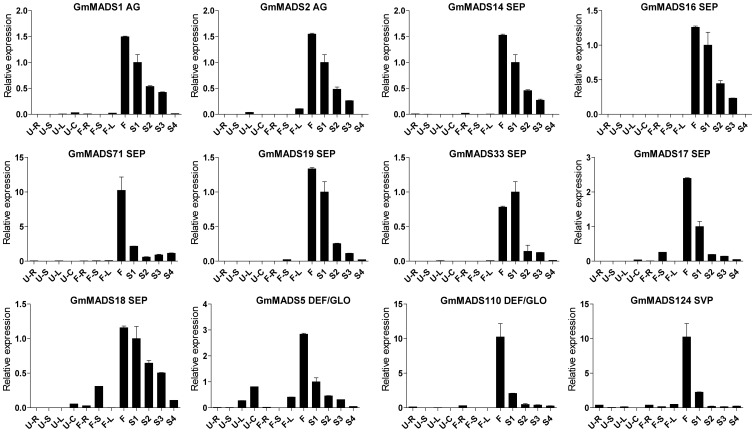
MIKC genes are well known of the importance in the flower development. In the soybean, transcriptions of MIKC genes were also detectable in the flowers (Figure 7, 8 and 9). For example, DEF/GLO (GmMADS6-10), SQUA (GmMADS24-27), AGL6 (GmMADS22 and 23) and SEP (GmMADS136) were most abundant only in flowers (Figure 7). But except in the flowers, SEP genes (GmMADS14, 16, 17, 18, 19, 33, and 71), AG genes (GmMADS1and 2), DEF/GLO (GmMADS5 and 10), SVP (GmMADS124) strongly expressed in the seed development (Figure 8). That indicated these MIKCC genes played important roles in reproductive tissues.
Figure 7. Expression peaks in the flowers through RT-qPCR. Notes as Figure 6.
Figure 8. High expression in the seeds and flowers through RT-qPCR.
Notes as Figure 6.
Figure 9. High expression in the flowers and leaves through RT-qPCR.
Notes as Figure 6.
There were also some genes having relative high abundance in multiple tissues besides the flowers (Figure 9). Two FLC genes (GmMADS73 and 15) and two SVP genes (GmMADS50 and 128) showed relatively high expression in the leaves at the seedling stage and at the flowering time respectively. The high level of transcripts of GmMADS69 (AGL6) and GmMADS84 (AGL17) were in roots, besides in flowers.
GmMADS Gene Expression Peaks in the Soybean Leaves
Expression peaks of 8 GmMADS genes occurred mainly in the leaves at the seedling and/or flowering stages (Figure 10). GmMADS49 and 51 (SVP), GmMADS44 (SOC) and GmMADS134 (MIKC*) highly expressed both at the seedling stage and flowering time; GmMADS28, 29 and 32 (SQUA) strongly expressed in the leaves and flowers at the flowering time; GmMADS137 highly expressed not only in the leaves, but in roots, stems and seeds.
Figure 10. Expression peaks in the leaves through RT-qPCR.
Notes as Figure 6.
GmMADS Gene Expression Peaks in the Soybean Stems
GmMADS31 (SQUA), GmMADS75 (Mα), GmMADS105 (Mγ), and GmMADS130 (Mβ) highly expressed in the stem (Figure 11). The relative expressions of GmMADS31 were strongly detected in the seedling stage stems and lower in other tissues. GmMADS130 highly expressed in the seedling stage stems, but high in the seedling stage roots and leaves and flowering stage roots. The highest transcriptions of GmMADS75 and GmMADS105were in the stems at the flowering time, and they showed relatively high expressions in the roots. And Low transcriptions of all the four genes can also be detected in flower and seeds.
Figure 11. Expression peaks in the stems through RT-qPCR.
Notes as Figure 6.
Expression Peaks of GmMADS Genes in Roots
There were about 16 MADS genes, AGL6 (GmMADS21, 34, and 91), SEP (GmMADS56), SOC (GmMADS42, 45, and GmMADS53), AGL17 (GmMADS46, 47, and 64), AGL12 (GmMADS40 and 41), SQUA (GmMADS30), AG (GmMADS74), Mβ (GmMADS67), Mγ (GmMADS113), showing high levels in roots at vegetative and/or reproductive stages (Figure 12). According to the transcription patterns, eight MIKCC genes expressed more highly in the root at the flowering times than at the seedling stage, and 4 MIKCC gene and one Mγ gene more highly expressed in the seedling stage roots. Expressions of two AGL6 genes were abundant in the roots and other tissues at the flowering time. And GmMADS67 expressed only in the roots at the seedlings. That indicated 16 genes were more importance of the roots than the flowers and seeds.
Figure 12. Expression peaks in the roots through RT-qPCR.
Notes as Figure 6.
Expression Divergences of the Paralog Gene Pairs
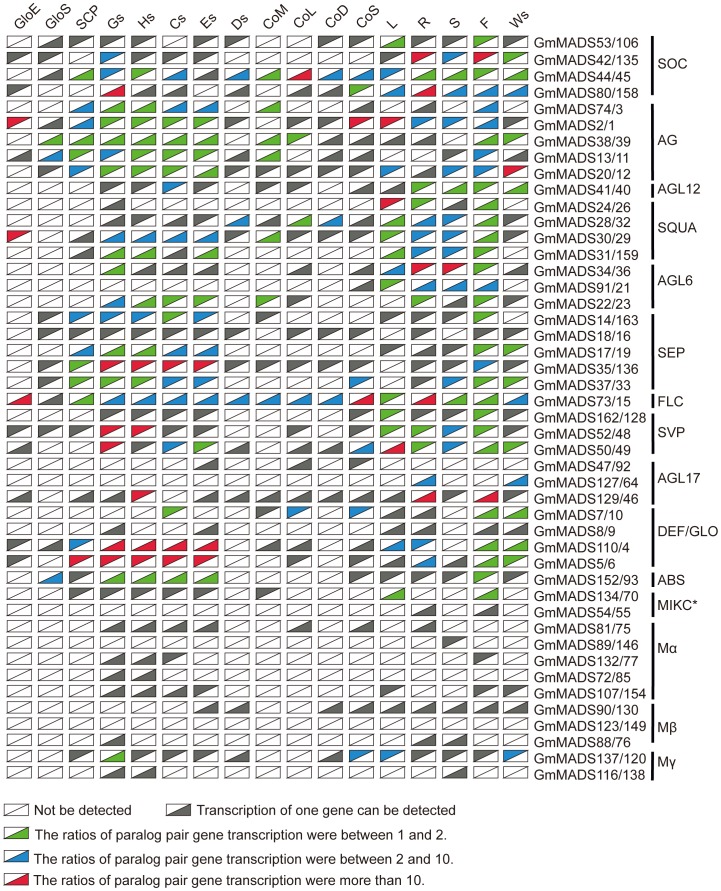
From the results above, there were 46 paralog gene pairs found in the soybean MADS gene family. Based on in silico expression data of these pairs in 17 soybean tissues, expression divergence in a sample was obviously evidenced. For example, 236 (about 30.2%) of gene pairs showed only one gene expressed while the other was undetectable; 113 (about 14.5%) of gene pairs showed the ratios between paralog gene pairs were between 1 and 2, 83 (about 10.6%) between 2 and 10, 38 (about 4.9%) above 10 (Figure 13). The MIKC paralog pair gene expressed high or low in same tissues, whereas most one of type I MADS paralog gene pair did not expressed (Figure 13 and Table S3). Our results indicated that, during evolutional progress, MIKC paralog gene pairs underwent sub-funtionalization and type I MADS paralog gene pairs underwent non-functionalization.
Figure 13. Expression divergence of paralog gene pairs.
The upper triangles showed the expression of the lift genes of the paralog gene pairs, and the lower triangle the expression of the right genes of the paralog gene pairs. The raw relative expressions of 163 MADS genes were in the Tab S6. Other notes were similar to Figure 3.
Discussion
Soybean MADS Subfamilies Experiencing Different Selection Pressure
At least one ancestral MADS-box gene was present in the common ancestor of plants, animals, and fungi, and probably the duplication that gave rise to the animal MEF2- and SRF-like genes occurred after animals diverged from plants but before fungi diverged from animals about 1000 million year ago (MYA) [6], [8]. Plant MIKC-type genes and animal MEF2-like genes are monophyletic, not so as plant type I and animal SRF-like genes do [6], [8], [16]. Subfamilies of MIKC, such as AG-, AGL6-, AGL12-, DEF/GLO, GGM13- (B(s)), STMADS11- and TM3-like genes, very likely existed already in the most recent common ancestor of angiosperms and gymnosperms about 300 MYA, and AGL2-, AGL17-, and SQUA-like genes, existed at least already in the most recent common ancestor of monocots and eudicots about 200 MYA [5]. So some important events during the phylogeny of species, especially spermatophyte, can be shown through the evolution of MADS gene family [55]. And the soybean genome has experienced two WGD events, the legume WGD and Glycine WGD, after the Gamma WGT event, when the monocots and eudicots diverged [27], [28]. So the trace of all soybean MADS genes experienced the three rounds of the WGD events should been found in the soybean genome during the soybean evolution process. But some evidences were found only in the MIKC gene evolutions. The homologous blocks containing MADS genes showed that ancestors of 6 soybean MIKCC subfamilies, SOC (TM3-like), SQUA, AGL6, SVP (STMADS11-like), AGL17, DEF/GLO, existed at least before the Gamma WGT event (Figure S5), and that AG and SEP (AGL2-like) at least before the legume WGD event (Figure 3). The evolution traces of blocks embodying 3 subfamilies of MIKC (AGL12, FLC and ABS), MIKC* family and all the type I MADS genes were only found after the Glycine WGD event (Figure 3 and 4 and Table S3). That indicated that in the soybean genome evolution, type I MADS-box genes and three MIKC subfamilies and MIKC* family had experienced faster birth-and-death evolution than some MIKC subfamilies.
More Early Divergence of Paraog genes, More Significant Difference of Expressions
In the soybean, duplication events result in a genome with approximately 46,430 ‘high-confidence’ genes, of which 75% are present as more than one copy [28]. And approximately 50% of paralogs from the recent WGD event differentially expressed and thus had undergone expression sub-functionalization through RNA-seq expressions of 7 tissues [29]. Based on our results, most MIKC paralogs from the recent WGD event showed the similar expression profiles, and one of the paralog pair expressed more highly in the most tissue than the other (Figure 13). But for the paralogs from much deeper duplications, the detached transcriptions were more obvious. For example, three paralog gene pairs of AGL6 subfamily diverged into two clades after the Gamma WGT event, one clade experienced the two WGD events, and then formed one paralog gene pair, GmMADS22/23, and the other was composed of GmMADS34/36 and GmMADS21/91(Figure 3). The former paralog gene pair strongly expressed in the flower and seeds (Figure 3 and 7), but the two latter paralog genes detached after the legume WGD event highly expressed in the roots at the flowering time (Figure 3 and 12). And other paralog genes of MIKCC subfamilies from different divergence events show similar expression divergences to AGL6 subfamily. That indicated the Gamma WGT event had important roles in vaiable functions of paralogs.
MADS Genes have Important Potential Roles in the Seed Development
Compared with type II MADS, very little is known about type I MADS, but recent studies display a key regulatory role for type I MADS factors in plant reproduction, in particular in specifying female gametophyte, embryo, and endosperm development [21], [48], [49], [56]–[58]. The rice and Arabidopsis have 28 and 61 type I MADS genes, respectively (Table S7). According to microarray expressions of MADS genes in rice or Arabidopsis (Figure S6 and S7), 10 and 32 type I MADS genes highly express in the rice or Arabidopsis seed development processes respectively. In addition, activities of promoters of 38 type I MADS genes are detected in the female gametophyte and seed development processes through their own promoters and 20 type I MADS genes are not detected [49]. In the soybean, 45 out of 75 type I MADS genes expressed in the seed tissues and transcriptions of 22 genes were not detected (Figure 4).
Since the first MADS genes are found in Arabidopsis and Antirrhinum respectively [3], [4], the importance of best-studied MIKCC genes is well known for floral homeotic functions during the ontogeny of flowers. Floral organ identity genes have been subdivided into five different classes, termed as class A (e.g. AP2 and AP1), B (e.g. PI, AP3, GLO, DEF), C (e.g. AG), D (e.g. SHP and STK), and E (e.g. SEP) genes, which are required in different combinations to specify sepals, petals, stamens, carpels and ovules [10], [25], [59]–[61]. Based on in silico analysis and our RT-qPCR results, GmMADS genes displayed high transcriptions in the soybean flower. For example, 7 members of DEF/GLO subfamily highly expressed in the flowers (Figure 3 and 7). Transcriptions of all SQUA subfamily genes, especially the AP1 ortholog genes GmMADS24, 25, 26, and 27, were also detected in the flowers (Figure 3 and 7).
But some MIKCC subfamilies with known floral homeotic functions displayed high relative expression not only in the flowers, but in the seed tissues, especially in the early stage seeds (Figure 3 and 8). Among 11 members of SEP subfamily, 7 SEP-like genes (GmMADS14, 16–19, 33, and 71) relatively highly expressed in the flowers, but all members of SEP subfamily strongly expressed in the seeds at different developmental stages (Figure 3, 6 and 8). In the rice, high transcriptions of four SEP genes (OsMADS1, 5, 7/45, 8/24,) were detected both in the rice panicle and seed development and OsMADS34 were detected only in the rice panicle development (Figure S6). In Arabidopsis, SEP1, 2 and 3 expressed in embryonic culture tissues [48], and SEP1 and 2 expressed both in the seed and in the flowers, while SEP4 highly expressed in the flowers (Figure S7). That suggested that soybean SEP genes may play a fundamental role in the development of all floral organs and seeds.
Homeotic C-class gene AG ortholog genes, GmMADS1 and 2 showed relatively high expression in the flowers and seeds (Figure 8), however, another AG ortholog gene, GmMADS3 highly expressed not in the flowers but in the early seeds, as well as 2 STK-like genes (a paralogous gene pair, GmMADS38/39) and 4 SHP2-like genes (two paralogous gene pairs, GmMADS11/13 and GmMADS12/20) did (Figure 6). And in the rice, compared with transcriptions in flowers, four rice AG members, such as OsMADS3, 13, 21 and 66, highly expressed in the seeds (Figure S6). In Arabidopsis, STK (AGL11) is not only detected in inflorescence but in the developing silique tissues, and redundantly with SHP1, SHP2 and ABS regulate the seed development (Figure S7) [62]–[65]. The results indicated that GmMADS genes underwent different evelutional progress from that of Arabidopsis MADS gene did and acquired new function in seed development. Another SHP1 ortholog gene GmMADS74 were detected in the seeds and flowers at low level, but high expressed in the roots and leaves (Figure 12), inferring its function beyond flowers and seeds.
The embryo proper represents new sporophytic generation and contains the shoot and root meristems. It is well known of the MIKC genes, AGL15 mRNA accumulates primarily in the embryo and the seed, and has an important component of the regulatory circuitry directing seed-specific processes in the developing embryo [62], [66], [67], and according to Figure S6, the expressions of AGL15 and AGL18 were increasing during the process of the seed mature. GmAGL15 (GmMADS122) is also preferentially expressed in developing embryos, but not in the flowers and yang pods [68]. Both AGL15 subfamily genes, GmMADS122 and 43, expressed in the seed tissues or flowers based on our RT-qPCR results, (Figure 6 and Table S6); two AGL17 subfamily members GmMADS64 and 160 highly accumulated in the globular stage embryo and low in the seed development (Table S6). In the rice, three AGL17-like genes, OsMADS25, 59 and 61, highly expressed in the later stage of the seed development. But in Arabidopsis, three AGL17-like genes, AGL17, 21 and ANR, highly expressed only in the roots, and low expressions of AGL16 can be detected in seeds (Figure S7). The suspensor is a terminally differentiated structure that supports and nourishes the embryo proper and degenerates later in development. GmMADS51 and 52 (SVP), GmMADS106 (SOC) and GmMADS127 (AGL17) highly expressed in suspensors and had potential roles in the function of the suspensors.
Materials and Methods
Plant Materials
The soybean (Glycine max) cultivar Kennong 18 was employed in all experiments. Plants were grown in a growth chamber under short day conditions (8 hr light/16 hr dark) at a temperature 25°C ∼ 28°C. Under the normal conditions, tissues were separately harvested at different stages for gene expression analysis. The seeds were sampled at day 7, 14 and 21 after flowering and when the seeds became yellow. At least five individual plants per sample were then harvested and frozen in liquid nitrogen and stored at −80°C until used. And all experiments were repeated three times under the consistent conditions.
Identification, Classification and Motif of Soybean MADS Genes
According to the HMM model of SRF-type MADS transcription factor (PF00319), HMMER v3.0 [69] were employed to identify 163 soybean MADS genes through searching the soybean genome protein database (V1.01, http://www.phytozome.net/soybean.php). The fragment genes were predicted the whole CDS by FGENESH (http://linux1.softberry.com/berry.phtml) or blasting the NCBI Ref-RNA database (Table S1). And 163 candidate genes were retrieved and named as GmMADS1 through 163 (Table S1).
V. vinifera and M.truncatula V3.5 whole-genome protein sequences were retrieved from Phytozome v8.0 (http://www.phytozome.net/) and the website (http://www.medicagohapmap.org) respectively, to investigate MADS inter-species colinearity. And their MADS families were also screened through HMMER v3.0 (Table S7). Some of 54 V.vinifera MADS family were named according to Diaz-Riquelme, et al. [42] and other named as VvMADS55 through 78, and M. truncatula MADS family were named as MtMADS1 through 93.
Because diversity of MADS genes in Arabidopsis is rather ancient and representative for other flowering plants [5], 108 Arabidopsis [14] and 75 rice MADS genes [41] (Table S7) were selected to classify 163 soybean MADS proteins into 5 families, MIKCC, MIKC*, Mα, Mβ and Mγ, and soybean genes most similar to Arabidopsis MADS genes were considered as the Arabidopsis ortholog genes, as well as the classification of M. truncatula and V.vinifera MADS family. And a topology tree (Figure S1) was constructed to investigate the relationship of the soybean and Arabidopsis MADS proteins through ClustalW1.8 and MAGE 5.0 using Neighbor-Joining method [70].
To analyze the specific motifs of different MADS families, 10 motifs were identified by MEME v 4.9.0 with default parameters [71] (http://meme.nbcr.net/meme/cgi-bin/meme.cgi) among the soybean, rice, Arabidopsis, V.vinifera and M. truncatula after removing redundant sequences by Purge tool [72], and then MAST v 4.9.0 was used to identify motif organizations of 163 soybean MADS proteins (Figure S2).
Investigating QTLs Relative to Soybean MADS Genes
To determine the co-localization of MADS genes with the QTLs, available SSR or RFLP markers linked to the QTLs were downloaded from Soybase (http://www.soybase.org/dlpages/index.php) [46], and markers’ sequences were mapped to the soybean genome (v1.09) through Blast. Furthermore, the QTL physical locations are usually uncertain due to the recombination frequency being affected by population size, even if the linked marker sequences and their genomic positions are known. Therefore, genes within a 2-Mb genomic region flanking markers were associated with a QTL.
Total RNA Isolation and Quantitative Reverse Transcription-PCR
The procedure used for RNA extraction, cDNA synthesis, and PCR was as described by Hu, et al [73]. According to the specificity and efficiency of the primer pairs, 96 soybean MADS genes were designed by Beacon Designer 7.9, and at least one primer was specific for the target gene primer pairs (Table S1). GmSKIP (Glyma12g05510), GmUKNII (Glyma14g08990) and GmUKN1 (Glyma12g02310) were selected as reference genes for all the experiments [73]. And primers used as controls or for analysis had an efficiency of greater than 90% by LinRegPCR (http://LinRegPCR.HFRC.nl) [74].
In silico Expression Analysis of MADS Genes
The tissue-specific transcript characteristics of 163 MADS genes were investigated based on the RNA-seq data from 17 soybean tissues (http://seedgenenetwork.net/soybean), such as three seed compartments at the seed globular stage (GSE29162), whole seeds at five stages of seed development (globular, heart, cotyledon, early-maturation, dry), and vegetative (leaves, roots, stems, seedlings) and reproductive (floral buds) tissues (GSE29163), cotyledons of mid-maturation and late maturation seeds, whole dry seeds, and cotyledons of seedlings six days after imbibitions (GSE29134).
For the whole expression analyses of soybean MADS, the RPKM method was employed to correct for biases in total gene exon size and to normalize for the total short read sequences obtained in each tissue library [53], [54]. And the geometrical average of RPKM values of the selected reference genes in RT-qPCR experiments was a reference gene value, and the ratios of target gene and a reference gene value were the relative expression level of the target gene in each sample (Table S6). A hierarchical clustering analysis of gene-wide normalizations of 138 gene transcription profiles in 17 tissues using a Pearson correlation was computed by Gensis1.7.5 [75].
Compared with expression profiles of the soybean MADS in the seed development, the microarray data, GSE6893 for rice [76] and GSE680 for Arabidopsis [77], were downloaded from the GEO database, and then cDNAs of MADS genes were selected to identify special probe sets through Probe Match tool (http://www.affymetrix.com/analysis/index.affx). At last, expression values of 55 rice and 73 Arabidopsis MADS probe sets were computed by Genespring 11.5 respectively (Figure S6 and 7). A hierarchical clustering analysis of gene-wide normalizations of transcription profiles used a Pearson correlation by Gensis1.7.5 [75].
Collinear Relationships of 163 MADS Genes in the Soybean Genome
MCScanX [52] was employed to identify syntenic regions containing MADS genes among soybean (Table S3), V. vinifera and M. truncatula. Briefly, BLASTP with e-value ≤1e−10 was applied to find intra-species paralogous pairs and inter-species homologous pairs, and the homologous blocks involved at least 5 collinear gene pairs and the gap gene pairs number was not more than 20. Based on the average Ks value of homologous blocks, the divergence times of the blocks were computed to investigate the evolution of soybean MADS genes in the soybean genome evolution. For example, if the average Ks is less than 0.3, divergence of the homologous blocks is about after the Glycine WGD event, and Ks is more than 1.5, the divergence time is after the Gamma WGT event, and Ks is between 0.3 and 1.5, the divergence time is after the Legume WGD event and before the Glycine WGD event.
Besides the WGD duplication, Soytedb (http://www.soybase.org/soytedb/) [35] was employed to identified the nearest transposable elements around some soybean MADS genes to analyze the importance of the transposable duplications to the soybean MADS gene evolutions (Table S4).
Supporting Information
Phylogenetic relationship of MADS genes between Glycine max and Arabidopsis . The deduced full-length amino acid sequences of 163 Glycine and 108 Arabidopsis genes were aligned by Clustal X 1.83 and the phylogenetic tree was constructed using MEGA 5.0 by the Neighbor-Joining (NJ) method with 1,000 bootstrap replicates. Lines of each GmMADS subfamily are in a specific color or in different color background.
(TIF)
Motifs of 163 soybean MADS proteins. Motif 1 and 2 are equivalent to the MADS-box domain (PF00319), and motif 3 and motif 6 are equivalent to the part of the I-domain and K-box domain (PF01486) for type II MADS proteins, respectively. Other motifs was unknown. Ten motifs were identified through MEME (http://meme.nbcr.net/meme/), and then motif organizations of 163 soybean MADS were investigated through MAST (http://meme.nbcr.net/meme/).
(TIF)
Expression heatmap of 138 MADS genes in the 17 tissues through RNA-seq. A hierarchical clustering analysis of gene-wide normalizations of 138 gene transcription profiles in 17 tissues using a Pearson correlation by Gensis1.7.5 suggested 138 genes can be grouped into 13 expression clusters. Clusters in same color showed the genes expressed mainly in the same tissues. And other notes as Figure 3.
(TIF)
Expression heatmap of 82 MADS genes in the 12 tiessues through RT-qPCR. The lines in same colors showed the genes expressed mainly in the same tissues. A hierarchical clustering analysis of gene-wide normalizations using a Pearson correlation by Gensis1.7.5 suggested 82 genes can be grouped into 5 clusters. And other notes as Figure 6.
(TIF)
The evolution model of blocks embodying MADS genes in the soybean. The black blocks were as the ancestors before the Gamma WGT event, and red, blue and green blocks showed the traces of the Gamma WGT, Legume WGD and Glycine WGD event respectively, and the bars were the MADS genes in the blocks. And the blocks without color showed the blocks were lost in the genome evolutionary history. Green blocks without bar were that the MADS genes were lost after the WGD events.
(TIF)
Microarray expressions of rice MADS genes. The microarray data (GSE6893) were from the NCBI GEO database. Special probe sets for 55 rice MADS genes and expression values were computed through Genespring 11.5. A hierarchical clustering analysis of gene-wide normalizations of 55 gene transcription profiles in 14 tissues using a Pearson correlation by Gensis1.7.5. SAM, P1 to P6 were up to 0.5 mm, 0–3 cm, 3–5 cm, 5–10 cm, 10–15 cm, 15–22 cm and 22–30 cm of panicles, respectively. And S1, S2, S3, S4 and S5 were seeds at 0–2, 3–4, 5–10, 11–20 and 21–29 days after pollination, respectively. Root and Young leaves was the roots and leaves from 7-d-old seedlings, respectively. Young leaves were as the control.
(TIF)
Microarray expressions of Arabidopsis MADS genes. The microarray data (GSE680) were from the NCBI GEO database. Special probe sets for 73 rice MADS genes and expression values were computed through Genespring 11.5. A hierarchical clustering analysis of gene-wide normalizations of 73 gene transcription profiles in 11 tissues using a Pearson correlation by Gensis1.7.5. Seedling was as the control.
(TIF)
The information of 163 GmMADS genes.
(XLSX)
The best match sequence of the soybean MADS motif.
(XLSX)
Intra- or inter-species synteny of soybean MADS.
(XLSX)
Proximal transposable elements of some soybean MADS genes.
(XLSX)
QTLs relative to soybean MADS genes.
(XLSX)
The normaliztion transcriptions of soybean MADS genes through RNA-seq analysis.
(XLSX)
The information of MADS family in A. thaliana , M. truncatula and V. vinifera.
(XLSX)
Acknowledgments
We thank Dr. Tao Zhao and Dr. Rongzhi Zhang (Institute of Crop Sciences, Chinese Academy of Agricultural Sciences) for good advises in the evolution analysis of soybean MADS genes.
Funding Statement
This work was partly supported by National High Technology Research and Development Program '863' (2013AA102602),Transgenic program (2009ZX08009-133B), the Chinese National Key Basic Research Program'973' (2010CB125906), and the National Natural Science Foundings (31000680 and 31000681). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Passmore S, Maine GT, Elble R, Christ C, Tye BK (1988) Saccharomyces cerevisiae protein involved in plasmid maintenance is necessary for mating of MAT alpha cells. J Mol Biol 204: 593–606. [DOI] [PubMed] [Google Scholar]
- 2. Norman C, Runswick M, Pollock R, Treisman R (1988) Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell 55: 989–1003. [DOI] [PubMed] [Google Scholar]
- 3. Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, et al. (1990) The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346: 35–39. [DOI] [PubMed] [Google Scholar]
- 4. Schwarz-Sommer Z, Huijser P, Nacken W, Saedler H, Sommer H (1990) Genetic Control of Flower Development by Homeotic Genes in Antirrhinum majus. Science 250: 931–936. [DOI] [PubMed] [Google Scholar]
- 5. Becker A, Theissen G (2003) The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol 29: 464–489. [DOI] [PubMed] [Google Scholar]
- 6. Alvarez-Buylla ER, Pelaz S, Liljegren SJ, Gold SE, Burgeff C, et al. (2000) An ancestral MADS-box gene duplication occurred before the divergence of plants and animals. Proc Natl Acad Sci U S A 97: 5328–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nam J, Kim J, Lee S, An G, Ma H, et al. (2004) Type I MADS-box genes have experienced faster birth-and-death evolution than type II MADS-box genes in angiosperms. Proceedings of the National Academy of Sciences of the United States of America 101: 1910–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Theissen G, Kim JT, Saedler H (1996) Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J Mol Evol 43: 484–516. [DOI] [PubMed] [Google Scholar]
- 9. Kaufmann K, Melzer R, Theißen G (2005) MIKC-type MADS-domain proteins: structural modularity, protein interactions and network evolution in land plants. Gene 347: 183–198. [DOI] [PubMed] [Google Scholar]
- 10. Riechmann JL, Krizek BA, Meyerowitz EM (1996) Dimerization specificity of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA, and AGAMOUS. Proc Natl Acad Sci U S A 93: 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henschel K, Kofuji R, Hasebe M, Saedler H, Munster T, et al. (2002) Two ancient classes of MIKC-type MADS-box genes are present in the moss Physcomitrella patens. Mol Biol Evol 19: 801–814. [DOI] [PubMed] [Google Scholar]
- 12. Vandenbussche M, Theissen G, Van de Peer Y, Gerats T (2003) Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations. Nucleic Acids Res 31: 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwantes M, Liebsch D, Verelst W (2012) How MIKC* MADS-box genes originated and evidence for their conserved function throughout the evolution of vascular plant gametophytes. Mol Biol Evol 29: 293–302. [DOI] [PubMed] [Google Scholar]
- 14. Parenicova L, de Folter S, Kieffer M, Horner DS, Favalli C, et al. (2003) Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell 15: 1538–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kofuji R, Sumikawa N, Yamasaki M, Kondo K, Ueda K, et al. (2003) Evolution and divergence of the MADS-box gene family based on genome-wide expression analyses. Mol Biol Evol 20: 1963–1977. [DOI] [PubMed] [Google Scholar]
- 16. De Bodt S, Raes J, Florquin K, Rombauts S, Rouze P, et al. (2003) Genomewide structural annotation and evolutionary analysis of the type I MADS-box genes in plants. J Mol Evol 56: 573–586. [DOI] [PubMed] [Google Scholar]
- 17. Smaczniak C, Immink RG, Angenent GC, Kaufmann K (2012) Developmental and evolutionary diversity of plant MADS-domain factors: insights from recent studies. Development 139: 3081–3098. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida H, Nagato Y (2011) Flower development in rice. Journal of Experimental Botany 62: 4719–4730. [DOI] [PubMed] [Google Scholar]
- 19. Teeri TH, Uimari A, Kotilainen M, Laitinen R, Help H, et al. (2006) Reproductive meristem fates in Gerbera. J Exp Bot 57: 3445–3455. [DOI] [PubMed] [Google Scholar]
- 20. Gramzow L, Theissen G (2010) A hitchhiker's guide to the MADS world of plants. Genome Biol 11: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masiero S, Colombo L, Grini PE, Schnittger A, Kater MM (2011) The Emerging Importance of Type I MADS Box Transcription Factors for Plant Reproduction. The Plant Cell 23: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verelst W, Saedler H, Munster T (2007) MIKC* MADS-protein complexes bind motifs enriched in the proximal region of late pollen-specific Arabidopsis promoters. Plant Physiol 143: 447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verelst W, Twell D, de Folter S, Immink R, Saedler H, et al. (2007) MADS-complexes regulate transcriptome dynamics during pollen maturation. Genome Biol 8: R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geuten K, Irish V (2010) Hidden variability of floral homeotic B genes in Solanaceae provides a molecular basis for the evolution of novel functions. Plant Cell 22: 2562–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Theissen G, Melzer R (2007) Molecular mechanisms underlying origin and diversification of the angiosperm flower. Ann Bot 100: 603–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irish VF, Litt A (2005) Flower development and evolution: gene duplication, diversification and redeployment. Curr Opin Genet Dev 15: 454–460. [DOI] [PubMed] [Google Scholar]
- 27. Severin AJ, Cannon SB, Graham MM, Grant D, Shoemaker RC (2011) Changes in twelve homoeologous genomic regions in soybean following three rounds of polyploidy. Plant Cell 23: 3129–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- 29.Roulin A, Auer PL, Libault M, Schlueter J, Farmer A, et al.. (2012) The fate of duplicated genes in a polyploid plant genome. Plant J. [DOI] [PubMed]
- 30. Buggs RJ, Elliott NM, Zhang L, Koh J, Viccini LF, et al. (2010) Tissue-specific silencing of homoeologs in natural populations of the recent allopolyploid Tragopogon mirus. New Phytol 186: 175–183. [DOI] [PubMed] [Google Scholar]
- 31. Freeling M (2009) Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annual review of plant biology 60: 433–453. [DOI] [PubMed] [Google Scholar]
- 32. Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ganko EW, Meyers BC, Vision TJ (2007) Divergence in expression between duplicated genes in Arabidopsis. Molecular biology and evolution 24: 2298–2309. [DOI] [PubMed] [Google Scholar]
- 34. Cusack BP, Wolfe KH (2007) Not born equal: increased rate asymmetry in relocated and retrotransposed rodent gene duplicates. Mol Biol Evol 24: 679–686. [DOI] [PubMed] [Google Scholar]
- 35. Du J, Grant D, Tian Z, Nelson RT, Zhu L, et al. (2010) SoyTEdb: a comprehensive database of transposable elements in the soybean genome. BMC Genomics 11: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodhouse MR, Pedersen B, Freeling M (2010) Transposed genes in Arabidopsis are often associated with flanking repeats. PLoS genetics 6: e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Wang X, Tang H, Tan X, Ficklin SP, et al. (2011) Modes of gene duplication contribute differently to genetic novelty and redundancy, but show parallels across divergent angiosperms. PLoS One 6: e28150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Immink RG, Ferrario S, Busscher-Lange J, Kooiker M, Busscher M, et al. (2003) Analysis of the petunia MADS-box transcription factor family. Mol Genet Genomics 268: 598–606. [DOI] [PubMed] [Google Scholar]
- 39. Hileman LC, Sundstrom JF, Litt A, Chen M, Shumba T, et al. (2006) Molecular and phylogenetic analyses of the MADS-box gene family in tomato. Mol Biol Evol 23: 2245–2258. [DOI] [PubMed] [Google Scholar]
- 40. Leseberg CH, Li A, Kang H, Duvall M, Mao L (2006) Genome-wide analysis of the MADS-box gene family in Populus trichocarpa. Gene 378: 84–94. [DOI] [PubMed] [Google Scholar]
- 41. Arora R, Agarwal P, Ray S, Singh AK, Singh VP, et al. (2007) MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genomics 8: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diaz-Riquelme J, Lijavetzky D, Martinez-Zapater JM, Carmona MJ (2009) Genome-wide analysis of MIKCC-type MADS box genes in grapevine. Plant Physiol 149: 354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y, Li X, Chen W, Peng X, Cheng X, et al. (2010) Whole-genome survey and characterization of MADS-box gene family in maize and sorghum. Plant Cell, Tissue and Organ Culture (PCTOC) 105: 159–173. [Google Scholar]
- 44. Hu L, Liu S (2012) Genome-wide analysis of the MADS-box gene family in cucumber. Genome 55: 245–256. [DOI] [PubMed] [Google Scholar]
- 45. de Folter S, Immink RG, Kieffer M, Parenicova L, Henz SR, et al. (2005) Comprehensive interaction map of the Arabidopsis MADS Box transcription factors. Plant Cell 17: 1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grant D, Nelson RT, Cannon SB, Shoemaker RC (2010) SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res 38: D843–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- 48. Lehti-Shiu MD, Adamczyk BJ, Fernandez DE (2005) Expression of MADS-box genes during the embryonic phase in Arabidopsis. Plant Mol Biol 58: 89–107. [DOI] [PubMed] [Google Scholar]
- 49. Bemer M, Heijmans K, Airoldi C, Davies B, Angenent GC (2010) An atlas of type I MADS box gene expression during female gametophyte and seed development in Arabidopsis. Plant Physiol 154: 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young ND, Debelle F, Oldroyd GE, Geurts R, Cannon SB, et al. (2011) The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, et al. (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449: 463–467. [DOI] [PubMed] [Google Scholar]
- 52. Wang Y, Tang H, DeBarry JD, Tan X, Li J, et al. (2012) MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Research 40: e49–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, et al. (2008) The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 320: 1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628. [DOI] [PubMed] [Google Scholar]
- 55.Vekemans D, Proost S, Vanneste K, Coenen H, Viaene T, et al.. (2012) Gamma paleohexaploidy in the stem-lineage of core eudicots: significance for MADS-box gene and species diversification. Mol Biol Evol. [DOI] [PubMed]
- 56. Kang IH, Steffen JG, Portereiko MF, Lloyd A, Drews GN (2008) The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. Plant Cell 20: 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Colombo M, Masiero S, Vanzulli S, Lardelli P, Kater MM, et al. (2008) AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. Plant J 54: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 58. Portereiko MF, Lloyd A, Steffen JG, Punwani JA, Otsuga D, et al. (2006) AGL80 is required for central cell and endosperm development in Arabidopsis. Plant Cell 18: 1862–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF (2004) The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol 14: 1935–1940. [DOI] [PubMed] [Google Scholar]
- 60. Theißen G, Becker A (2004) Gymnosperm orthologues of class B floral homeotic genes and their impact on understanding flower origin. Critical Reviews in Plant Sciences 23: 129–148. [Google Scholar]
- 61. Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405: 200–203. [DOI] [PubMed] [Google Scholar]
- 62. Rounsley SD, Ditta GS, Yanofsky MF (1995) Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7: 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, et al. (2003) Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424: 85–88. [DOI] [PubMed] [Google Scholar]
- 64. Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, et al. (2000) SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404: 766–770. [DOI] [PubMed] [Google Scholar]
- 65. Mizzotti C, Mendes MA, Caporali E, Schnittger A, Kater MM, et al. (2012) The MADS box genes SEEDSTICK and ARABIDOPSIS Bsister play a maternal role in fertilization and seed development. Plant J 70: 409–420. [DOI] [PubMed] [Google Scholar]
- 66. Heck GR, Perry SE, Nichols KW, Fernandez DE (1995) AGL15, a MADS domain protein expressed in developing embryos. Plant Cell 7: 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Perry SE, Nichols KW, Fernandez DE (1996) The MADS domain protein AGL15 localizes to the nucleus during early stages of seed development. Plant Cell 8: 1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thakare D, Tang W, Hill K, Perry SE (2008) The MADS-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in Arabidopsis and soybean. Plant Physiol 146: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Finn RD, Clements J, Eddy SR (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Research 39: W29–W37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bailey TL, Boden M, Buske FA, Frith M, Grant CE, et al. (2009) MEME Suite: tools for motif discovery and searching. Nucleic Acids Research 37: W202–W208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Neuwald AF, Liu JS, Lawrence CE (1995) Gibbs motif sampling: detection of bacterial outer membrane protein repeats. Protein Sci 4: 1618–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hu R, Fan C, Li H, Zhang Q, Fu YF (2009) Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Molecular Biology 10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ruijter J, Ramakers C, Hoogaars W, Karlen Y, Bakker O, et al. (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic acids research 37: e45–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sturn A, Quackenbush J, Trajanoski Z (2002) Genesis: cluster analysis of microarray data. Bioinformatics 18: 207–208. [DOI] [PubMed] [Google Scholar]
- 76. Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, et al. (2007) F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol 143: 1467–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Le BH, Cheng C, Bui AQ, Wagmaister JA, Henry KF, et al. (2010) Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc Natl Acad Sci U S A 107: 8063–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic relationship of MADS genes between Glycine max and Arabidopsis . The deduced full-length amino acid sequences of 163 Glycine and 108 Arabidopsis genes were aligned by Clustal X 1.83 and the phylogenetic tree was constructed using MEGA 5.0 by the Neighbor-Joining (NJ) method with 1,000 bootstrap replicates. Lines of each GmMADS subfamily are in a specific color or in different color background.
(TIF)
Motifs of 163 soybean MADS proteins. Motif 1 and 2 are equivalent to the MADS-box domain (PF00319), and motif 3 and motif 6 are equivalent to the part of the I-domain and K-box domain (PF01486) for type II MADS proteins, respectively. Other motifs was unknown. Ten motifs were identified through MEME (http://meme.nbcr.net/meme/), and then motif organizations of 163 soybean MADS were investigated through MAST (http://meme.nbcr.net/meme/).
(TIF)
Expression heatmap of 138 MADS genes in the 17 tissues through RNA-seq. A hierarchical clustering analysis of gene-wide normalizations of 138 gene transcription profiles in 17 tissues using a Pearson correlation by Gensis1.7.5 suggested 138 genes can be grouped into 13 expression clusters. Clusters in same color showed the genes expressed mainly in the same tissues. And other notes as Figure 3.
(TIF)
Expression heatmap of 82 MADS genes in the 12 tiessues through RT-qPCR. The lines in same colors showed the genes expressed mainly in the same tissues. A hierarchical clustering analysis of gene-wide normalizations using a Pearson correlation by Gensis1.7.5 suggested 82 genes can be grouped into 5 clusters. And other notes as Figure 6.
(TIF)
The evolution model of blocks embodying MADS genes in the soybean. The black blocks were as the ancestors before the Gamma WGT event, and red, blue and green blocks showed the traces of the Gamma WGT, Legume WGD and Glycine WGD event respectively, and the bars were the MADS genes in the blocks. And the blocks without color showed the blocks were lost in the genome evolutionary history. Green blocks without bar were that the MADS genes were lost after the WGD events.
(TIF)
Microarray expressions of rice MADS genes. The microarray data (GSE6893) were from the NCBI GEO database. Special probe sets for 55 rice MADS genes and expression values were computed through Genespring 11.5. A hierarchical clustering analysis of gene-wide normalizations of 55 gene transcription profiles in 14 tissues using a Pearson correlation by Gensis1.7.5. SAM, P1 to P6 were up to 0.5 mm, 0–3 cm, 3–5 cm, 5–10 cm, 10–15 cm, 15–22 cm and 22–30 cm of panicles, respectively. And S1, S2, S3, S4 and S5 were seeds at 0–2, 3–4, 5–10, 11–20 and 21–29 days after pollination, respectively. Root and Young leaves was the roots and leaves from 7-d-old seedlings, respectively. Young leaves were as the control.
(TIF)
Microarray expressions of Arabidopsis MADS genes. The microarray data (GSE680) were from the NCBI GEO database. Special probe sets for 73 rice MADS genes and expression values were computed through Genespring 11.5. A hierarchical clustering analysis of gene-wide normalizations of 73 gene transcription profiles in 11 tissues using a Pearson correlation by Gensis1.7.5. Seedling was as the control.
(TIF)
The information of 163 GmMADS genes.
(XLSX)
The best match sequence of the soybean MADS motif.
(XLSX)
Intra- or inter-species synteny of soybean MADS.
(XLSX)
Proximal transposable elements of some soybean MADS genes.
(XLSX)
QTLs relative to soybean MADS genes.
(XLSX)
The normaliztion transcriptions of soybean MADS genes through RNA-seq analysis.
(XLSX)
The information of MADS family in A. thaliana , M. truncatula and V. vinifera.
(XLSX)