Abstract
The multiple cellular targets affected by proteasome inhibition implicate a potential role for bortezomib, a first-in-class proteasome inhibitor, in enhancing antitumor activities in hematologic malignancies. Here, we examined the antitumor activity and drug targets of bortezomib in leukemia cells. Human leukemia cell lines were used for in vitro studies. Drug efficacy was evaluated by apoptosis assays and associated molecular events assessed by Western Blot. Gene silencing was performed by small interference RNA. Drug was tested in vivo in xenograft models of human leukemia cell lines and in primary leukemia cells. Clinical samples were assessed by immunohistochemical staining. Bortezomib differentially induced apoptosis in leukemia cells that was independent of its proteasome inhibition. Cancerous inhibitor of protein phosphatase 2A, a cellular inhibitor of protein phosphatase 2A, mediated the apoptotic effect of bortezomib. Bortezomib increased protein phosphatase 2A activity in sensitive leukemia cells (HL-60 and KG-1), but not in resistant cells (MOLT-3 and K562). Bortezomib’s downregulation of cancerous inhibitor of protein phosphatase 2A and phospho-Akt correlated with its drug sensitivity. Furthermore, cancerous inhibitor of protein phosphatase 2A negatively regulated protein phosphatase 2A activity. Ectopic expression of CIP2A up-regulated phospho-Akt and protected HL-60 cells from bortezomib-induced apoptosis, whereas silencing CIP2A overcame the resistance to bortezomib-induced apoptosis in MOLT3 and K562 cells. Importantly, bortezomib exerted in vivo antitumor activity in HL-60 xenografted tumors and induced cell death in some primary leukemic cells. Cancerous inhibitor of protein phosphatase 2A was expressed in leukemic blasts from bone marrow samples. Cancerous inhibitor of protein phosphatase 2A plays a major role in mediating bortezomib-induced apoptosis in leukemia cells.
Introduction
Intensive chemotherapy followed by consolidative chemotherapy remains the backbone of treatment for most acute leukemia.1 For refractory or relapsed acute leukemia, allogeneic hematopoietic stem cell transplantation plays an important salvaging role.1
However, prognosis of the comorbid elderly or patients ineligible for transplants remains poor.2–4 Moreover, chemoresistance manifested by low complete remission rates and short response durations to conventional salvaging chemotherapy regimens are common and challenging problems, highlighting the need for novel molecular targeted strategies for acute leukemia. Bortezomib, a modified dipeptidyl boronic acid, is a specifically designed reverse-inhibitor of the 26S proteasome (a key barrel-shaped multiprotein for degradation of ubiquitylated proteins). It is well-known that the nuclear factor-κB (NF-κB) transcription factor plays a vital role in cell proliferation, apoptosis, tumor cell invasiveness and metastasis, tumorigenesis and angiogenesis, particularly in multiple myeloma.5–7 Inactive NF-κB is bound in the cytoplasm to its inhibitor IκB and upon activation IκB is phosphorylated, ubiquinated, and degraded by the proteasome thereby releasing NF-κB to locate to the nucleus. Through a blockade on the proteasome degradation of IκB, bortezomib has shown remarkable in vitro and clinical anti-tumor activity against multiple myeloma and mantel cell lymphoma that has resulted in the approval of the drug for clinical treatment of these two hematologic malignancies.6,7
Moreover, the multiple cellular targets affected by proteasome inhibition implicate a potential role for bortezomib in enhancing antitumor activities in hematologic malignancies. Bortezomib combined with conventional chemotherapy or other novel investigational agents is currently undergoing several early (I or II) phase trials for treatment of relapsed or refractory leukemia (NCT clinical trials: NCT00440726, NCT01075425, NCT00666588, NCT01127009).
Interestingly, more and more studies are showing that bortezomib exerts different cytotoxic effects on different cancers (solid tumors and hematologic malignancies),8–12 suggesting that its mechanism of action may not necessarily depend on its proteasome inhibitory effect. Indeed, our previous study showed that downregulation of phospho-Akt (P-Akt) plays a key role in determining the sensitivity of hepatocellular carcinoma (HCC) cells to bortezomib-induced apoptosis.13 Importantly, we found that the differential cytotoxic effects of bortezomib on HCC are independent of its proteasome inhibition.13 Recently, we found that protein phosphatase 2A (PP2A), a major endogenous negative regulator of Akt signaling, may play a major role in regulating the bortezomib induced downregulation of P-Akt and subsequent apoptosis in HCC cells.14 Our data illustrated that okadaic acid, a PP2A inhibitor, reversed the downregulation of P-Akt in bortezomib-treated cells, and PP2A knockdown by small interference RNA (siRNA) also reduced apoptosis induced by a combination of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and bortezomib, indicating that bortezomib-induced P-Akt downregulation may be PP2A dependent.14
PP2A is a serine/threonine protein phosphatase complex that regulates cell proliferation via dephosphorylation of oncogenic kinases such as Akt and Erk.15 PP2A consists of a scaffold subunit (A), a catalytic subunit (C, the AC core enzyme), and a wide variety of regulatory subunits (B).16 The substrate specificity, cellular localization and enzymatic activity of PP2A are believed to be largely regulated by the regulatory B subunit.15 Several cellular inhibitors of PP2A have been identified, including SET,17,18 and cancerous inhibitor of protein phosphatase 2A (CIP2A).19 CIP2A, originally named KIAA1524 or P90, has been cloned from patients with HCC.20 CIP2A has been shown to promote anchorage-independent cell growth and in vivo tumor formation by inhibiting PP2A activity toward c-Myc.19 In addition to HCC, CIP2A is over-expressed in several human malignancies including gastric cancer, head and neck cancer, colon cancer, breast cancer, prostate cancer and non-small cell lung cancer.19–25 Similarly, Come et al.25 found that CIP2A is associated with clinical aggressiveness in human breast cancer and promotes the malignant growth of breast cancer cells. Of note, very recently CIP2A has been found to be related to hematologic malignancies; in the first case recorded, an MLL-KIAA1524 fusion gene was identified in an infant with acute myeloid leukemia (AML).26 Importantly, CIP2A is also over-expressed in patients with newly diagnosed/relapsed AML,27 and has been shown to be an important determinant of disease progression to blast crisis in chronic myeloid leukemia (CML).28
In this study, we confirmed that bortezomib-induced apoptosis in leukemia cells occurs via upregulation of PP2A activity and subsequent P-Akt downregulation. We further demonstrated that CIP2A, through inhibition of PP2A-dependent P-Akt inactivation, mediates the apoptotic effect of bortezomib in leukemia cells. Bortezomib down-regulates CIP2A in sensitive leukemia cells but not in resistant ones. More importantly, knockdown of CIP2A by siRNA restores the effects of bortezomib on PP2A-dependent P-Akt inactivation in resistant leukemia cells. CIP2A is, therefore, a major molecular determinant of bortezomib-induced apoptosis in leukemia cells and may thus have value as a predictive biomarker of clinical response to bortezomib in leukemia.
Design and Methods
Reagents and antibodies
Bortezomib (Velcade®) was kindly provided by Millennium Pharmaceuticals. For in vitro studies, bortezomib at various concentrations was dissolved in dimethyl sulfoxide (DMSO) and then added to cells in RPMI-1640 medium (Invitrogen) supplemented with 5% fetal bovine serum. Final DMSO concentration was 0.1% after addition to the medium. Okadaic acid and 1,9 dideoxy-forskolin were purchased from Cayman Chemical (Ann Arbor, MI, USA). Antibodies for immunoblotting such as anti-Akt1, NF-κB and CIP2A were purchased from Santa Cruz Biotechnology (San Diego, CA, USA). Other antibodies such as anti-caspase-3, p-Erk 1/2 and Erk 1/2 and P-Akt (Ser473 & Thr308) were from Cell Signaling (Danvers, MA, USA).
Statistical analysis
Data are expressed as mean ± SD or SE. Statistical comparisons were based on non-parametric tests. P<0.05 was considered significant. All statistical analyses were performed using SPSS for Windows version 12.0 software (SPSS, Inc., Chicago, IL, USA).
Further details of Design and Methods are described in the Online Supplementary Appendix.
Results
Differential anti-leukemic effects of bortezomib on leukemia cells
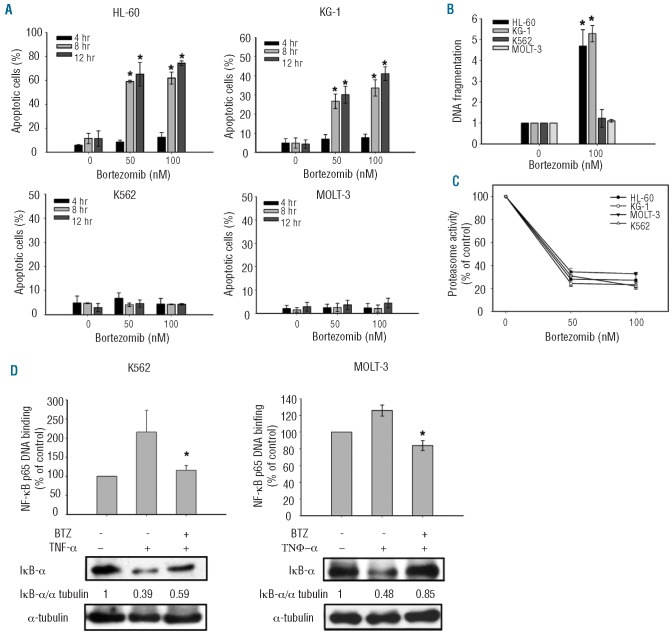
To investigate the antitumor effect of bortezomib on leukemia cells, we first assessed the apoptotic effect of bortezomib in a panel of four human leukemia cell lines, HL-60, KG-1, K562 and MOLT-3, at clinically relevant concentrations.29 Flow cytometry analysis of sub-G1 cells showed that bortezomib induced differential apoptotic effects on the four leukemia cell lines (Figure 1A). Bortezomib induced apoptosis at 8 h and 12 h of treatment in HL-60 and KG-1 cells, whereas no apparent apoptotic effects were elicited by bortezomib in K562 and MOLT-3 cells (Figure 1A). Examination of DNA fragmentation through a cell death ELISA assay confirmed that bortezomib induced differential apoptosis in these cells (Figure 1B). After 12 h of treatment, bortezomib killed HL-60 and KG-1 cells (P<0.05) but not K562 and MOLT-3 cells (Figure 1B). These data indicated that HL-60 and KG-1 cells were sensitive to the cytotoxic activity of bortezomib, whereas K562 and MOLT-3 cells were resistant.
Figure 1.
Differential anti-leukemic effects of bortezomib on leukemia cells. (A) Dose-and time-escalation effects of bortezomib (50 nM or 100 nM) on apoptosis in four leukemia cell lines (HL-60, KG-1, K562, and MOLT-3). Cells were exposed to bortezomib at the indicated doses for 4, 8 and 12 h. Apoptotic cells were determined by flow cytometry. Columns, mean (n=3); bars, SD; *P<0.05. (B) Effects of bortezomib on DNA fragmentation in four leukemia cells. Cells were treated with bortezomib (100 nM) for 12 h and DNA fragmentation was analyzed by using a cell death ELISA kit. Columns, mean (n=3); bars, SD; *P<0.05. (C) Bortezomib exerts efficient and similar dose-dependent effect on proteasome inhibition in the four leukemia cells. Cells were exposed to bortezomib at the indicated doses for 6 h before measurement of proteasome activity. (D) Bortezomib still inhibits the proteasome degradation of IkB (inhibitor of NF-κB) in resistant cells. In resistant K562 (left) and MOLT-3 cells (right), bortezomib abolished the NF-κB activation induced by TNF-α, as evidenced by decreased nuclear NF-κB p65 subunit binding activity, and associated increased IκB-α protein level. Cells were exposed to 20 ng/mL of TNF-α for 1 h and then treated with DMSO or 100 nM bortezomib for 24 h. Nuclear extracts were prepared and assayed for NF-κB p65 subunit binding activity by ELISA kit. Columns, mean (n=3); bars, SD. *P<0.05. Cytoplasmic extracts were prepared and assayed for IκB-α by Western blot. Representative of 3 independent experiments.
Bortezomib exerts similar, efficient proteasome inhibition in both sensitive and resistant leukemia cells
To explore the mechanism by which bortezomib induces apoptosis in these leukemia cell lines, we first examined the proteasome inhibitory effects of bortezomib in the four cell lines. Proteasome activity was measured in the four cell lines after 6 h of treatment with bortezomib. Bortezomib treatment resulted in similar dose-dependent effects on proteasome inhibition in all four leukemia cells (Figure 1C). In addition, previous studies have shown that bortezomib inhibited NF-κB signaling through inhibiting the proteasome degradation of IkB (inhibitor of NF-κB), therefore we examined the DNA-binding activity of the nuclear NF-κB subunit p65 and protein levels of IκB in resistant K562 and MOLT-3 cells treated with bortezomib. Since in cancers NF-kB signaling is often stimulated by tumor-associated cytokines, such as TNF-alpha, we used TNF-α stimulation to mimic the biological event in vivo. TNF-α stimulation increased the nuclear NF-κb binding activity in association with downregulation of IkB (Figure 1D). Notably, bortezomib abolished NF-κB activation induced by TNF-α, in association with increased cytoplasmic protein levels of IκB-α even in the resistant cells, suggesting similar proteasome inhibition in all these cells (Figure 1D). These results suggest that the differential induction of apoptosis by bortezomib in leukemia cells may not necessarily be dependent on proteasome inhibition, which is consistent with our previous findings in HCC cells.13
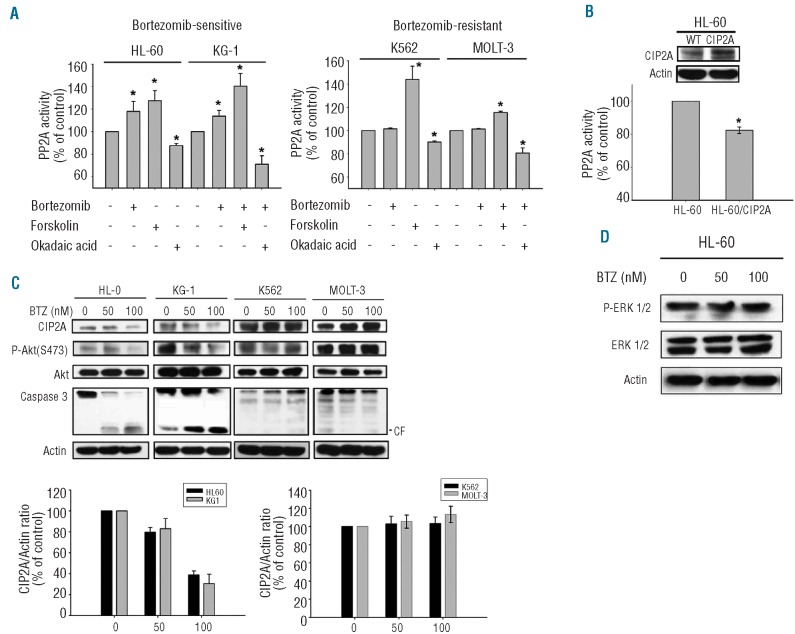
Bortezomib enhances PP2A activity, which is regulated by CIP2A, and down-regulates CIP2A and P-Akt in sensitive leukemia cells
Our previous study demonstrated that PP2A mediated the sensitizing effect of bortezomib on TRAIL-induced cell apoptosis in HCC.14 Here we investigated the effects of bortezomib on PP2A, P-Akt and CIP2A in association with apoptosis in leukemia cells. As shown in Figure 2A, bortezomib significantly increased the phosphatase activity of PP2A in bortezomib-sensitive leukemia cell lines, HL-60 and KG-1, whereas bortezomib did not influence the phosphatase activity in resistant K562 and MOLT-3 cell lines (Figure 2A). In addition, okadaic acid, a PP2A inhibitor acting as a negative control, decreased the phosphatase activity of PP2A in these four cell lines; while forskolin, a PP2A agonist acting as a positive control, increased PP2A activity in these cells (Figure 2A). Moreover, consistent with previous findings in HCC,14,30 the expression of PP2A complex including subunits A, B56γ and C was not significantly altered by bortezomib (data not shown). Next, we examined PP2A activity in HL-60 cells with ectopic expression of CIP2A (HL-60-CIP2A) by transfection with CIP2A. Ectopic expression of CIP2A reduced PP2A activity in HL-60-CIP2A cells as compared with wild-type HL-60 cells (Figure 2B). This result supports the previously known regulatory role of CIP2A on PP2A.19 We then further examined the molecular events associated with apoptosis in bortezomib-treated leukemia cells. Bortezomib decreased protein levels of CIP2A and induced apoptosis in sensitive HL-60 and KG-1 cells in a dose-dependent manner, but did not show similar effects in resistant K562 and MOLT-3 cells (Figure 2C). Furthermore, inhibition of CIP2A was associated with downregulation of P-Akt and induction of apoptosis which is evidenced by the activation (cleavage) of caspase-3 in sensitive cells (Figure 2C). On the contrary, bortezomib did not affect protein levels of CIP2A, P-Akt, Akt or induction of apoptosis in resistant K562 and MOLT-3 cells (Figure 2C). Moreover, since P-Akt may also be regulated by Erk 1/2 activity,31,32 another pathway linked to cell survival, we examined the phosphorylation of Erk 1/2 in HL60 cells treated with bortezomib and found it made no significant alteration of Erk phosphorylation (Figure 2D). These results suggest that inhibition of CIP2A is the major determinant of bortezomib-induced apoptosis in leukemia cells.
Figure 2.
Bortezomib enhances PP2A activity, which is regulated by CIP2A, and down-regulates CIP2A and P-Akt in sensitive leukemia cells. (A) Analysis of PP2A activity in drug-treated leukemia cells. Columns, mean (n=3); bars, SD; *P<0.05. Cells were treated with DMSO or bortezomib at 50 nM or okadaic acid at 20 nM (as negative control) or forskolin 40 μM (as a positive control) for 24 h. Cell lysates were prepared for detecting PP2A activity. (B) Analysis of PP2A activity in CIP2A-overexpressed HL-60 cells. Ectopic expression of CIP2A reduced PP2A activity in HL-60 cells. Columns, mean (n=3); bars, SD; *P<0.05. (C) (Top) Dose-dependent analysis of CIP2A, P-Akt and caspase-3. Cells were exposed to bortezomib (BTZ) for 6 h at the indicated doses. Cell lysates were prepared and assayed for these molecules by Western blotting. Representative of 3 independent experiments. CF: cleaved form (activated form). (Bottom) Ratio of CIP2A to actin levels. Immunoblots were scanned by a UVP BioSpectrum AC image system and quantitated using VisionWork LS software. Columns, mean (n=3); bars, SD. (D) Bortezomib did not significantly alter the phosphorylation of Erk 1/2 in HL-60 cells. Cells were exposed to bortezomib at the indicated doses for 6 h. Cell lysates were prepared and assayed for p-Erk 1/2 and Erk by Western blotting. Representative of 3 independent experiments.
Target validation of CIP2A as a molecular determinant in bortezomib-induced apoptosis
To validate the role of CIP2A signaling in mediating the apoptotic effect of bortezomib in leukemia cells, we first knocked down protein expression of CIP2A in resistant MOLT-3 and K562 cells by using siRNA. Downregulation of CIP2A by bortezomib is associated with downregulation of P-Akt and sensitized the resistant MOLT-3 and K562 cells to bortezomib-induced apoptosis (Online Supplementary Figure S1A and B). Notably, knocked down CIP2A expression by CIP2A siRNA alone was insufficient to induce significant apoptosis either in MOLT-3 or in K562 cells, despite P-Akt also being down-regulated (Online Supplementary Figure S1A and B). This suggests that other mechanisms may also participate in bortezomib-induced apoptosis. Next, we generated HL-60-Akt cells that constitutively express ectopic myc-tagged Akt (Online Supplementary Figure S1C). Similarly, we generated HL-60-CIP2A cells that constitutively express ectopic CIP2A (Online Supplementary Figure S1D). Notably, HL-60-CIP2A cells also expressed constitutively activated P-Akt (Online Supplementary Figure S1D). Constitutive ectopic expression of either myc-tagged Akt or CIP2A protected sensitive HL-60 cells from apoptotic death induced by bortezomib (Online Supplementary Figure S1C and D). These results indicate that CIP2A plays a key role in mediating the apoptotic effect of bortezomib in leukemia cells.
Bortezomib down-regulates transcription of CIP2A in leukemia cells
To examine the effects of bortezomib on CIP2A expression, we first examined whether bortezomib could affect CIP2A elimination (degradation) when translation was blocked by the protein synthesis inhibitor cycloheximide. Our data showed that after protein translation was blocked by cyclohexamide, the rate of CIP2A degradation did not change significantly with or without bortezomib treatment in HL-60 cells (Online Supplementary Figure 2A) suggesting that the effect of bortezomib on CIP2A may occur at the pre-translation level and that CIP2A might not be proteasome degradation substrate. We next investigated whether bortezomib affected CIP2A transcription. mRNA levels of CIP2A (as measured by a semi-quantitative nested polymerase chain reaction, PCR), decreased in a dose-dependent manner upon treatment with bortezomib in sensitive HL-60 cells (Online Supplementary Figure 2B). However, in resistant K562 cells, CIP2A mRNA was not inhibited by bortezomib (Online Supplementary Figure 2B). Failure of inhibiting CIP2A transcription by bortezomib suggests drug resistance. Further studies are needed to unravel the mechanisms underlying resistance to bortezomib-induced downregulation of CIP2A in K562 and MOLT3 cells.
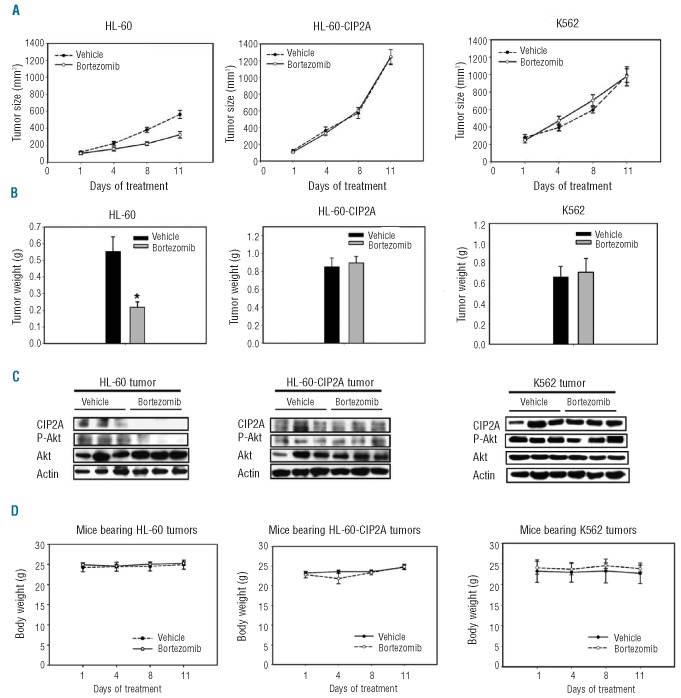
Effect of bortezomib on leukemia xenograft tumor growth in vivo
To confirm that the effect of bortezomib on CIP2A has potentially relevant clinical implications in leukemia, we assessed the in vivo effect of bortezomib on leukemia xenograft tumors. HL-60 xenografted, HL-60-CIP2A (HL-60 with ectopic over-expressed CIP2A) xenografted and resistant K562 xenografted tumor mice were generated to validate CIP2A’s role in vivo. Tumor-bearing mice were treated with vehicle or bortezomib intraperitoneally at the clinically relevant dose of 0.5 mg/kg twice a week for two weeks. Bortezomib significantly inhibited HL-60 tumor growth (P<0.05) and the mean tumor size in the bortezomib treatment group was approximately 50% that of control at the end of treatment (Figure 3A). The mean tumor weight was also significantly reduced in bortezomib-treated mice (Online Supplementary Figure 1B). In contrast, bortezomib did not inhibit HL-60-CIP2A nor K562 tumor growth, as measured by tumor size (Figure 3A) and tumor weight (Figure 3B). Notably, HL-60-CIP2A tumors grew larger and weighed more than wild-type HL-60 tumors (Figure 3A and B, P<0.05), suggesting more aggressive tumor behavior in leukemia cells with over-expressed CIP2A. To correlate biological response with the mechanism of action identified in vitro, the effect of bortezomib on CIP2A and P-Akt in these tumors was examined by Western blot. Figure 3C shows Western blots of Akt and P-Akt in the homogenates of three representative HL-60, HL-60-CIP2A, and K562 tumors. Overall, there was a significant decrease in CIP2A and P-Akt in HL-60 tumors treated with bortezomib, whereas no significant changes were observed in control (vehicle) (Figure 3C). In addition, CIP2A protein levels were not significantly altered by bortezomib in resistant HL-60-CIP2A tumors or K562 tumors (Figure 3C). Importantly, all animals tolerated the treatments well without observable signs of toxicity and had stable body weights throughout the course of the study (Figure 3D). No gross pathological abnormalities of major organs such as liver, lung, spleen, intestine and heart were noted at mice necropsy examination.
Figure 3.
In vivo effect of bortezomib on HL-60, HL-60-CIP2A (HL-60 with ectopic over-expressed CIP2A), and K562 xenograft nude mice. (A) Bortezomib decreased the size of HL-60 tumors (left), but had no anti-tumor effects on HL-60-CIP2A tumors (middle) or on K562 tumors (right). Points, mean (n = 6); bars, SE; *P<0.05. (B) Bortezomib reduced the weight of HL-60 tumors (left), but had no effect on that of HL-60-CIP2A tumors (middle) or that of K562 tumors (right). Columns, mean (n = 6); bars, SD; *P<0.05. (C) Western blot analysis of CIP2A and P-Akt in HL-60, HL-60-CIP2A and K562 tumors. (D) Body weight of xenograft mice bearing HL-60 tumors (left) and HL-60-CIP2A tumors (middle) and K562 tumors (right) during the in vivo experiment. Points, mean (n = 6); bars, SE. ).
Effects of bortezomib in primary leukemia specimens
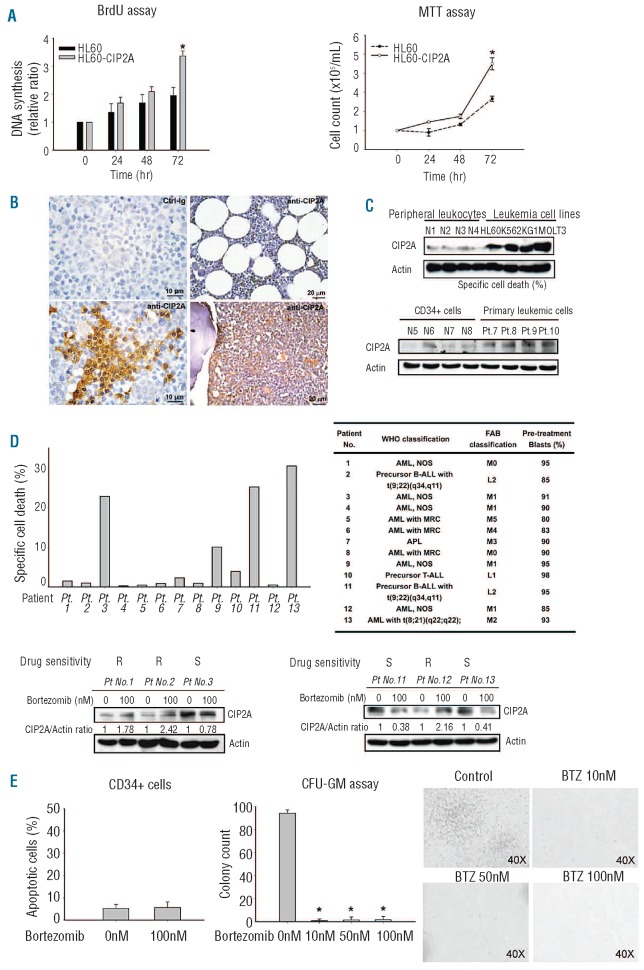
Several studies have indicated the oncogenic role of CIP2A in several solid tumors;19,22,25 however, it remains unclear whether CIP2A also contributes to leukemia aggressiveness. As the in vivo xenografted HL-60-CIP2A tumor grew larger and faster (Figure 3A and B), we further checked DNA synthesis and cell proliferation in HL-60-CIP2A cells. DNA synthesis and cell proliferation in HL-60-CIP2A cells was significantly increased at 72 h in comparison with wild-type HL-60 cells (Figure 4A). These results suggest that CIP2A promotes leukemia cell DNA synthesis and proliferation. Next, we performed immunohistochemical staining for CIP2A in clinical bone marrow biopsy specimens and found that CIP2A antibody stained only weakly and focally in the cells in the marrow from 2 patients with idiopathic thrombocytopenic purpura (Figure 4B). In contrast, leukemic cells were diffusely positive for CIP2A in samples from 2 patients with over 80% AML blasts (Figure 4B); focal but strong staining for CIP2A was also found in the marrow of an AML patient with around 25% blasts (Figure 4B). Furthermore, CIP2A expression was significantly higher in the four leukemic cell lines than in the peripheral leukocytes from 4 normal healthy volunteers (Figure 4C). Similarly, CIP2A expression is higher in primary leukemic cells than in CD34+ normal hematopoietic cells (Figure 4C). Importantly, we tested effects of bortezomib in primary leukemic cells from 13 patients with leukemia whose characteristics (see Figure 4D for patients’ characteristics) and found that bortezomib showed some efficacy (shown as specific cell death) in these cells, which correlated with its downregulation of CIP2A (Figure 4D). Due to limited samples, no further analysis on the correlation with drug efficacy and disease diagnosis could be made. Further studies on the role of CIP2A in leukemogenesis and leukemia tumor behavior are warranted. Finally, we examined the effect of bortezomib on normal hematopoietic cells and found that bortezomib did not induce significant apoptosis in normal hematopoietic cells but did inhibit CFU-GM colony growth (Figure 4E). The inhibition of colonogenic assay by bortezomib treatment is consistent with bortezomib-induced myelosuppression as reported in clinical trials.33
Figure 4.
Effects of bortezomib in primary leukemia specimens. (A) (Left) DNA synthesis of HL-60 and HL-60-CIP2A cells as measured by BrdU incorporation assay. Columns, mean (n=3); bars, SD; *P<0.05. (Right) cell proliferation curve of HL-60 and HL-60-CIP2A cells as measured by MTT assay. Points, mean (n=3); bars, SE; *P<0.05. (B) CIP2A immunohistochemical staining in paraffin-embedded bone marrows from patients with acute myeloid leukemia (AML) and from controls. (Upper left) No signal was detected in an AML bone marrow stained with control rabbit IgG as a control. (Upper right) Weak and focal stainings for CIP2A in the bone marrow tissue of a patient with idiopathic thrombocytopenic purpura (ITP). A similar result was seen in another patient with ITP (not shown). (Lower left) The same AML bone marrow in upper left panel showed positive signals for CIP2A in tumor cells. (Lower right) Diffuse and intense CIP2A expression was detected in the bone marrow of another patient with AML. Scale bars are shown. (C) Comparison of CIP2A expression by Western blot analysis in peripheral leukocytes from normal healthy volunteers (N1-N4) and in the four leukemia cells (upper) and comparison of CIP2A expression in CD34+ normal hematopoietic cells (N5-N6) and in primary leukemic cells (Patient ns.7–10) (lower). The CIP2A expression is significantly higher in the human leukemic cell lines than in the normal peripheral leukocytes. Similarly, CIP2A expression is higher in primary leukemic cells than in CD34+ normal hematopoietic cells. Representative of 3 independent experiments. (D) Effects of bortezomib in primary leukemic cells. Primary leukemic cells from 13 patients were collected and treated with bortezomib 100 nM for 12 h. Specific cell death by bortezomib was defined in Design and Methods. (Upper left) Bortezomib induced significant apoptosis in some primary leukemic cells. (Upper right) The WHO and FAB classifications of 13 patients (ns. 1–13) with leukemia and percentage of leukemic blasts in marrow. (Lower panel) Selective Western blot analysis of primary leukemic cells treated with bortezomib. In bortezomib-sensitive samples (patient ns. 3, 11 and 13), bortezomib-induced cell death correlates with downregulation of CIP2A. In bortezomib-resistant samples (patient ns. 1, 2 and 12), bortezomib fails to down-regulate CIP2A expression. Representative of 3 independent experiments. Pt.: patient; AML NOS: acute myeloid leukemia not otherwise specified; ALL: acute lymphocytic leukemia; AML with MRC: acute myeloid leukemia with myelodysplasia-related changes; S: sensitive; R: resistant. (E) Effect of bortezomib on normal hematopoietic stem cells. Short-term treatment (6 h) with bortezomib did not significantly induce apoptosis in CD34+ normal hematopoietic cells (left). Bortezomib suppressed colony-forming ability of CD34+ normal hematopoietic cells (middle). Columns, mean (n=3); bars, SD; *P<0.05. Representative photocopies of clonogenic (CFU-GM) assay were shown (right). For CFU-GM assay, collected CD34+ normal hematopoietic cells were treated with bortezomib 0, 10, 50 and 100 nM for 6 h and cultured in MethoCult® methylcellulose-based medium as described in Online Supplementary Design and Methods. Colonies were scored after 14 days of culture.
Discussion
This study uncovers a novel mechanism by which bortezomib induces apoptosis in leukemia cells: the CIP2A-PP2A-P-Akt regulatory machinery. This finding has several potential important implications. First, we identified CIP2A as a novel molecular determinant of cell sensitivity to bortezomib-induced apoptosis, and demonstrated that bortezomib-induced apoptosis does not necessarily depend on its proteasome inhibition. Although bortezomib exerts excellent anti-cancer activity in multiple myeloma and mantle cell lymphoma through its proteasome inhibitory effects, clinical trials have shown that bortezomib is less efficient or shows transient anti-cancer activity in solid tumors or other hematologic malignancies.9–12 Our findings may partly explain this discrepancy. Bortezomib has been shown to cause G2/M arrest which is associated with its inhibition of the proteasome-mediated degradation of cell cycle regulators, such as p27, cyclin E, etc. which is in turn linked to bortezomib-mediated apoptosis.34,35 In the current study, we observed similar G2/M arrest during flow cytometry analysis of sub-G1 apoptotic cells in bortezomib sensitive or resistant leukemia cell lines (data not shown). Buzzeo et al. have also demonstrated that G2/M arrest induced by bortezomib’s proteasome inhibition is not correlated with the sensitivity of cells to bortezomib-induced apoptosis.36 We showed that bortezomib efficiently inhibits proteasome activity in all tested leukemia cells, as demonstrated by proteasome activity analysis and by efficient NF-kB inhibition even in bortezomib-resistant leukemia cells (Figure 1C and D). However, bortezomib induces differential apoptotic effects in these leukemia cells. This differential apoptotic effect in leukemia cells is not associated with bortezomib’s proteasome inhibition, but is clearly associated with CIP2A downregulation. We demonstrated that in sensitive leukemia cells bortezomib increases PP2A activity through downregulation of CIP2A. We thus propose that, in addition to the several known mechanisms of bortezomib resistance in cancers,6,37 the CIP2A-PP2A-P-Akt pathway may contribute to bortezomib resistance. We and others have shown that sorafenib restores the apoptotic effects of bortezomib in various tumor cells through downregulation of P-Akt in co-treated cancer cells.38,39 Furthermore, novel Akt inhibitors such as perifosine, have also been shown to synergize with bortezomib and thus may be able to overcome bortezomib resistance in cancer cells.40,41
Our study also suggests that CIP2A, as a newly discovered oncoprotein, may be a potential drug target for anti-cancer agents. Our findings indicate that CIP2A may be a molecular determinant of bortezomib’s sensitivity in leukemia cells. Recently Choi et al.42 also showed that doxorubicin could down-regulate CIP2A. Therefore, anti-cancer agents that target CIP2A may be an attractive alternative anti-cancer strategy. However, how these agents down-regulate CIP2A remains unknown and further mechanistic studies are necessary to consolidate CIP2A as a useful drug target. In addition, future immunohistochemical study of in vivo leukemia blasts and the correlation between CIP2A expression and drug-sensitivity in leukemia patients may help to establish a clinical role for CIP2A as a predictive factor.28 We also found that bortezomib enhances phosphatase (PP2A) activity through downregulation of CIP2A, suggesting that targeting phosphatase may be a novel anti-cancer strategy. It is known that protein kinases play important roles in regulating most cellular functions, such as proliferation/cell cycle, cell metabolism, survival/apoptosis, DNA damage repair, cell motility, response to the microenvironment, etc.43 Kinases such as c-Src, c-Abl, mitogen activated protein kinase, phosphotidylinositol-3-kinase (PI3K), Akt, and the epidermal growth factor receptor are commonly activated in cancer cells, and these important oncogenic pathways have been hot targets of novel targeted anti-cancer agents. Through dephosphorylation, protein phosphatases play extremely important roles in regulating the activities of protein kinases and their upstream or downstream kinases and thus a large number of cellular activities. In this regard, PP2A regulates oncogenic kinases such as Myc, Akt and Erk. Interestingly, Cristobàl et al.44 recently reported that PP2A inactivation is a recurrent event in AML and that restoration of PP2A activity with forskolin in AML cells could down-regulate P-Akt and induce apoptosis. In line with their finding, we also found that forskolin enhances PP2A activity in leukemia cells and further showed that bortezomib is a feasible agent to enhance PP2A activity. Our data suggest that using bortezomib as a lead to discover novel compounds that enhance PP2A activity might be a novel approach for anti-cancer drug development.
Our data showed that CIP2A promotes leukemia-cell DNA synthesis and proliferation and facilitates xenograft tumor growth. Previous studies have suggested that CIP2A is an oncoprotein that promotes tumor aggressiveness in several solid tumors.19,22,24–25,45 Junttila et al.19 found that through inhibition of the interaction of PP2A with c-Myc, CIP2A stabilizes the oncogenic activity c-Myc, a mechanism that may explain the promotion of tumor aggressiveness by CIP2A. Moreover, Lucas et al. showed that CIP2A levels at diagnosis can consistently predict CML patients who will progress to blast crisis.28 They also demonstrated that siRNA-mediated knockdown of CIP2A expression in K562 cells results in increased PP2A activity, decreased c-Myc levels, and a decrease in Bcr-Abl tyrosine kinase activity.28 In addition to this known molecular interaction, our recent results30 and those from the current study strongly suggest that CIP2A also influences the interaction between PP2A and its other important substrates (in this study, P-Akt). Our results suggest that, in addition to the inhibition of c-Myc-associated PP2A activity, CIP2A might also exert its oncogenicity through the regulation of P-Akt and its downstream signaling, thereby increasing drug resistance. Interestingly, recently a novel MLL-KIAA1524 fusion gene was identified in an infant with AML.26 MLL is a histone methyltransferase that has been shown to be a positive global regulator of gene transcription and is involved in the epigenetic maintenance of transcriptional memory.46 In the future, it will be important to find out whether rearrangement of MLL with CIP2A is associated with aberrant CIP2A expression and aggressive leukemia.
Our data show that bortezomib is able to decrease CIP2A transcription but does not affect the half-life of CIP2A protein degradation (Online Supplementary Figure S2), suggesting that the effect of bortezomib on CIP2A occurs pre-translation and is possibly irrelevant to its proteasome inhibition. There are several possible mechanisms through which bortezomib may affect the transcription of CIP2A, such as direct or indirect promoter regulation of CIP2A mRNA, epigenetic regulation of the CIP2A gene by DNA methylation or micro-RNA machinery, or affecting other uncovered molecules that regulate CIP2A expression. Future studies on how bortezomib affects CIP2A transcription are needed. In addition, although we used cycloheximide (a common laboratory agent used to inhibit protein synthesis) to determine whether bortezomib could affect the half-life of CIP2A protein, from our experiment design it is not known whether bortezomib would affect CIP2A protein synthesis as cycloheximide does.
Current study showed that K562 cells are resistant to bortezomib-induced apoptosis. It is known that in K562 cells Bcr–Abl can maintain its active tyrosine-phosphorylated form by inducing expression of SET, another inhibitor of PP2A,17 and that inhibition of PP2A activity diminishes SHP1 activity, a tyrosine phosphatase that dephosphorylates Bcr–Abl. Thus, SET suppresses the PP2A and SHP1 activities.18,47 A recent report further showed that Bcr–Abl does not directly activate expression of SET protein; rather Bcr-Abl activates Jak2 and in turn induces expression of SET.48 Therefore, PP2A can be controlled by molecules other than CIP2A. In this regard, our previous data showed that bortezomib did not significantly influence SET expression.30 Although it is also possible that the resistance of K562 cells to bortezomib might be associated with SET signaling, we showed that knockdown of CIP2A in K562 cells restores bortezomib-induced apoptosis in K562 cells (Online Supplementary Figure S1). Our data suggested a role of CIP2A in mediating bortezomib-induced apoptosis and did not conflict with the SET signaling pathway. Another interesting finding is that the short-term incubation of normal CD34+ cells with bortezomib does not induce apoptosis at a concentration of 100 nM. In contrast by using these CD34+ cells in CFU-GM assay, a significant reduction is shown at 10nM (Figure 4E). Therefore, it would be of interest to know whether patients’ AML cells that are defined as unresponsive in short-term culture assay would have the same pattern in the CFU-assay. To address this, we tested 3 additional primary AML cells in which bortezomib induced little apoptotic cell death in short-term suspension culture (Online Supplementary Figure S3). We found that bortezomib reduced colony formation of these primary leukemia cells by various degrees at the indicated doses (10, 50 and 100 nM). Meanwhile, the numbers of colonies for drug-free controls also showed a wide variation (Online Supplementary Figure S3). These results suggested that bortezomib also affects the more immature clonogenic leukemic subset within the hierarchically organized AML-cell population, consistent with previous studies.49
In summary, this study identified CIP2A as a major molecular determinant of the sensitivity of leukemia cells to bortezomib-induced apoptosis, suggesting that CIP2A may be a potential drug target in leukemia. This study also suggests that focusing on the interactions of oncoproteins, phosphatase and kinases could be a potential anti-leukemia strategy. Future studies defining the clinical and biological roles of CIP2A in leukemia and delineating the machinery by which bortezomib affects CIP2A expression may improve targeted therapy for leukemia and further clarify bortezomib’s anti-leukemic activity.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This research was supported by grants from the Taiwan Clinical Oncology Research Foundation; NTUH-TVGH Joint Research Program NTUH-VN-100-07, NTUH-VN-101-03 from National Taiwan University Hospital; NSC98-2314-B-002-067-MY3 and NSC100-2314-B-075-052 from the National Science Council, Taiwan; TVGH-NTUH Joint Research Program VN-100-07 VN-101-03 and V99-B1-016 from Taipei Veterans General Hospital; and The Hematology Society of Taiwan.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hubel K, Weingart O, Naumann F, Bohlius J, Fresen MM, Engert A, et al. Allogeneic stem cell transplant in adult patients with acute myelogenous leukemia: a systematic analysis of international guidelines and recommendations. Leuk Lymphoma. 2011; 52(3):444–57 [DOI] [PubMed] [Google Scholar]
- 2.Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br J Haematol. 2011;152(5):524–42 [DOI] [PubMed] [Google Scholar]
- 3.Marks DI. Treating the “older” adult with acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:13–20 [DOI] [PubMed] [Google Scholar]
- 4.Kolb HJ, Simoes B, Schmid C. Stem cell transplants for patients with relapsed/refractory leukaemia. Curr Opin Hematol. 2009;16(6):444–52 [DOI] [PubMed] [Google Scholar]
- 5.Orlowski RZ, Baldwin AS., Jr NF-κB as a therapeutic target in cancer. Trends Mol Med. 2002;8(8):385–9 [DOI] [PubMed] [Google Scholar]
- 6.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008; 14(6):1649–57 [DOI] [PubMed] [Google Scholar]
- 7.Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006;46:189–213 [DOI] [PubMed] [Google Scholar]
- 8.Milano A, Perri F, Caponigro F. The ubiquitin-proteasome system as a molecular target in solid tumors: an update on bortezomib. Onco Targets Ther. 2009;2:171–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel RH, Brown JA, Von Roenn JH, O’Regan RM, Bergan R, Badve S, et al. A phase II study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Invest. 2007;25(8):733–7 [DOI] [PubMed] [Google Scholar]
- 10.Davis NB, Taber DA, Ansari RH, Ryan CW, George C, Vokes EE, et al. Phase II trial of PS-341 in patients with renal cell cancer: a University of Chicago phase II consortium study. J Clin Oncol. 2004;22(1):115–9 [DOI] [PubMed] [Google Scholar]
- 11.Cortes J, Thomas D, Koller C, Giles F, Estey E, Faderl S, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004; 10(10):3371–6 [DOI] [PubMed] [Google Scholar]
- 12.Belch A, Kouroukis CT, Crump M, Sehn L, Gascoyne RD, Klasa R, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol. 2007;18(1):116–21 [DOI] [PubMed] [Google Scholar]
- 13.Chen KF, Yeh PY, Yeh KH, Lu YS, Huang SY, Cheng AL. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008;68(16):6698–707 [DOI] [PubMed] [Google Scholar]
- 14.Chen KF, Yeh PY, Hsu C, Hsu CH, Lu YS, Hsieh HP, et al. Bortezomib overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance in hepatocellular carcinoma cells in part through the inhibition of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2009; 284(17):11121–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bielinski VA, Mumby MC. Functional analysis of the PP2A subfamily of protein phosphatases in regulating Drosophila S6 kinase. Exp Cell Res. 2007;313(14):3117–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353(Pt 3):417–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271(19):11059–62 [DOI] [PubMed] [Google Scholar]
- 18.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8(5):355–68 [DOI] [PubMed] [Google Scholar]
- 19.Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130(1):51–62 [DOI] [PubMed] [Google Scholar]
- 20.Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21(32):5006–15 [DOI] [PubMed] [Google Scholar]
- 21.Vaarala MH, Vaisanen MR, Ristimaki A. CIP2A expression is increased in prostate cancer. J Exp Clin Cancer Res. 2010;29:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna A, Bockelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst. 2009;101(11):793–805 [DOI] [PubMed] [Google Scholar]
- 23.Katz J, Jakymiw A, Ducksworth MK, Stewart CM, Bhattacharyya I, Cha S, et al. CIP2A expression and localization in oral carcinoma and dysplasia. Cancer Biol Ther. 2010;10(7):694–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong QZ, Wang Y, Dong XJ, Li ZX, Tang ZP, Cui QZ, et al. CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann Surg Oncol. 2011;18(3):857–65 [DOI] [PubMed] [Google Scholar]
- 25.Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15(16):5092–100 [DOI] [PubMed] [Google Scholar]
- 26.Coenen EA, Zwaan CM, Meyer C, Marschalek R, Pieters R, van der Veken LT, et al. KIAA1524: A novel MLL translocation partner in acute myeloid leukemia. Leuk Res. 2010;35(1):133–5 [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Li W, Li L, Yu X, Jia J, Chen C. CIP2A is over-expressed in acute myeloid leukaemia and associated with HL60 cells proliferation and differentiation. Int J Lab Hematol. 2011;33(3):290–8 [DOI] [PubMed] [Google Scholar]
- 28.Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood. 2011;117(24):6660–8 [DOI] [PubMed] [Google Scholar]
- 29.Leveque D, Carvalho MC, Maloisel F. Review. Clinical pharmacokinetics of bortezomib. In Vivo. 2007;21(2):273–8 [PubMed] [Google Scholar]
- 30.Chen KF, Liu CY, Lin YC, Yu HC, Liu TH, Hou DR, et al. CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene. 2010;29(47):6257–66 [DOI] [PubMed] [Google Scholar]
- 31.Hayashi H, Tsuchiya Y, Nakayama K, Satoh T, Nishida E. Down-regulation of the PI3-kinase/Akt pathway by ERK MAP kinase in growth factor signaling. Genes Cells. 2008;13(9):941–7 [DOI] [PubMed] [Google Scholar]
- 32.Ripple MO, Kalmadi S, Eastman A. Inhibition of either phosphatidylinositol 3-kinase/Akt or the mitogen/extracellular-regulated kinase, MEK/ERK, signaling pathways suppress growth of breast cancer cell lines, but MEK/ERK signaling is critical for cell survival. Breast Cancer Res Treat. 2005;93(2):177–88 [DOI] [PubMed] [Google Scholar]
- 33.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–72 [DOI] [PubMed] [Google Scholar]
- 34.Colado E, Alvarez-Fernandez S, Maiso P, Martin-Sanchez J, Vidriales MB, Garayoa M, et al. The effect of the proteasome inhibitor bortezomib on acute myeloid leukemia cells and drug resistance associated with the CD34+ immature phenotype. Haematologica. 2008;93(1):57–66 [DOI] [PubMed] [Google Scholar]
- 35.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17(3):590–603 [DOI] [PubMed] [Google Scholar]
- 36.Buzzeo R, Enkemann S, Nimmanapalli R, Alsina M, Lichtenheld MG, Dalton WS, et al. Characterization of a R115777-resistant human multiple myeloma cell line with cross-resistance to PS-341. Clin Cancer Res. 2005;11(16):6057–64 [DOI] [PubMed] [Google Scholar]
- 37.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11(4–5):164–79 [DOI] [PubMed] [Google Scholar]
- 38.Chen KF, Yu HC, Liu TH, Lee SS, Chen PJ, Cheng AL. Synergistic interactions between sorafenib and bortezomib in hepatocellular carcinoma involve PP2A-dependent Akt inactivation. J Hepatol. 2010;52(1):88–95 [DOI] [PubMed] [Google Scholar]
- 39.Yu C, Friday BB, Lai JP, Yang L, Sarkaria J, Kay NE, et al. Cytotoxic synergy between the multikinase inhibitor sorafenib and the proteasome inhibitor bortezomib in vitro: induction of apoptosis through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer Ther. 2006;5(9):2378–87 [DOI] [PubMed] [Google Scholar]
- 40.Richardson P, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin DH, et al. Phase I/II Results of a Multicenter Trial of Perifosine (KRX-0401) + Bortezomib in Patients with Relapsed or Relapsed/Refractory Multiple Myeloma Who Were Previously Relapsed from or Refractory to Bortezomib. ASH Annual Meeting Abstracts. 2008;112(11):870 [Google Scholar]
- 41.Schmidt-Hieber M, Dabrowski R, Weimann A, Aicher B, Lohneis P, Busse A, et al. In vitro cytotoxicity of the novel antimyeloma agents perifosine, bortezomib and lenalidomide against different cell lines. Invest New Drugs. 2012;30(2):480–9 [DOI] [PubMed] [Google Scholar]
- 42.Choi YA, Park JS, Park MY, Oh KS, Lee MS, Lim JS, et al. Increase in CIP2A expression is associated with doxorubicin resistance. FEBS Lett. 2011;585(5):755–60 [DOI] [PubMed] [Google Scholar]
- 43.Lazo JS, Wipf P. Phosphatases as targets for cancer treatment. Curr Opin Investig Drugs. 2009;10(12):1297–304 [PubMed] [Google Scholar]
- 44.Cristobal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD. PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent antileukemic effect. Leukemia. 2011;25(4):606–14 [DOI] [PubMed] [Google Scholar]
- 45.Li W, Ge Z, Liu C, Liu Z, Bjorkholm M, Jia J, et al. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14(12):3722–8 [DOI] [PubMed] [Google Scholar]
- 46.Ansari KI, Mandal SS. Mixed lineage leukemia: roles in gene expression, hormone signaling and mRNA processing. FEBS J. 2010;277(8):1790–804 [DOI] [PubMed] [Google Scholar]
- 47.Perrotti D, Neviani P. ReSETting PP2A tumour suppressor activity in blast crisis and imatinib-resistant chronic myelogenous leukaemia. Br J Cancer. 2006;95(7): 775–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samanta AK, Chakraborty SN, Wang Y, Kantarjian H, Sun X, Hood J, et al. Jak2 inhibition deactivates Lyn kinase through the SET-PP2A-SHP1 pathway, causing apoptosis in drug-resistant cells from chronic myelogenous leukemia patients. Oncogene. 2009;28(14):1669–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stapnes C, Døskeland AP, Hatfield K, Ersvaer E, Ryningen A, Lorens JB, et al. The proteasome inhibitors bortezomib and PR-171 have antiproliferative and proapoptotic effects on primary human acute myeloid leukaemia cells. Br J Haematol. 2007;136 (6):814–28 [DOI] [PubMed] [Google Scholar]