Abstract
High-dose chemotherapy followed by autologous stem cell transplantation has been shown to be feasible and highly effective in newly diagnosed primary central nervous system lymphoma. In this retrospective multicenter study, we investigated prognosis and baseline risk factors in patients with primary central nervous system lymphoma who underwent this treatment approach. We retrospectively analyzed 105 immunocompetent patients with primary central nervous system lymphoma who underwent high-dose chemotherapy followed by autologous stem cell transplantation with or without whole brain radiotherapy as first-line consolidation treated at 12 German centers between 1997 and 2011. We estimated survival rates and investigated the impact of age, performance status, serum lactate dehydrogenase level, and deep brain involvement on overall and progression-free survival. Patients were additionally categorized into three prognostic groups according to the Memorial Sloan Kettering Cancer Center prognostic model. After a median follow up of 47 months, median progression-free survival and overall survival was reached after 85 and 121 months; 2- and 5-year survival rates were 82% and 79%, respectively. The Memorial Sloan Kettering Cancer Center prognostic model did not predict survival. Only age revealed some evidence of prognostic relevance. Overall response rate was 95%; of those patients with progressive disease before high-dose chemotherapy, 7 of 20 achieved ongoing complete remission after therapy without whole brain radiation therapy. Transplantation-associated mortality was 2.8%. High-dose chemotherapy followed by autologous stem cell transplantation is a highly effective and safe treatment modality for selected primary central nervous system lymphoma patients. Superiority compared to standard chemotherapy still warrants further investigation.
Introduction
Primary central nervous system lymphoma (PCNSL) is a highly aggressive disease with rising incidence over the past 30 years.1,2 High-dose methotrexate (MTX) combined with high-dose cytarabine (AraC) is currently regarded standard treatment.3 It has been suggested that consolidating whole brain radiotherapy has no additional benefit regarding overall survival (OS) after high-dose MTX alone or in combination with ifosfamide.4 Nevertheless, ongoing trials compare whole brain radiotherapy with high-dose chemotherapy followed by autologous stem cell transplantation (auto-SCT) as consolidation (NCT01011920, NCT00863460). Similar to other hematologic diseases, the rationale for consolidation in PCNSL is the elimination of minimal residual disease. Beside whole brain radiotherapy, the application of high-dose chemotherapy with carmustine (BCNU) and thiotepa followed by auto-SCT has been shown to be feasible and highly effective in newly diagnosed eligible patients, but also in the salvage situation.5–8 Two international prognostic scores have been developed to predict outcome in PCNSL: i) the International Extranodal Lymphoma Study Group (IELSG) score, which distinguishes three prognostic groups based on serum lactate dehydrogenase (LDH), age, Eastern Cooperative Oncology Group (ECOG) performance status, involvement of Deep Brain Structures (periventricular regions, basal ganglia, brainstem, and/or cerebellum), and cerebrospinal fluid (CSF) protein concentration;9 and ii) the Memorial Sloan Kettering Cancer Center (MSKCC) score, which also distinguishes three groups but only according to age and Karnofsky Performance Status (KPS).10 In recent years, several other factors such as serological markers, tumor characteristics, and pharmacokinetic MTX parameters have been proposed to potentially identify risk groups,11–14 but most of these findings still lack external validation from larger cohorts. Due to improved therapy and supportive care, risk factors are likely to change over time, and it is unclear whether the established risk models still predict prognosis in selected patients who received high-dose chemotherapy followed by auto-SCT. In this retrospective multicenter study, we investigated survival rates and the prognostic relevance of baseline risk factors in PCNSL patients who underwent high-dose chemotherapy followed by auto-SCT as first-line consolidating therapy.
Design and Methods
Patient selection criteria and data collection
Eligibility criteria for inclusion into this retrospective multicenter analysis were: i) a novel histologically or cytologically-proven PCNSL; ii) exclusion of systemic lymphoma manifestation by computerized tomography body scan and bone marrow examination; iii) no evidence of immunodeficiency; iv) completed high-dose chemotherapy followed by auto-SCT application for first-line therapy. In the present analyses, we pooled individual patient data from different sources. Data of 34 patients were from one pilot and one phase II trial6,7 conducted between 1998 and 2003. Data of further eligible patients from Freiburg University Hospital, who were treated before or after these trials, were extracted from the electronic patient documentation system. Patient data from 11 co-operating German centers were collected using a pre-specified case report form that recorded anonymized data about patient and tumor characteristics at baseline, treatment, toxicity, transplantation-specific data, objective response, site and date of relapse or progression, neurotoxicity, progression-free survival (PFS) and OS (altogether n=36 variables). All identified eligible patients from the co-operating centers were included. Having received these sheets, data were checked for consistency purposes and queries rechecked with the corresponding investigational site before entering the data in our central database. The 34 patients from the pilot and phase II trials provided written informed consent for the performance of institution initiated research studies and specifically for analyses of clinical outcome studies according to our institutional review board guidelines (Freiburg University Medical Center). The remaining 71 patients provided informed consent for the documentation of clinical and therapeutic data and the use for scientific publication in anonymous form. Our local ethics committee approved the study protocol.
PCNSL assessment
Baseline examination before treatment and response assessment during treatment were carried out using gadolinium-enhanced brain magnetic resonance imaging (MRI) scans evaluated by one experienced (neuro-) radiologist. We used baseline status and response assessments as documented in clinical routine. Baseline MRI was obtained before initiating therapy and remission status was defined in the absence of glucocorticoid use. Complete remission (CR) was defined as the disappearance of all signal enhancements in MRI. Partial remission (PR) was defined as a 50% or more reduction in tumor size compared to baseline. Progressive disease (PD) was defined as 25% or more increase in tumor size or appearance of any new lesion. All other situations were considered as stable disease (SD).
Statistical analysis
Our main outcomes of interest were OS (time from diagnosis to death) and PFS (time from diagnosis to progress, relapse or death; whichever occurred first). Both end points were estimated using the Kaplan-Meier method; follow up was estimated using the inverse Kaplan-Meier method.15 For our primary multivariable analysis, we pre-specified the following baseline characteristics to investigate their impact on PFS and OS: KPS, age (both as continuous variables), involvement of Deep Brain Structures (yes vs. no), and elevated LDH serum level (yes vs. no). We used a Cox’s proportional hazard regression model for these prognostic analyses (assumption of proportional hazards was investigated using the Grambsch-Therneau test). Results are presented as adjusted and unadjusted Hazard Ratios with 95% confidence intervals (CI) and P values. Patients with missing data of either of the 4 baseline characteristics (n=28) had to be excluded from the multivariable analyses. To illustrate the predictive value of the MSKCC score, we categorized patients accordingly10 and calculated corresponding Kaplan-Meier plots. For exploratory purposes, we also calculated PFS and OS probabilities for rituximab (yes vs. no), whole brain radiation therapy (yes vs. no), and remission status before high-dose chemotherapy followed by auto-SCT (CR vs. PR). Calculations for these exploratory analyses were only of a descriptive nature and have not been statistically tested. All tests of significance were two-sided and P<0.05 was considered significant. Statistical analyses were carried out using STATA version 12.2 (StataCorp LP, Texas, USA).
Results
Patients’ characteristics and therapeutic management
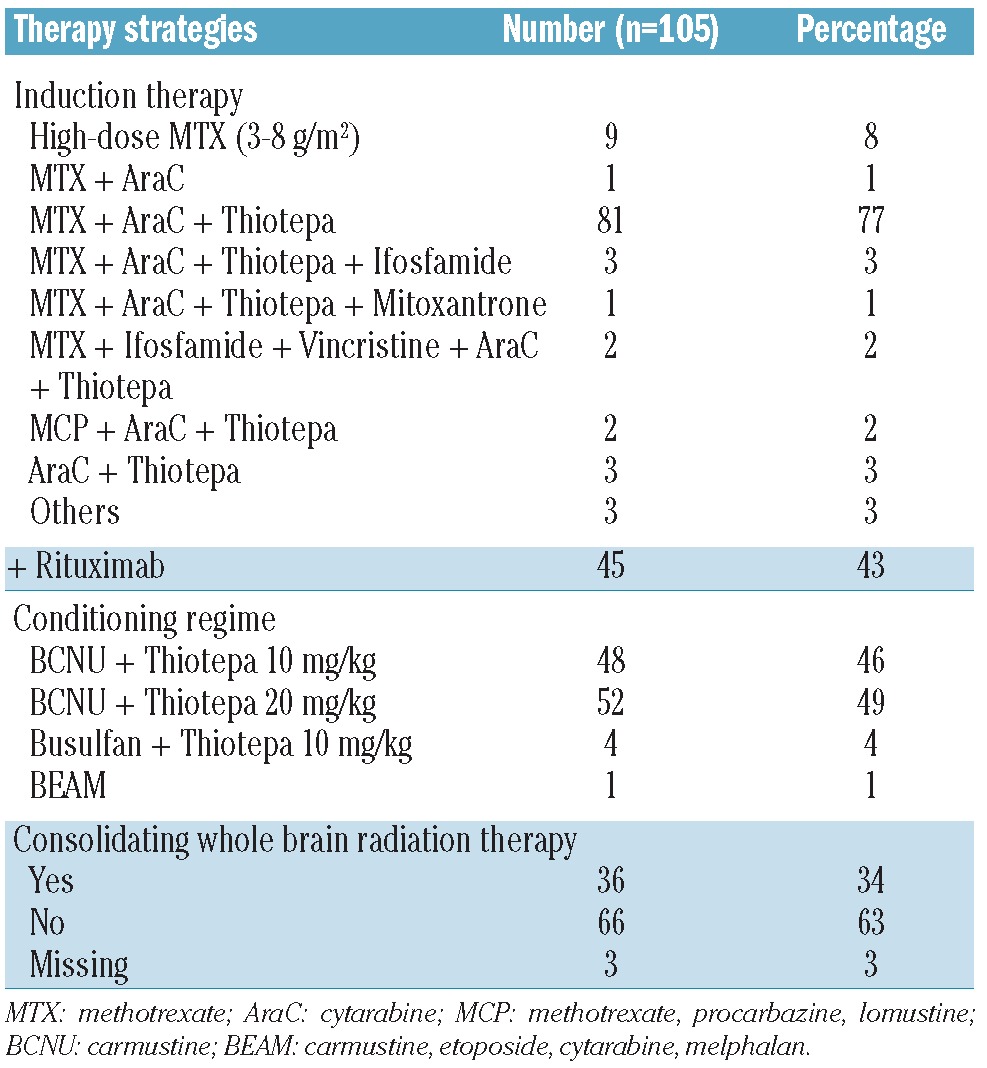
Data of 105 eligible patients diagnosed with PCNSL between 1997 and 2011 were included. Patients’ baseline characteristics at time of diagnosis are summarized in Table 1. Information about induction treatment was available in 97 of 105 patients; most of them were treated according to high-dose MTX based protocols (96%). Regarding the conditioning regimen, the majority were treated according to protocols containing carmustine and thiotepa (96%). Thirty-six percent received consolidating whole brain radiation therapy as part of first-line therapy. Seven patients received intrathecal therapy with cytarabine. None of the patients received intraventricular therapy. The various treatment regimes are summarized in Table 2.
Table 1.
Patients’ basic characteristics at time of diagnosis.
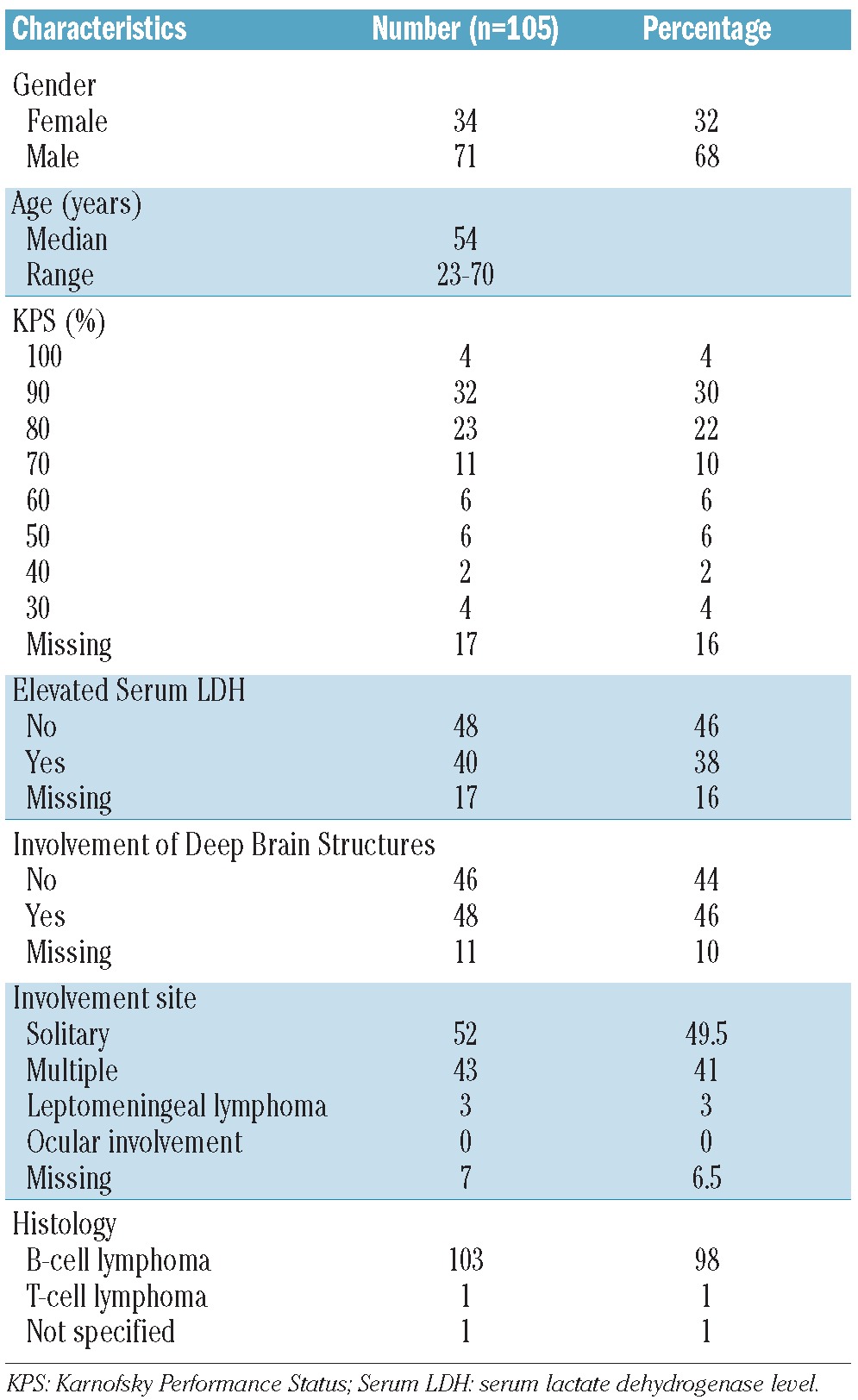
Table 2.
Treatment parameters.
Treatment response
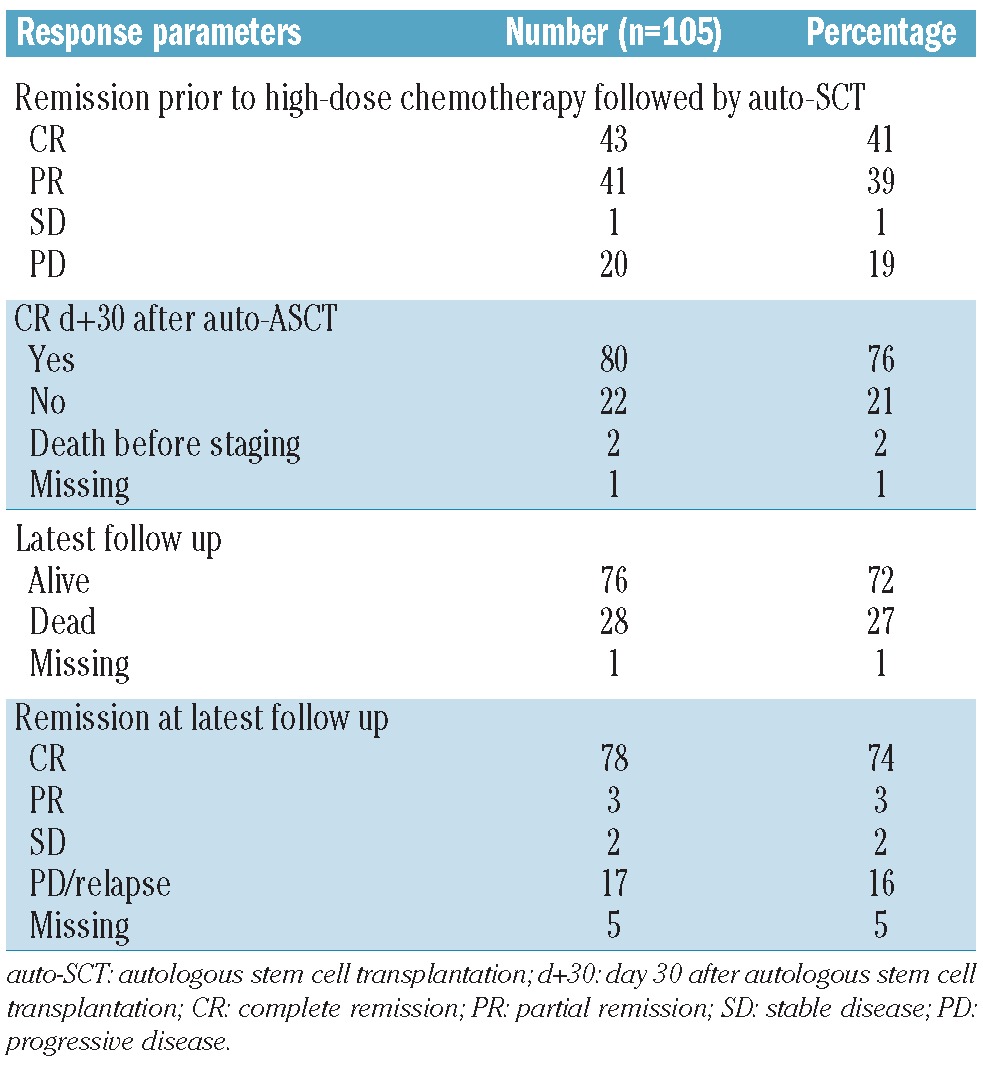
In 3 of 105 patients no response data were available. Overall, 100 of 102 assessable patients achieved an objective response (80 of 102 CR and 20 of 102 PR), 2 developed PD on Day 30 after high-dose chemotherapy followed by auto-SCT. Before entering high-dose chemotherapy followed by auto-SCT, 43 of 105 patients (41%) achieved CR, 41 of 105 (39%) PR, one of 105 (1%) SD, and 20 of 105 (19%) showed PD following induction treatment (Table 3). Of those patients with PD before high-dose chemotherapy followed by auto-SCT, 5 of 20 achieved ongoing CR (PFS 7–58 months) after auto-SCT without consolidating or salvage treatment. Of those patients with CR after auto-SCT, 65 of 80 (81%) remained free of progression (PFS 2–86 months).
Table 3.
Response parameters.
Treatment-related mortality
Overall treatment-related mortality associated with high-dose chemotherapy followed by auto-SCT was observed in 3 of 105 patients (2.8%); all of them died early, within 100 days after auto-SCT due to fatal infectious complications during neutropenia.
Survival and risk factor analysis
After a median follow up of 47 months, 77 of 105 patients (73%) were alive. Median PFS and OS were reached after 85 months and 121 months, respectively (Figures 1 and 2). Results of the multivariable analysis regarding the main outcomes PFS and OS are summarized in Table 4. The low P value of the factor age suggests some evidence of a higher risk of progression or death with increasing age. All other risk factors were not significant. The MSKCC prognostic classification system did not identify significant survival differences (Figure 3).
Figure 1.
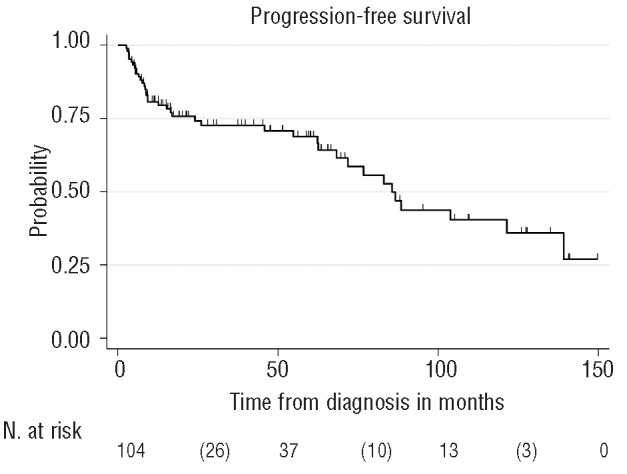
Kaplan-Meier plot. Progression-free survival from time of initial diagnosis of all evaluable patients.
Figure 2.
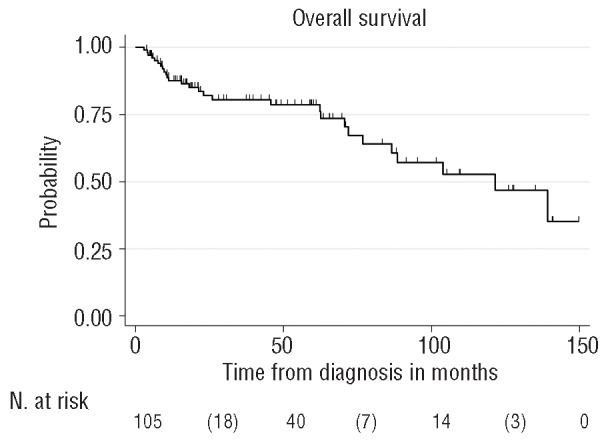
Kaplan-Meier plot. Overall survival from time of initial diagnosis of all patients.
Table 4.
Cox’s regression analysis for progression-free and overall survival.
Figure 3.
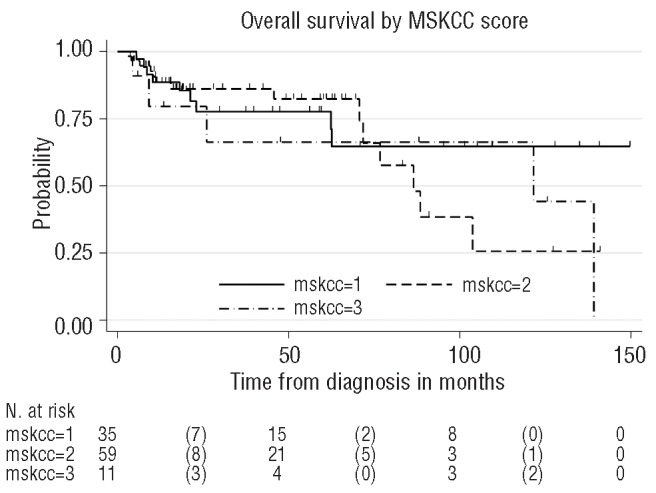
Kaplan-Meier plot. Overall survival according to the Memorial Sloan Kettering prognostic score.
Impact of whole brain radiation therapy, rituximab, and response status on OS and PFS
There was a strong difference in follow-up times between patients who received whole brain radiation therapy as consolidation (n=36, median 109 months) and those who did not (n=66, median 21 months), thus we explored the survival rates in these groups only descriptively. In 3 patients, information about potential consolidating whole brain radiation therapy was not available. Of those patients who received whole brain radiation therapy as consolidation, 18 of 36 were irradiated being in CR, 18 of 36 were in PR (Table 2). Two and 5-year OS rates were 85% (95%CI: 68%–93%) and 82% (95%CI: 63%–91%) in the group who received whole brain radiation therapy, respectively. In the 66 patients without whole brain radiation therapy, corresponding OS rates were 82% (95%CI: 68%–90%) and 77% (95%CI: 61%–88%). Similar to whole brain radiation therapy, the follow-up times between those patients who received rituximab (n=45) and those who did not (n=60) differed too much for survival probabilities to be statistically compared. Two and 5-year OS rates for patients who received rituximab were both 84% (95%CI: 68%–93%). For patients not receiving rituximab, corresponding OS rates were 82% (95%CI: 70%–90%) and 78% (95%CI: 65%–87%). Similar results are seen for PFS: with rituximab 70% (95%CI: 52%–83%), and without rituximab respectively 77% (95%CI: 64%–86%) and 71% (95%CI: 57%–81%). In patients achieving CR before high-dose chemotherapy followed by auto-SCT, OS rate was 89% (95%CI: 73%–96%) after two and five years. Those patients only achieving PR before high-dose chemotherapy followed by ASCT revealed a 2- and 5-year OS of 85% (95%CI: 67%–93%) and 77% (95%CI: 58%–89%). Similar rates were seen for PFS.
Discussion
To the best of our knowledge, this is the largest cohort reporting data of PCNSL patients who underwent high-dose chemotherapy followed by auto-SCT for first-line therapy. The remarkably high efficacy of this approach is reflected by a response rate of 95% and median overall survival rates of around ten years.
Similar to the International Prognostic Index for systemic high-grade lymphoma,16 patient-specific characteristics, namely age and performance status, have been shown to be robust prognostic factors in PCNSL over recent decades.17–19 They are also the fundamentals of the MSKCC prognostic score.10 In addition, the IELSG score takes specific tumor-spreading characteristics into account. Its development was based on 105 completely assessable patients from several centers.9 Compared to our cohort, all these patients received various different treatments and thus are not comparable to the population of patients analyzed herein, because our cohort represents a highly selected population of patients supposed to benefit from an aggressive therapy like high-dose chemotherapy followed by auto-SCT. Interestingly, a recent investigation on elderly PCNSL patients (n=174, age ≥65 years) identified only age and performance status as outcome-determining baseline factors, whereas serum LDH, involvement of Deep Brain Structures, and cerebrospinal fluid protein elevation had no impact on survival.20 However, one needs to consider that there is some patient overlap with the cohort the MSKCC prognostic score was developed from. Thus, the conclusion that tumor specific characteristics are not predictive for elderly patients in general needs to be taken with caution.
Some preliminary evidence5–7 and our present analysis suggest that high-dose chemotherapy followed by auto-SCT in eligible PCNSL patients leads to high response and long survival rates. Granted, one may object that these eligible patients constitute a favorable prognostic subgroup because of relatively young age and good clinical performance at diagnosis, and this may lead to overestimating the effect of high-dose chemotherapy followed by auto-SCT. In fact, it was age (a cut off at 65 years) that mainly influenced whether patients were put on track for high-dose chemotherapy followed by auto-SCT in our studies.6,7 However, most of the patients in this analysis were not categorized in the most favorable MSKCC prognostic group as one would have expected, but rather in the second (n=59). Furthermore, although the decision to apply therapy or not is mostly driven by patient specific characteristics (e.g. age and performance), factors such as time of diagnosis and center policies need to be considered as well, because they may potentially imply an indication bias. For example, the threshold of treating elderly but otherwise fit patients may have been different between centers, or the threshold may have been lowered over time in general. All these issues can have an impact on survival prognosis.
A recent publication of 31 patients reported no prognostic discrimination by the MSKCC score, which is similar to our findings. However, the IELSG score still distinguished between the 2nd and 3rd risk group in this previous report.21
Rituximab is a standard agent for treating systemic B-cell lymphomas,22 but in PCNSL, although already in wide use, the value of rituximab rests mainly on evidence from systemic lymphoma trials. It is now under investigation in two large ongoing randomized PCNSL studies (NCT01011920, NTR2427). In our analysis, the addition of rituximab does not seem to have an impact on overall and progression-free survival. The response status (CR or PR) after completing induction treatment does not seem to greatly influence overall survival; however, in general, any estimation of survival based on response status needs to be regarded with caution, because response may be just a surrogate for prognostically favorable patients.23 Because of this, we only provided estimates and did not conduct statistical testing. Our study has three limitations. First, even though this is the largest cohort to investigate prognosis and the impact of risk factors in PCNSL patients who underwent high-dose chemotherapy followed by auto-SCT, the number of patients is still relatively small, especially with respect to the suggested ratio of events per variable tested in the multivariable model. Second, the data quality of our cohort is limited due to its retrospective nature and associated missing values, especially data on cerebrospinal fluid protein concentration which were available in only 24 patients; therefore we decided to exclude this factor from the analyses and could not assign patients to an IELSG prognostic group. The rate of concurrent meningeal involvement detected by cerebrospinal fluid cytology examination is estimated to be 16%, whereby isolated leptomeningeal lymphoma only represents less than 5% of all PCNSL.24,25 The high rate of missing values in our cohort may be explained by the fact that spinal tabs to obtain cerebrospinal fluid protein concentration or meningeal involvement are often not collected in routine clinical practice. Patients with PCNSL initially often present with space-consuming intracerebral masses with peri-focal edema and presumed increased intracranial pressure. Furthermore, positive findings in the CSF have no therapeutic consequences; therefore, in many centers, this invasive procedure is only performed when meningeal involvement is suspected. Thus, in a substantial proportion of patients, the IELSG score cannot be applied completely. Certainly, this lack of simplicity is a limitation of that score.
The third limitation is that we are not able to report data of the intent-to-treat population, the unselected group of patients considered to aim for high-dose chemotherapy followed by auto-SCT at time of diagnosis. Therefore, the question whether this treatment approach eliminates established risk factors cannot be answered with our dataset because we would need such an unselected population of patients to be able to introduce appropriate interaction terms to a much more complex analysis.
In summary, prospective trials or cohorts are needed to gain better insight into particular lymphoma-specific and probably also patients’ characteristics that allow for risk stratification independently of the therapy applied. The benefit of high-dose chemotherapy followed by auto-SCT compared to whole brain radiation therapy is currently under investigation in randomized trials (NCT00863460, NCT01011920). Furthermore, a randomized multicenter trial to compare the high-dose chemotherapy followed by auto-SCT approach to a conventional intensive poly-chemotherapy regimen will start in 2013. Besides providing a higher level of evidence of the efficacy of high-dose chemotherapy followed by auto-SCT, these randomized trials will also allow us to analyze treatment-effect modifiers based on patient- or tumor-specific characteristics.
Footnotes
Funding
Only in-house sources
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Makino K, Nakamura H, Kino T, Takeshima H, Kuratsu J. Rising incidence of primary central nervous system lymphoma in Kumamoto, Japan. Surg Neurol. 2006; 66(5):503–6 [DOI] [PubMed] [Google Scholar]
- 2.Haldorsen IS, Krossnes BK, Aarseth JH, Scheie D, Johannesen TB, Mella O, et al. Increasing incidence and continued dismal outcome of primary central nervous system lymphoma in Norway 1989–2003 : time trends in a 15-year national survey. Cancer. 2007;110(8):1803–14 [DOI] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374 (9700):1512–20 [DOI] [PubMed] [Google Scholar]
- 4.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–47 [DOI] [PubMed] [Google Scholar]
- 5.Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26(15):2512–8 [DOI] [PubMed] [Google Scholar]
- 6.Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24 (24):3865–70 [DOI] [PubMed] [Google Scholar]
- 7.Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93(1):147–8 [DOI] [PubMed] [Google Scholar]
- 8.Kasenda B, Schorb E, Fritsch K, Finke J, Illerhaus G. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma--a long-term follow-up study. Ann Oncol. 2012;23(10):2670–5 [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003; 21(2):266–72 [DOI] [PubMed] [Google Scholar]
- 10.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5 [DOI] [PubMed] [Google Scholar]
- 11.Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9(3):1063–9 [PubMed] [Google Scholar]
- 12.Ferreri AJ, Dell’Oro S, Capello D, Ponzoni M, Iuzzolino P, Rossi D, et al. Aberrant methylation in the promoter region of the reduced folate carrier gene is a potential mechanism of resistance to methotrexate in primary central nervous system lymphomas. Br J Haematol. 2004;126(5):657–64 [DOI] [PubMed] [Google Scholar]
- 13.Hottinger AF, Iwamoto FM, Karimi S, Riedel E, Dantis J, Park J, et al. YKL-40 and MMP-9 as serum markers for patients with primary central nervous system lymphoma. Ann Neurol. 2011;70(1):163–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joerger M, Huitema AD, Illerhaus G, Ferreri AJ. High-dose methotrexate in patients with primary central nervous system lymphoma: does drug exposure really matter? Leuk Lymphoma. 2011;52(10):1825–7 [DOI] [PubMed] [Google Scholar]
- 15.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6 [DOI] [PubMed] [Google Scholar]
- 16.Shipp MA, Harrington D, Armitage JO. A predictive model for aggressive non- Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329 (14):987–94 [DOI] [PubMed] [Google Scholar]
- 17.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18(17):3144–50 [DOI] [PubMed] [Google Scholar]
- 18.Reni M, Ferreri AJ, Garancini MP, Villa E. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol. 1997;8(3):227–34 [DOI] [PubMed] [Google Scholar]
- 19.Pollack IF, Lunsford LD, Flickinger JC, Dameshek HL. Prognostic factors in the diagnosis and treatment of primary central nervous system lymphoma. Cancer. 1989; 63(5):939–47 [DOI] [PubMed] [Google Scholar]
- 20.Ney DE, Reiner AS, Panageas KS, Brown HS, DeAngelis LM, Abrey LE. Characteristics and outcomes of elderly patients with primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2010;116(19):4605–12 [DOI] [PubMed] [Google Scholar]
- 21.Wieduwilt MJ, Valles F, Issa S, Behler CM, Hwang J, McDermott M, et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res. 2012;18(4):1146–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11): 1013–22 [DOI] [PubMed] [Google Scholar]
- 23.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26(24): 3913–5 [DOI] [PubMed] [Google Scholar]
- 24.Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–20 [DOI] [PubMed] [Google Scholar]
- 25.Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118(3):510–22 [DOI] [PubMed] [Google Scholar]


