Abstract
The risk profile and prognosis of patients with myelofibrosis is well described by the Dynamic International Prognostic Scoring System risk categorization. Allogeneic stem cell transplantation is considered for intermediate-2/high risk disease. However, indicators of prognosis after transplantation are still lacking. Seventy simultaneously collected pairs of trephine and blood samples were quantified for JAK2 p.V617F allele burden to compare test sensitivity. The course of 30 patients with JAK2 p.V617F-positive myeloproliferative neoplasia was correlated with allele burden after transplantation. Monitoring can be performed on full blood samples as well as trephine biopsies, provided that techniques with ample sensitivity (0.01% to 0.001%) are available. Measurement of allele burden on day 28 after transplantation discriminates two prognostic groups: patients with a JAK2 p.V617F allele burden >1% have a significantly higher risk of relapse of JAK2 p.V617F positive neoplasia (P=0.04) and a poorer overall survival (P<0.01). In conclusion, measurement of JAK2 p.V617F allele burden early after transplantation is an important predictive parameter in monitoring patients following this treatment. As this might provide an important tool in early management of imminent early relapse it will be important to define consensus guidelines for optimal monitoring.
Introduction
BCR-ABL-negative myeloproliferative neoplasms (MPN) are heterogeneous stem cell disorders with a variable risk of progression to secondary acute leukemia and to bone marrow failure due to bone marrow fibrosis.1 Allogeneic stem cell transplantation (SCT) is currently the only potentially curative procedure, but is still associated with significant mortality and morbidities.2–4 The curative potential of SCT relies heavily on the graft-versus-leukemia effect.5–7 This has led over the past decade to the adoption of reduced intensity conditioning SCT and to new curative possibilities even for elderly patients.8–11 However, following SCT 30–50% of all patients relapse and most of them die.12,13 Immunmodulation by titration of immunosuppression or use of donor lymphocyte infusions has been investigated in different diseases and may provide an option for avoiding hematologic relapse early after SCT.14,15 Such interventions, however, are only successful during early relapse and rely on the availability of appropriate techniques for monitoring molecular markers of the underlying disease. To date, molecular monitoring in patients with MPN has been limited, with real-time polymerase chain reaction (qPCR) being the most important tool for determining whether there is minimal residual disease in JAK2 p.V617F positive disease.16,17 Standardization of PCR protocols and documentation of adequate sensitivity and specificity for monitoring minimal residual disease in these patients have been initiated but not yet finalized.
We show here that both quantitative amplification refractory mutation system (ARMS) qPCR and allele-specific wild-type-blocking (AS-WTB) qPCR are sufficiently sensitive for monitoring minimal residual disease in patients with JAK2 p.V617F positive disease and that both methods can be performed on blood samples or bone marrow trephines with comparable results.
Applying these techniques we were able to show that allele burden measurement 28 days after allogeneic SCT was highly predictive of relapse of JAK2 p.V617F positive neoplasia and for overall survival. We, therefore, recommend urgent standardization of MPN monitoring following allogeneic SCT.
Design and Methods
Patients
In a first exploratory study, analyses were performed on samples from 14 patients with JAK2 p.V617F positive MPN and a median age of 61 years (range, 52–70 years) who were transplanted between 2000 and 2008 at the Department of Hematology and Oncology of the University of Leipzig. Patients were selected based on the availability of blood and bone marrow DNA for simultaneous analysis at different time points after allogeneic SCT. Following evaluation of these data, a second group of 16 patients with a comparable median age who had been transplanted over the same period, was examined for correlation of the post-transplantation disease course with JAK2 p.V617F allele burden at day 28. The clinical profiles of the patients are shown in Table 1, individual risk profile, MPN subtype and JAK2 p.V617F allele burden prior to SCT are presented in Table 2A/B. Applying the Dynamic International Prognostic Scoring System (DIPSS) for risk stratification, the patients with primary myelofibrosis (n=16) were classified into either the intermediate-2 risk group or the high risk group. The patients were followed for a median of 43.5 months (range, 20.3 – 104.4 months). All patients gave written informed consent to participation in the study in accordance with the Declaration of Helsinki, and the study was approved by the local ethics committee and the national health authorities. Within the first cohort the JAK2 p.V617F allele burden of one patient (UPN6) never exceeded 4%. This patient had an abnormal karyotype and atypical spindle-shaped mast cell aggregates were seen following allogeneic SCT. Together with the molecular pathological finding of a KIT p.D816V mutation the disease was identified as systemic mastocytosis associated with a JAK2 p.V617F-positive MPN. The other 13 patients were determined to have classical MPN which in four of them had transformed to secondary acute myeloid leukemia prior to the allogeneic SCT. All patients in the second cohort (n=16) had classical MPN which in seven cases had transformed to secondary AML prior to transplantation. Patients were transplanted due to progression under conventional treatment with at least one of the following features: secondary AML; increased transfusion frequency; pancytopenia or uncontrolled white blood cell counts under therapy.
Table 1.
Characteristics of the patients (cohort 1 and cohort 2), stem cell transplants and outcomes.
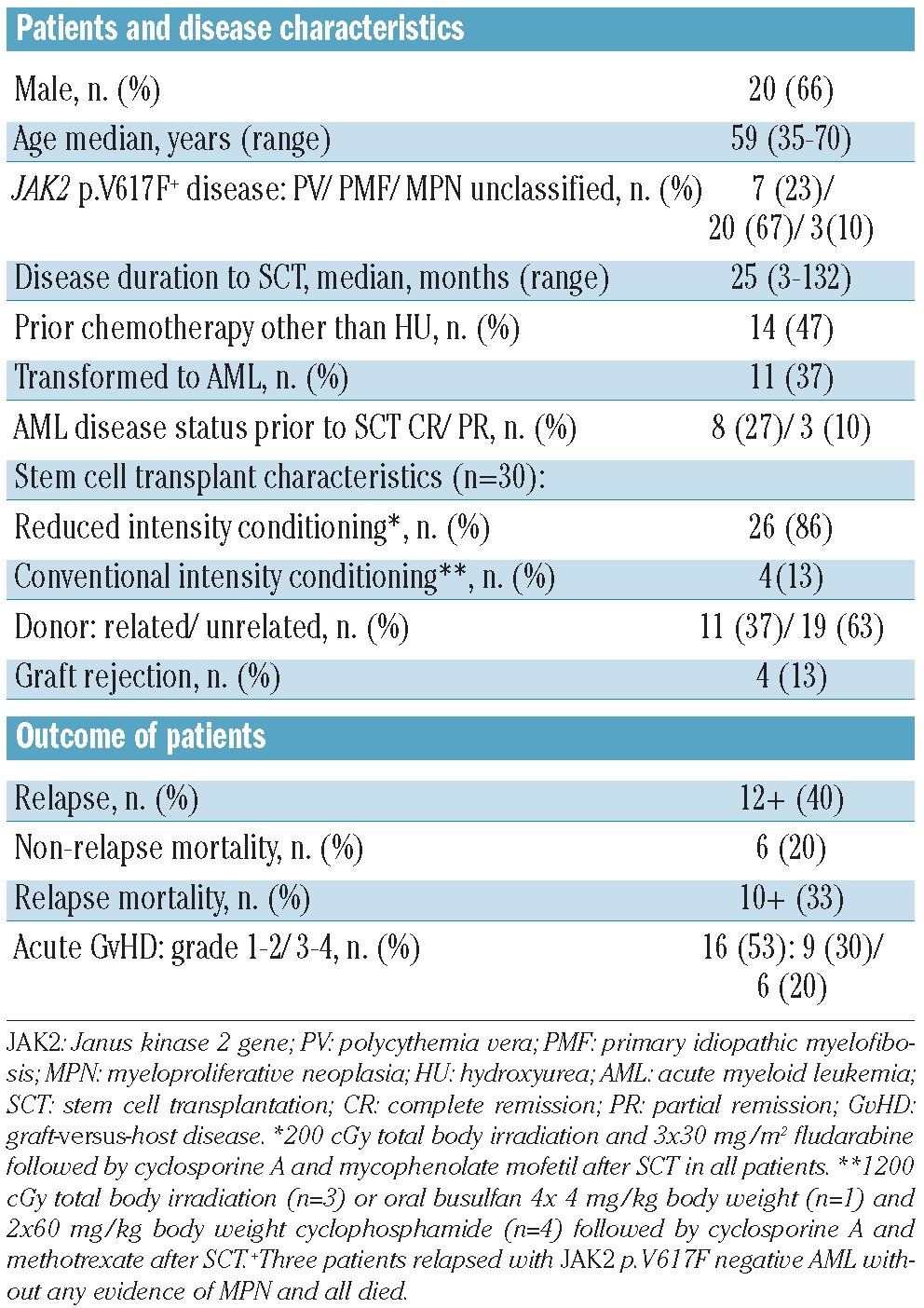
Table 2A.
Individual outcomes as a function of JAK2 status, observation period and age. Patients with JAK2 p.V617F allele burden >1%.
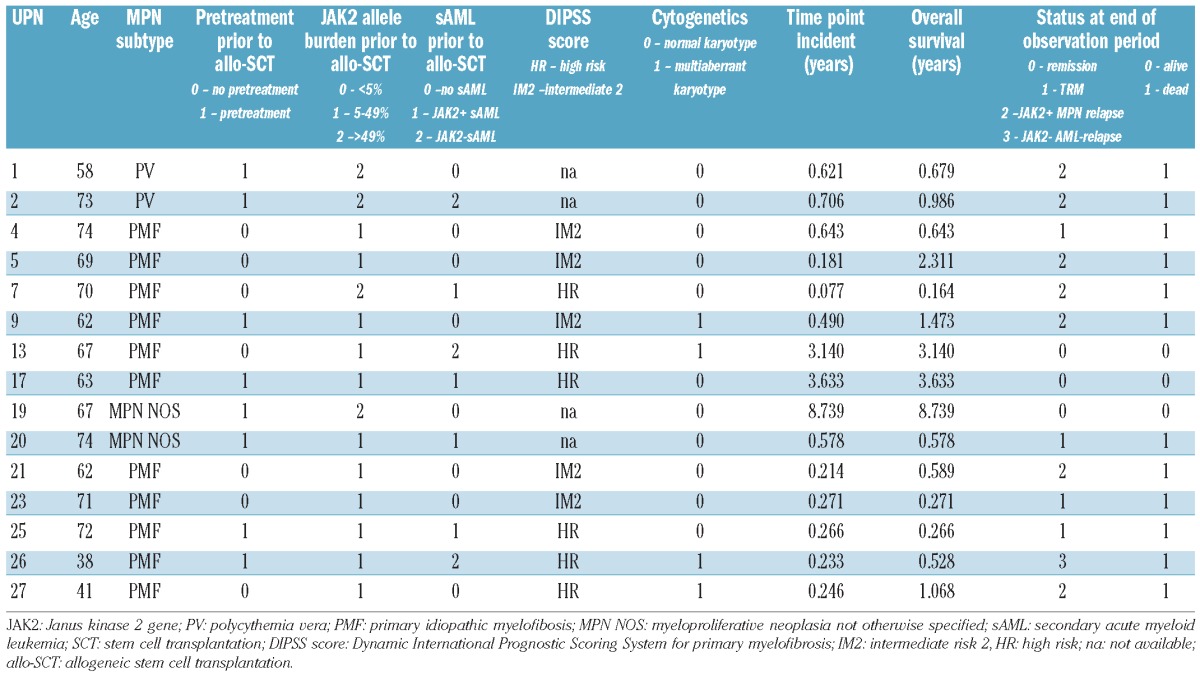
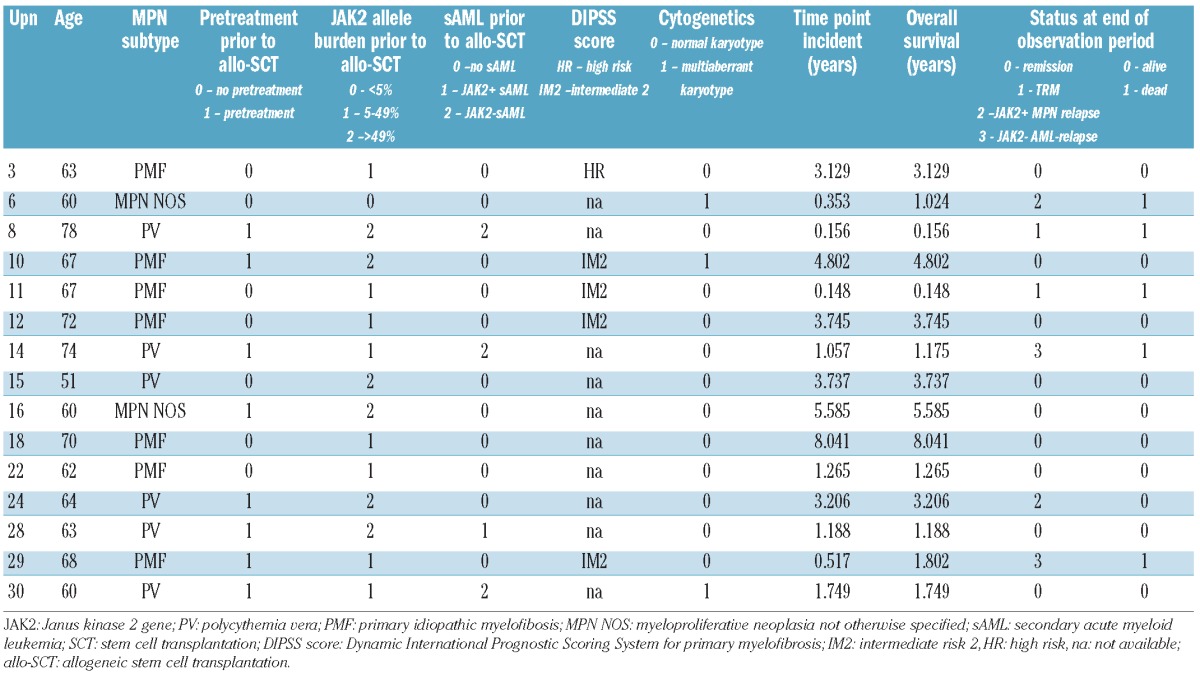
Table 2B.
Individual outcomes as a function of JAK2 status, observation period and age. Patients with JAK2 p.V617F allele burden ≤1%.
The success of allogeneic SCT was assessed on the basis of clinical data, cytological, histomorphological and laboratory findings. Relapse was indicated by the reappearance of MPN-typical changes in peripheral blood (i.e. progressive cytopenia, leukerythroblastic blood counts) and/or by clinical symptoms (i.e. increase in splenomegaly, constitutional symptoms) together with characteristic changes in the bone marrow such as progressive fiber density in a trephine biopsy or an increase of atypical blasts in the absence of uncontrolled graft-versus-host disease, graft rejection or poor bone marrow function and infections. Response or remission of MPN was indicated by constant or decreasing spleen size and blood count parameters as well as a return to normal histomorphology.
Samples
For the initial study of 14 patients a total of 70 peripheral blood samples were immediately frozen at −80°C. At the same time as the blood sampling, 76 bone marrow biopsies were obtained from the posterior iliac crest. The biopsies were fixed in phosphate-buffered formaldehyde solution (4%) for 12 h. Further processing included decalcification in 10% buffered ethylene-diamine tetra-acetic acid (EDTA), pH 7.2 for 6 h and embedding in paraffin. Early sampling was carried out at standardized intervals (1 month, 2 months, 3 months) and/or in response to clinical features. Decisions regarding sampling at later time points were based on clinicopathological findings. Overall, from 146 total samples, 142 were analyzed by both molecular assays (see below).
For the subsequent analysis of the 16 patients, JAK2 p.V617F allele burden was tested either in bone marrow biopsies (n=29) or in frozen peripheral blood probes (n=3) prior to and at day 28 after allogeneic SCT.
Reproducibility was demonstrated by performing the assay in quadruplicate at different time points. Concerning the cut-off of 1% JAK2 p.V617F allele burden, the initial classification could be confirmed in every case.
DNA extraction
Total DNA was extracted from peripheral blood cells that had been frozen at −80°C using the DNeasy Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations.
Genomic DNA was isolated from bone marrow biopsies by cutting one to three 8 μm paraffin wax-embedded sections and processing the sections using a DNeasy Micro Kit and QIAcube (Qiagen) according to the manufacturer’s instructions.
Amplification refractory mutation system – real-time polymerase chain reaction analysis
This quantitative, allele-specific oligonucleotide PCR assay, established on the principles of the ARMS.qPCR for JAK2 p.V617F mutated allele, was performed on 144 probes (in quadruplicate) as previously described14 with minor modifications but without the reported additional third-to-last-3′ point mutation which resulted in our hands in a cross reactivity to JAK2 p.V617F wild type allele (data not shown). The level of JAK2 p.V617F mutated allele was normalized to that of the HCK gene and expressed as JAK2 p.V617F mutated alleles per HCK allele to enable quantification. In all JAK2 p.V617F negative samples the HCK allele burden was noted separately.
Allele-specific wild-type blocking quantitative real-time polymerase chain reaction
As previously reported18 the combination of wild-type blocking PCR (employing locked nucleic acid-substituted oligonucleotides and the Taq polymerase Stoffel fragment) with allele-specific qPCR results in a two-step procedure combining high sensitivity with robustness. While the sensitivity of the AS-qPCR approach is around 1%, that of AS-WTB qPCR achieves a sensitivity of at least 0.001%. This assay can, therefore, be used to determine values around the cut-off of 1% very accurately. A total of 144 samples from the first cohort and a further 32 samples from the second cohort were analyzed with this method (in quadruplicate).
Statistical analysis
Endpoints were overall survival and incidence of JAK2 p.V617F-positive relapse. The characteristics of patients were expressed as median and range for continuous variables and frequencies for categorical variables. Categorical data were compared by Fisher’s exact test or the χ2 test. Survival curves were estimated by the Kaplan-Meier method and were compared using the log-rank test. The Gray test was used to compare cumulative incidence curves. JAK2 p.V617F allele burden on day 28 after allogeneic SCT was included in univariate analysis. The calculations were performed with SPSS version 20 and with the statistical software environment R, version 2.13.1, using the R package cmprsk.
Results
Comparison of two allele-specific polymerase chain reaction amplification methods
The two allele-specific assays described above were tested together in 70 peripheral blood and 72 bone marrow biopsy samples. All investigations were performed in quadruplicate at different time points.
The results revealed a high degree of consistency. When the categories negative, positive with JAK2 p.V617F ≤1%, and JAK2 p.V617F >1% were considered separately, 73% of all values showed a complete match in both assays. In 6% of the samples, the differences were due to inconsistent discrimination of the categories ≤ and >1% by the two assays and in these cases the higher value was counted. In 19% of the samples JAK2 p.V617F was detected only by AS-WTB qPCR and in 2% of the samples only by ARMS-qPCR indicating a slightly higher sensitivity for the AS-WTB qPCR.
Comparison of JAK2 p.V617F allele burden in simultaneously taken peripheral blood and bone marrow samples
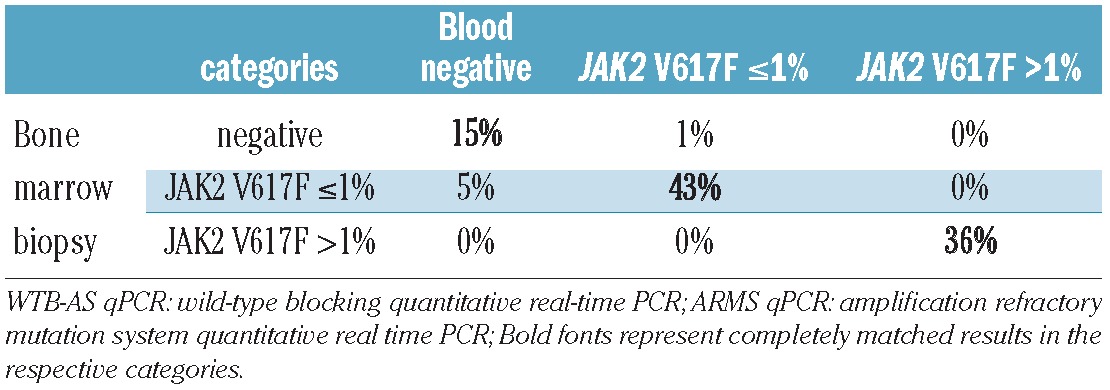
In order to select the optimal haematopoietic compartment for minimal residual disease analysis, molecular monitoring was carried out using bone marrow biopsies and peripheral blood samples taken at the same time point. Importantly, there was absolute agreement between the samples (100%) in the category JAK2 p.V617F allele burden >1%. Further results are shown in Table 3. We conclude that both peripheral blood and bone marrow biopsy are appropriate for minimal residual disease monitoring after allogeneic SCT as the prognostically important differentiation into high (>1%) and low (≤1%) allele burden was possible in every case.
Table 3.
Comparison of 70 simultaneously analyzed sample pairs (WTB-AS qPCR and ARMS qPCR).
Patients’ clinical outcome
Following allogeneic SCT 12 of 30 patients were still alive without hematologic neoplasia at the end of the observation period. Four patients experienced primary graft failure, defined as <10% donor T-cell chimerism at day 28 post-transplantation. Two of them had a hematologic relapse and two died of transplant-related causes 2 to 7 months after the allogenic transplant. Two patients died of graft-versus-host disease, related infection and sudden death 8 and 2 months after allogeneic SCT, respectively. The median follow-up of the remaining 24 patients was 32.1 months (range, 1.9–104.2 months). Altogether nine patients developed a hematologic relapse of the MPN, as defined above, in a median of 8.0 months (range, 0.8–38.5 months) after allogeneic SCT and seven patients died. Three patients relapsed with secondary AML in the absence of MPN and died (see Tables 1 and 2A,B).
Correlation of overall survival and molecular follow-up following allogeneic stem cell transplantation
JAK2 p.V617F allele burden prior to allogeneic SCT was quantified by ARMS-qPCR in all patients. At this assessment, the JAK2 p.V617F allele burden ranged from 15 to 85%, except in two patients: patient UPN 12 had only a single sample tested 8 days prior to transplantation and in this sample the JAK2 p.V617F burden was 1.3% by ARMS-qPCR, while in patient UPN 6 the JAK2 p.V617F allele burden prior to allogeneic SCT never exceeded 5%. This patient was diagnosed with systemic mastocytosis with an associated JAK2 p.V617F-positive MPN, carried a KIT p.D816V mutation and had multiple karyotypic aberrations.
In the first cohort of 14 patients molecular monitoring was performed using both methods on days 28, 56 and 84 post-transplantation. No molecular response was detected in the two patients with graft rejection. One of these patients (UPN 8) who died of transplant-related causes achieved intermittent molecular negativity, defined as undetectable JAK2 p.V617F alleles by both techniques. The other patient (UPN 4) consistently showed a significant JAK2 p.V617F allele burden with values above 1% but did not develop a clinical/hematologic relapse as defined above.
When patients were separated on the basis of JAK2 p.V617F allele burden, values >1% on day 28 after transplantation were seen in seven patients, six of whom died due to relapse (n=4), disease persistence with graft rejection (n=1) or transplant-related causes with significant persistence of JAK2 p.V617F but no hematologic relapse (n=1). In the single patient who survived (UPN 13), significant persistence of JAK2 p.V617F alleles with intermittent values >1% was seen until month 6 after SCT. Thereafter, the JAK2 p.V617F burden declined to values ≤1% and spleen size normalized. No evidence was found of hematologic disease recurrence. On the other hand, of the seven patients in whom the JAK2 p.V617F allele burden was ≤1% on day 28 after allogeneic SCT, six became molecularly negative during the observation period (conversion 2–26 months post-SCT) with undetectable JAK2 p.V617F alleles by both techniques at least at one time point. Four patients remained in hematologic remission of their MPN although one of these patients (UPN 14) experienced relapse of secondary JAK2 p.V617F-negative AML. Patient UPN 8 achieved intermittent molecular negativity but died of treatment-related causes. Patient UPN 6 already had a low JAK2 p.V617F allele burden prior to allogeneic SCT and demonstrated molecular negativity at day 40 and day 150, but died with hematologic relapse of complex MPN on day 372. Taken together, all patients who engrafted after allogeneic SCT exhibited episodes of molecular negativity if their JAK2 p.V617F allele burden was <1% one month after SCT.
Encouraged by these results, we proceeded to analyze a second cohort of 16 patients transplanted over the same time period. These patients were grouped on the basis of allele burden (> or ≤1%) on day 28 after allogeneic SCT. Among this cohort, three patients relapsed and one patient died 7 months after transplantation Two further patients relapsed with secondary JAK2 p.V617F-negative AML 6 and 21 months after their allogeneic SCT. Three patients died from transplant-related causes without evidence of MPN. Ten patients remain alive. Further details are given in Table 2A,B. Considering all patients together, the overall survival was significantly higher in the group with a low allele burden (P<0.01) indicating that two prognostic groups can successfully be discriminated based on this parameter (see also Figure 1). In contrast, univariate analysis revealed that MPN subtype, DIPSS score, initial JAK2 p.V617F allele burden, patient’s age and pretreatment did not have a significant influence on survival of the patients.
Figure 1.
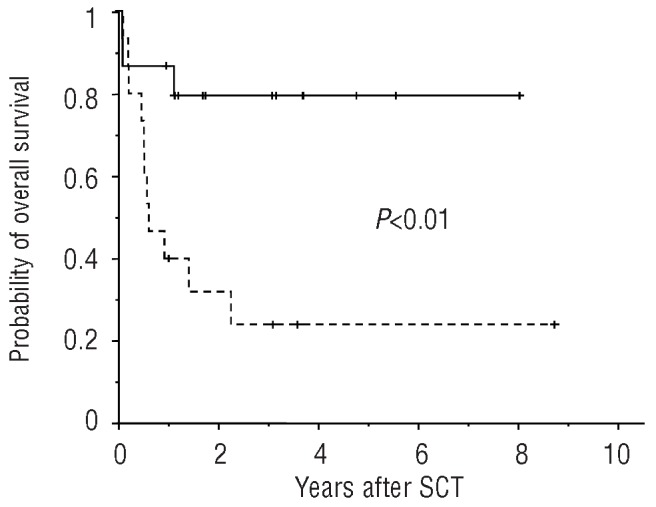
Overall survival according to the presence or absence of the risk factor JAK2 p.V617F allele burden >1% at day 28 after allogeneic SCT: 15 patients were grouped in the low-risk arm (continuous line), 15 patients in the high-risk arm (dashed line).
Probability of JAK2 p.V617F-positive relapse according to the allele burden on day 28 after transplantation
To further evaluate the impact of allele burden for the prediction of relapse of JAK2 p.V617F-positive MPN, the patients’ course was analyzed in more depth. Only the JAK2 p.V617F diseases were considered, with JAK2 p.V617F-negative AML being excluded.
Nine of the 30 patients developed a JAK2 p.V617F-positive relapse during a median follow-up of 32.1 months (range, 1.9–104.2 months) after SCT. Again, most of the patients (n=7) were grouped in the cohort with an allele burden >1%. The patient suffering from systemic mastocytosis with associated clonal hematologic non-mast-cell lineage and with only a low allele burden (<5%) preceding allogeneic SCT relapsed 4.2 months after the transplant. The other patient relapsed, after a long disease-free interval, 38.4 months after SCT (Table 2A,B). Prediction of MPN relapse on the basis of JAK2 p.V617F allele burden on day 28 was significant (P=0.04; Figure 2). Again, univariate analysis revealed no significant influence of MPN subtype, DIPSS score, initial JAK2 p.V617F allele burden, patient’s age or pretreatment on survival of the patients.
Figure 2.
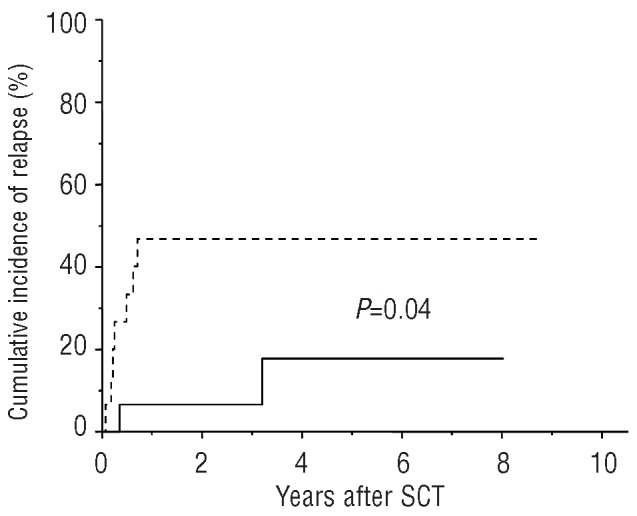
Probability of JAK2 p.V617F positive relapse according to the presence or absence of the risk factor JAK2 p.V617F allele burden >1% at day 28 after allogeneic SCT: 15 patients were grouped in the low-risk arm (continuous line), 15 patients in the high-risk arm (dashed line).
Discussion
In 2007 Kroger et al. observed that in 90% of all successfully treated patients with JAK2 p.V617F MPN, JAK2 p.V617F remained negative after a median follow-up of 20 months with JAK2 p.V617F status converting after a median of 89 days.14 In a more extensive cohort of patients, this group further demonstrated that achievement of JAK2 p.V617F clearance after allogeneic SCT was significantly associated with a decreased incidence of relapse.2 Although these studies were undoubtedly seminal, many questions still remain: (i) how important is the quantification of JAK2 p.V617F allele burden before and after allogeneic SCT and what are the sensitivity and robustness requirements for the PCR amplification procedures? (ii) is monitoring of peripheral blood samples sufficient for molecular monitoring? (iii) can we define a critical time point and a critical level of allele burden for predicting the patients’ course and outcome after allogeneic SCT? (iv) does post-transplant monitoring of JAK2 p.V617F allele burden have the potential to provide additional information not available from assessment of prognostic factors prior to allogeneic SCT?
To answer these questions we employed two previously described PCR amplification procedures with complementary strengths.14,18 The AS-WTB qPCR enables detection of JAK2 p.V617F down to trace levels after effective blocking of the wild-type allele. Semiquantitative analysis is possible since we know that AS-qPCR alone has a sensitivity of 1% and we can, therefore, conclude that positivity revealed solely with the AS-WTB qPCR indicates a level of mutated allele of less than 1%. The ARMS-qPCR is clearly superior for the quantification of allele burden but is less sensitive. As demonstrated here, the simultaneous analysis employing both assays provides a high level of sensitivity and diagnostic confidence.
Armed with these techniques we compared concurrently obtained peripheral blood and bone marrow biopsies for allele burden at defined time points. Here, we found that data concerning the discrimination of allele burden (>1% and ≤1%) matched perfectly and that molecular monitoring can be performed on peripheral blood. In this context it is important to emphasize that we employed exclusively anticoagulated full blood probes. Selection of granulocytes did not seem to be needed for routine molecular follow-up, as suggested by others.19,20
We then analyzed a cohort of 14 patients who underwent allogeneic SCT due to JAK2 p.V617F-positive MPN. To our surprise a critical JAK2 p.V617F allele burden of 1% on day 28 after allogeneic SCT distinguished two groups with clearly different outcomes and different risks of JAK2 p.V617F-positive MPN relapse. We next analyzed a further 16 patients and rechecked these findings in the whole group. In this analysis we found that a JAK2 p.V617F allele burden ≤1% on day 28 predicted a significantly higher overall survival and lower relapse risk of JAK2 p.V617F-positive MPN compared to those of patients with an allele burden >1% at this time point. Specifically, of the 15 patients with an allele burden ≤1% on day 28, only two experienced JAK2 p.V617F-positive relapses. One of these patients had a systemic mastocytosis with an associated JAK2 p.V617F-positive MPN and a JAK2 p.V617F allele burden that never exceeded 5% prior to SCT. We conclude that the accuracy of allele burden quantification as a prognostic marker can be increased still further when quantitative molecular data preceding allogeneic SCT are taken into account. The other patient developed a relapse of JAK2 p.V617F-positive MPN more than 3 years after allogeneic SCT following a long interval of molecular negativity. As the mechanisms leading to relapse differ in the early and later stages after allogeneic SCT, it is possible that the allele burden cutoff used in this study may prove to be less applicable for late relapse.
In recent years, several studies have clearly shown that a prognostic scoring system for MPN can predict the course of individual patients.21,22 Furthermore, several recently published studies suggest that post-transplant success is also dependent on pre-transplant DIPSS classification.12,23,24 Since DIPPS is only useful for primary myelofibrosis, while allogeneic SCT in our case was considered solely for intermediate-2/high-risk primary myelofibrosis or other high-risk MPN, we cannot confirm DIPSS as a predictive parameter for survival in our cohort of patients, 53% of whom suffered from primary myelofibrosis.
It should also be noted that our findings are relevant to estimating the risk of JAK2 p.V617F-positive MPN relapse and clearly cannot detect JAK2 p.V617F-negative secondary AML.
On the basis of risk identification, a reduction of immunosuppressive therapy or administration of donor lymphocyte infusions could be considered in high-risk cases at the stage of molecular relapse because these patients may benefit from a strong graft-versus-tumor effect. Similarly, immunosuppressive therapy could be tailored to minimize the risk of graft-versus-host disease as appropriate. As the JAK2 p.V617F allele burden also stratifies two groups with significantly different overall survival rates, it is possible that the adoption of further supportive measures might help to improve outcome in the cohort of patients with the less favorable outcome.
Acknowledgments
The authors would like to thank Juergen Thiele for his constant support.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355(23): 2452–66 [DOI] [PubMed] [Google Scholar]
- 2.Alchalby H, Badbaran A, Zabelina T, Kobbe G, Hahn J, Wolff D, et al. Impact of JAK2V617F mutation status, allele burden, and clearance after allogeneic stem cell transplantation for myelofibrosis. Blood. 2010;116(18):3572–81 [DOI] [PubMed] [Google Scholar]
- 3.Kroger N, Zabelina T, Schieder H, Panse J, Ayuk F, Stute N, et al. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofibrosis. Br J Haematol. 2005; 128(5):690–7 [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108(5):1497–503 [DOI] [PubMed] [Google Scholar]
- 5.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–33 [DOI] [PubMed] [Google Scholar]
- 6.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–50 [PubMed] [Google Scholar]
- 7.Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73(6):1720–8 [PubMed] [Google Scholar]
- 8.Barrett J, Childs R. Non-myeloablative stem cell transplants. Br J Haematol. 2000; 111(1):6–17 [DOI] [PubMed] [Google Scholar]
- 9.Craddock C. Nonmyeloablative stem cell transplants. Curr Opin Hematol. 1999;6 (6):383–7 [DOI] [PubMed] [Google Scholar]
- 10.Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28 (3):405–11 [DOI] [PubMed] [Google Scholar]
- 11.Sandmaier BM, McSweeney P, Yu C, Storb R. Nonmyeloablative transplants: preclinical and clinical results. Semin Oncol. 2000;27(2 Suppl 5):78–81 [PubMed] [Google Scholar]
- 12.Scott BL, Gooley TA, Sorror ML, Rezvani AR, Linenberger ML, Grim J, et al. The Dynamic International Prognostic Scoring System for myelofibrosis predicts out-comes after hematopoietic cell transplantation. Blood. 2012;119(11):2657–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patriarca F, Bacigalupo A, Sperotto A, Isola M, Soldano F, Bruno B, et al. Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Haematologica. 2008;93(10):1514–22 [DOI] [PubMed] [Google Scholar]
- 14.Kroger N, Badbaran A, Holler E, Hahn J, Kobbe G, Bornhauser M, et al. Monitoring of the JAK2-V617F mutation by highly sensitive quantitative real-time PCR after allogeneic stem cell transplantation in patients with myelofibrosis. Blood. 2007;109(3): 1316–21 [DOI] [PubMed] [Google Scholar]
- 15.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–9 [DOI] [PubMed] [Google Scholar]
- 16.Steckel NK, Koldehoff M, Ditschkowski M, Beelen DW, Elmaagacli AH. Use of the activating gene mutation of the tyrosine kinase (VAL617Phe) JAK2 as a minimal residual disease marker in patients with myelofibrosis and myeloid metaplasia after allogeneic stem cell transplantation. Transplantation. 2007;83(11):1518–20 [DOI] [PubMed] [Google Scholar]
- 17.Fiorini A, Reddiconto G, Farina G, Marietti S, Palladino M, Chiusolo P, et al. Eradication of JAK2 V617F mutation after allogeneic transplantation in a patient with myelofibrosis with myeloid metaplasia. Leukemia. 2006;20(12):2198–9 [DOI] [PubMed] [Google Scholar]
- 18.Siebolts U, Lange T, Niederwieser D, Wickenhauser C. Allele-specific wild-type blocker quantitative PCR for highly sensitive detection of rare JAK2 p.V617F point mutation in primary myelofibrosis as an appropriate tool for the monitoring of molecular remission following therapy. J Clin Pathol. 2010;63(4):370–2 [DOI] [PubMed] [Google Scholar]
- 19.Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110(12):4030–6 [DOI] [PubMed] [Google Scholar]
- 20.Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840–6 [DOI] [PubMed] [Google Scholar]
- 21.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901 [DOI] [PubMed] [Google Scholar]
- 22.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–8 [DOI] [PubMed] [Google Scholar]
- 23.Alchalby H, Yunus DR, Zabelina T, Kobbe G, Holler E, Bornhauser M, et al. Risk models predicting survival after reduced-intensity transplantation for myelofibrosis. Br J Haematol. 2012;157(1):75–85 [DOI] [PubMed] [Google Scholar]
- 24.Ditschkowski M, Elmaagacli AH, Trenschel R, Gromke T, Steckel NK, Koldehoff M, et al. Dynamic international prognostic scoring system scores, pre-transplant therapy and chronic graft-versus-host disease determine outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica. 2012;97(10):1574–81 [DOI] [PMC free article] [PubMed] [Google Scholar]


