Abstract
We investigated sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 18F-fluorodeoxyglucose-positron emission tomography in 170 cases with suspected or biopsy-proven posttransplant lymphoproliferative disorder. All solid organ and hematopoietic stem cell transplant recipients who underwent an 18F-fluorodeoxyglucose-positron emission tomography scan between 2003 and 2010 in our center for the indication posttransplant lymphoproliferative disorder, were retrospectively reviewed and results were compared with tissue biopsy whenever possible. One hundred and seventy positron emission tomography scans in 150 patients were eligible for evaluation. In 45 cases, the patient had a biopsy-confirmed posttransplant lymphoproliferative disorder before positron emission tomography scanning and positron emission tomography was performed for staging purposes. In the remaining 125 cases, positron emission tomography was performed to differentiate between posttransplant lymphoproliferative disorder and other diseases. 18F-fluorodeoxyglucose-uptake was quantitatively expressed by calculation of maximum and mean standardized uptake value in the most intense lesion or, in the absence of attenuation corrected positron emission tomography scans, by comparing uptake in target lesion to liver and mediastinal uptake. We found an overall sensitivity of 89%, specificity of 89%, positive predictive value of 91% and negative predictive value of 87% for posttransplant lymphoproliferative disorder detection by 18F-fluorodeoxyglucose-positron emission tomography. In a subanalysis of the 125 scans performed for differentiating posttransplant lymphoproliferative disorder from other diseases, sensitivity, specificity, positive predictive value and negative predictive value were 90%, 89%, 85% and 93%, respectively. 18F-fluorodeoxyglucose-uptake in posttransplant lymphoproliferative disorder was generally high with a median mean and maximum standardized uptake value of 9.0 (range 2.0–18.6) and 17.4 (range 2.6–26.4). Posttransplant lymphoproliferative disorder often had an atypical presentation on positron emission tomography with high incidence of extranodal involvement. In conclusion, from these data, we can conclude that 18F-fluorodeoxyglucose-positron emission tomography is highly sensitive for detecting posttransplant lymphoproliferative disorder and has an excellent ability to differentiate posttransplant lymphoproliferative disorder from non-malignant diseases.
Introduction
Posttransplant lymphoproliferative disorder (PTLD) comprises a wide spectrum of lymphoproliferative conditions following solid organ and hematopoietic stem cell transplantation.1 The disorder represents an important cause of morbidity and mortality in transplant recipients. The strongest risk factors for occurrence of PTLD are primary Epstein Barr virus (EBV)-infection, type of transplanted organ, T-cell depletion, and total burden of immunosuppression.2,3
Current diagnosis of PTLD is based on clinical and biochemical suspicion and (at least in some cases) on increasing EBV viral replication rates, with tissue biopsy being the gold standard. Following diagnosis, disease staging is performed by means of conventional computed tomography (CT) scanning, bone marrow biopsy and, in case of clinical evidence, central nervous system imaging and cerebrospinal fluid examination. However, diagnosis and staging of PTLD is characterized by some specific difficulties. Firstly, obtaining a representative tissue biopsy is not always easy due to severe thrombocytopenia, especially in the case of hematopoietic stem cell transplantation. Secondly, many physicians tend to start therapy (either reduction of immunosuppression or a monoclonal anti-CD20 antibody such as rituximab) in an early phase before all diagnostic procedures have been performed. Finally, PTLD is often characterized by frequent extranodal involvement, rendering conventional CT scan less appropriate for adequate staging and less suitable for image-guided biopsy.
Recently, 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) combined with CT has emerged as the most sensitive and specific imaging modality for diagnosis and staging of both Hodgkin’s and different subtypes of aggressive non-Hodgkin’s lymphoma.4 The role of FDG-PET scan in PTLD, however, has not yet been established.
Our aim was to evaluate the accuracy and clinical performance of FDG-PET scan in the diagnosis of PTLD in a large cohort of transplant recipients.
Design and Methods
Data collection
We conducted a monocentric retrospective analysis of all solid organ and hematopoietic stem cell transplant recipients who underwent a PET scan between January 2003 and December 2010 in order to confirm or to eliminate the clinical and/or biochemical suspicion of PTLD.
Positron emission tomography (PET)
FDG-PET scanning was performed from the skull to the mid-thigh. In the first years of this survey, only non-attenuated PET scans (Siemens ECAT HR+) were available. In 73 (43%) and 97 (57%) cases, a PET scan without CT or a combined PET/CT scan were performed, respectively. Scanning protocol has changed slightly over time since the introduction of a hybrid PET/CT imaging system (Biograph 40 TruePoint with True V, Siemens Medical Solutions). Standard administered FDG-dose was 4 × body weight (kg) + 20 MBq in all patients.
All PET scans were evaluated by 2 experienced nuclear physicians (LB and RV) and blindly scored visually according to a 4-point scale. In cases in which no arguments for lymphoma were withheld, a score of 0 was allocated; if only a few arguments could be retrieved, a score of 1 was given. This was often the case when the reviewer was in doubt of inflammatory changes. A score of 2 was reserved if there was a clear preference for lymphoma and, finally, when PET scan was very suggestive for lymphoma, a score of 3 was allocated. In order to improve and facilitate the interpretation scores, 0 and 1 were considered ‘PET negative’, while scores 2 and 3 were designated as ‘PET positive’. For every attenuation corrected PET scan, mean and maximum standardized uptake value (SUV) was determined. SUV mean was determined by a visual 3D-delineation of the most active lesion undoubtedly attributed to PTLD. For non-attenuated PET, FDG intensity was compared to mediastinal and liver uptake. Whenever possible, results of the PET scan were compared to histological data. All biopsies were reviewed by 3 expert hematopathologists (CDWP, XS, TT) and, in case of PTLD, classified according to the WHO 2008 classification.1 In ideal circumstances, a biopsy of all suspected lesions should have been obtained. However, from a practical and ethical point of view, this was rarely possible. In cases in which a tissue biopsy was lacking, correlations between the PET results and the findings on CT scan and clinical characteristics were performed. The final diagnosis of PTLD in patients without biopsy was considered unlikely if another cause of biochemical/clinical PTLD suspicion was found, or if rapid spontaneous regression occurred without any intervention. Besides PET data, baseline characteristics of the patients were collected from the medical files. These included the type of transplantation, the presence of clinical symptoms (fever, night sweats, weight loss, pain, fatigue) and biochemical abnormalities (elevated lactate dehydrogenase, anemia, leukopenia, thrombocytopenia, Epstein Barr virus viral load). Clinical symptoms and biochemical abnormalities were considered to be present if at least one clinical symptom or biochemical abnormality was observed. This study was approved by the Ethical Committee of the University Hospitals Leuven and was conducted according to the Declaration of Helsinki.
Statistical analysis
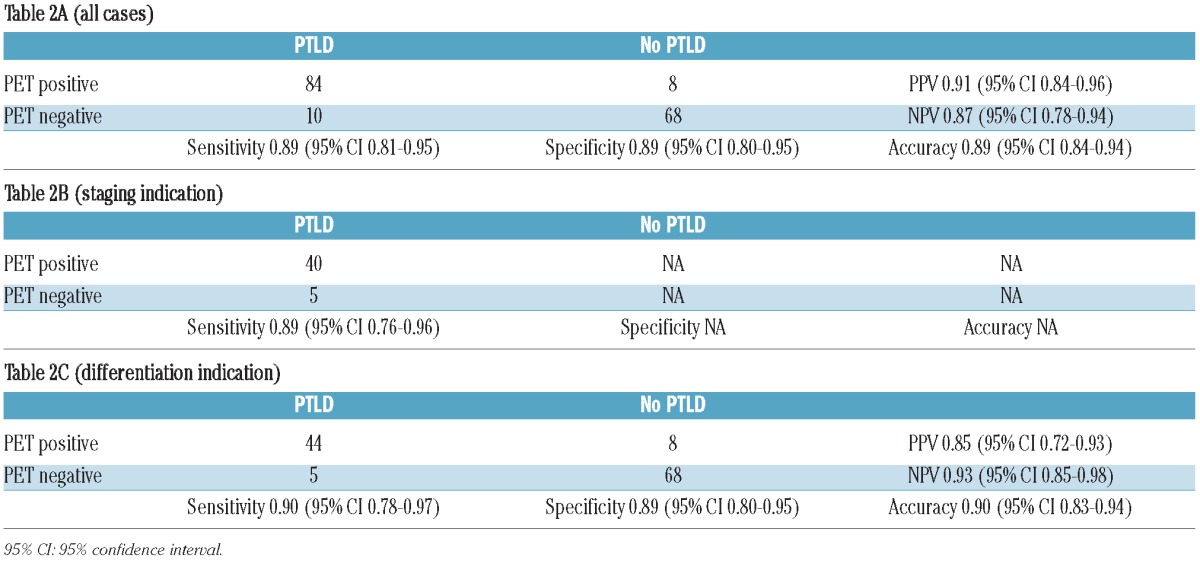
Sensitivity was defined as: [number of true positive cases]/[total number of true positive and false negative cases]). Specificity was defined as: [number of true negative cases]/[total number of false positive and true negative cases]. Positive predictive value was defined as: PPV, [number of true positive cases]/[total number of true positive and false positive cases]. Negative predictive value (NPV) was defined as: [number of true negative cases]/[total number of true negative and false negative cases]. Overall accuracy [total number of true positive and true negative cases] / [all cases]) was calculated for different settings of PET imaging. PPV and NPV were only defined for this particular studied population. Exact 95% confidence intervals (CI) based on the binomial distribution were calculated for each of these indices. Separate analyses were performed for the evaluation of PET as: a) a tool for the detection of lesions of suspected PTLD (all patients); b) a tool for the differentiation of PTLD versus other diseases (no biopsy-confirmed diagnosis before PET scan); and c) a staging tool for biopsy-confirmed PTLD (before PET scan was performed).
Results
Between January 2003 and December 2010, 240 PET scans were performed for the indication PTLD. After a first selection, which was performed by revising the initial clinical request forms and ensuring the quality of data for correct interpretation, 185 scans remained. Afterwards, a second selection excluded an additional 15 PET scans. The main reason for exclusion during the second selection was the inability to obtain a correct diagnosis based on histology or clinical characteristics in 8 patients. Three patients with one single PTLD localization had a PET scan following resection, whereas in 2 patients a second malignancy was diagnosed at the same time. Finally, two PET scans were excluded because of the different characteristics of the lymphoproliferative disorder: one patient had an indolent NHL occurring more than twenty years following transplantation, whereas in another patient the lymphoproliferative disorder was already present before transplantation, as previously published.5 In 10 patients, two episodes of PTLD suspicion were reported, and in 5 patients, three different PET scans (at different time points) were recorded. Eventually, a final evaluation was made on 170 PET scans in 150 different transplant patients.
In 45 (26.5%) of the 170 selected PET scans a biopsy-confirmed diagnosis of PTLD was made before the PET scan was performed. In these cases, PET was considered as staging tool. In the remaining 125 (73.5%) cases, PTLD was suspected but not confirmed at time of PET scan.
Male:female ratio was 1.5:1 which is an exact reflection of the gender distribution of transplant recipients in our center (60% males). Median time between transplantation and PET scan was 68.71 months (range 1–322). The majority of PET scans were performed in kidney transplant recipients (34%), followed by liver (15%), lung (15%), heart (15%), allogeneic hematopoietic stem cell (15%), combined (5%) and bowel (1%) transplant recipients. Table 1 shows the different clinical and biochemical characteristics of the study cases. A biopsy was obtained in 119 (70%) cases. Biopsy-proven PTLD cases included early lesions in 11 (12%), polymorphic PTLD in 3 (3%), diffuse large B-cell lymphoma (DLBCL) type monomorphic PTLD in 64 (69%), non-DLBCL type monomorphic PTLD in 12 (13%), and Hodgkin-like PTLD in 3 (3%) cases.
Table 1.
Clinical and biochemical characteristics (n=170).
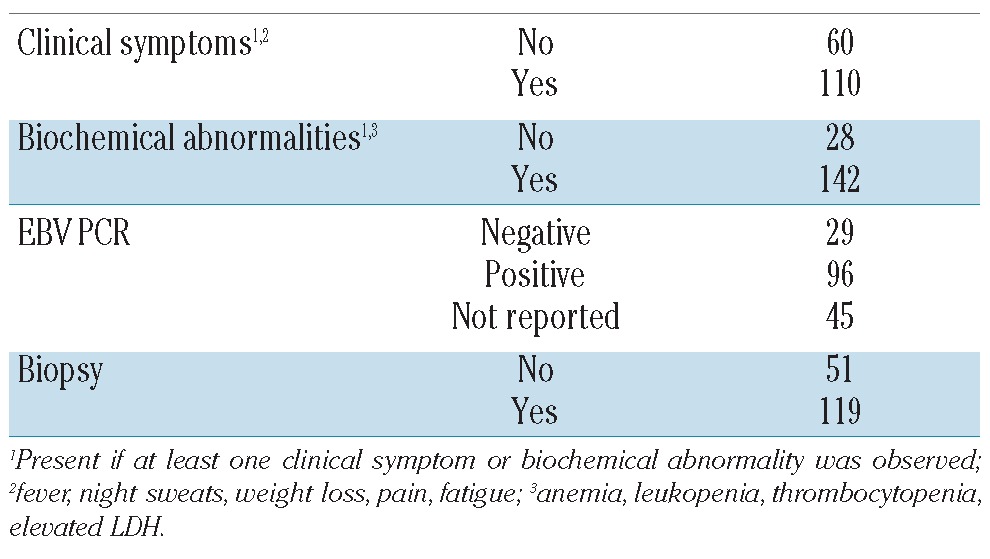
Table 2A shows data on sensitivity, specificity, positive predictive value, negative predictive value and accuracy of PET scan for the detection of PTLD in all 170 PET scans. Table 2B shows sensitivity for the 45 patients in whom the diagnosis of PTLD was already confirmed before PET scan (staging indication). Finally, Table 2C shows the same parameters of the remaining 125 patients who had PET for differentiating PTLD versus other disease. False positive results (n=8) were mainly due to infectious (3 cases) or inflammatory conditions (3 cases). In the 2 remaining cases, diagnosis remained unknown but PTLD was considered very unlikely due to spontaneous regression without any therapeutic intervention. False negative results (n=10) were mainly reported in limited stage early lesions (4 cases), with 2 cases presenting with respectively isolated (kidney and heart) graft localization. Other subtypes presenting with negative PET scan included early stage diffuse large B-cell non-Hodgkin’s lymphoma (3 cases), primary central nervous system PTLD (2 cases) and limited stage Hodgkin’s lymphoma (1 case).
Table 2.
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy of PET scan in PTLD.
Visualized lesions on PET scan showed an intense FDG uptake with a median mean standardized uptake value (SUV) of 9.0 (range 2.0–18.6) and a median maximum SUV of 17.4 (range 2.6–26.4). Atypical extranodal presentation was a frequent finding with diffuse pulmonary involvement, gastrointestinal lesions and kidney, bone, liver, spleen, muscle and skin invasion (Figures 1, 2 and 3).
Figure 1.
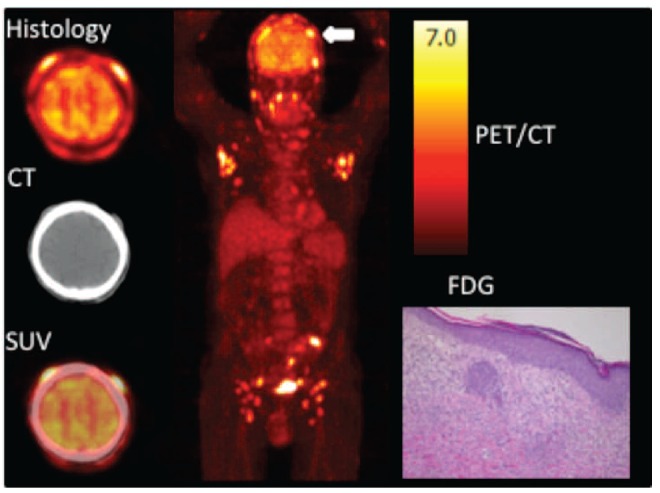
A 64-year old man presented with infiltrative skin lesions ten years following second kidney transplantation. Transaxial FDG-PET, CT and fused PET/CT images of the skull (left), maximum intensity projection (center) and histology of subcutaneous nodule (right). FDG PET/CT showed multiple cutaneous lesions (white arrow) and both axillary and inguinal lymphadenopathies. Skin biopsy revealed a CD8-positive epidermotropic EBV-negative T-cell lymphoproliferative disorder, extending in the subcutis, compatible with a monomorphic cytotoxic T-cell PTLD. SUV: standardized uptake value.
Figure 2.
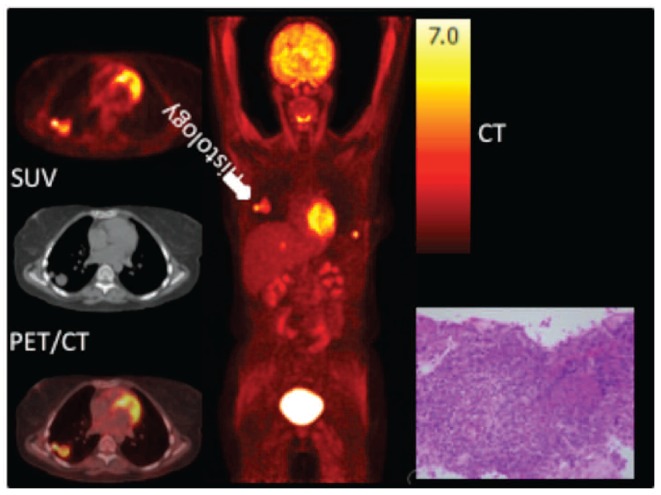
A 28 year-old woman presented with asymptomatic pulmonary nodules associated with increasing EBV viral load in the peripheral blood four months following lung transplantation. Transaxial FDG-PET, CT and fused PET/CT images of the chest (left), maximum intensity projection (center) and histology of pulmonary nodule (right). FDG PET/CT showed a bilobar pulmonary nodule in the right lung (white arrow) and a lesion in the liver and left chest wall. Histological analysis of a core needle biopsy of the pulmonary nodule revealed an EBV-driven diffuse large CD20-positive lymphoid proliferation, compatible with a monomorphic PTLD, type DLBCL, NOS of non-germinal center B-cell origin. SUV: standardized uptake value.
Figure 3.
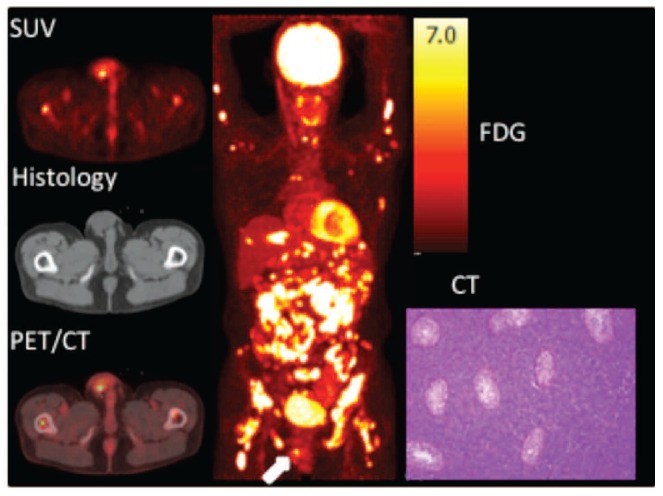
A 44-years old man presented with bone pain and B symptoms 14 years following kidney transplantation. Transaxial FDG-PET, CT and fused PET/CT images of the scrotum (left), maximum intensity projection (center) and histology of testis (right). FDG PET/CT showed a focal FDG-avide lesion in the right scrotum (white arrow) and both nodal, extranodal and bone marrow involvement in supra-and infradiaphragmatic locations. Histological analysis of the right testis revealed a large B-cell lymphoproliferation, diffusely growing between the seminiferous tubules, compatible with an EBV-negative monomorphic B-cell PTLD, type primary testicular DLBCL of germinal center B-cell origin. SUV: standardized uptake value.
Discussion
Historically long-term outcome of solid organ and hematopoietic stem cell transplant recipients was limited by low graft and patient survival, mainly due to acute organ rejection, acute graft-versus-host disease and infectious complications. However, due to improvement in immunosuppressive therapy and clinical prophylactic management strategies, overall outcome has improved. Posttransplant malignancies and cardiovascular disorders have emerged as the most important long-term complications, in particular, following solid organ transplantation. The incidence of malignancies in solid organ transplant patients is estimated to be 20% after ten years of chronic immunosuppression.6 Skin cancer and lymphoproliferative disorders are the two most frequent malignancies in this specific patient population.7
Posttransplant lymphoproliferative disorder (PTLD) is a rare but life-threatening disorder, characterized by an uncontrolled proliferation of lymphocytes, caused by immunosuppressive drug-induced diminished immune surveillance. According to the World Health Organization (WHO) 2008 classification, PTLD can be classified into early lesions, polymorphic PTLD, monomorphic PTLD and Hodgkin-like PTLD.1
Metabolic imaging with FDG-PET, combined with CT is widely accepted for the diagnosis and staging of both Hodgkin’s and different subtypes of aggressive non-Hodgkin’s lymphoma.4 The role of PET scan in PTLD, however, is less clear.
The staging system most frequently used for lymphoma is the Ann Arbor classification, classifying patients based on the number of involved lymph node regions, the localization of nodal involvement and the presence of organ invasion.8 However, as extranodal involvement is a frequent feature of PTLD, CT scan may not be the most appropriate staging tool. Another potential problem is the need for intravenous contrast with CT, which is relatively contra-indicated in transplant patients due to the relative frequent co-existing renal (allograft) impairment.9 These drawbacks have stimulated growing interest in the use of FDG-PET for detecting and staging of PTLD. However, as PET scan lacks anatomic detail, recent years have seen the development of hybrid PET/CT and this is now considered state of the art imaging for both staging and response assessment in DLBCL and HL.4,10
Experience with the use of PET in PTLD has been limited to case reports and rather small single-center case series.11–17 These reports reveal that PET scan may have a high sensitivity for the detection of PTLD lesions. Our study included a large number of transplant patients for whom a PET scan was ordered because of clinical PTLD suspicion (diagnostic PET scan) or biopsy-proven PTLD (staging PET scan) and showed a high sensitivity, specificity, positive predictive value and negative predictive value. However, probably due to specific immunosuppression-related problems, PET scan seems more difficult to interpret compared to metabolic imaging of lymphomas occurring in immunocompetent patients. These problems include the presence of diffuse pulmonary involvement, non-typical organ invasion and concomitant infectious problems. These difficulties seem comparable to those observed in PET scans in HIV associated lymphomas, with HIV-associated nodal reactive hyperplasia and infections confounding correct PET interpretation.18
From a clinical point of view, the high sensitivity is important. However, three specific problems leading to false negative results need to be taken into account. Firstly, although not considered real PTLD according to the WHO 2008 classification, indolent lymphomas frequently lack FDG avidity.19 However, their incidence might increase in the future due to improved patient survival and given the higher age of transplant recipients.20 Secondly, PET scan is not the preferred examination in case of suspicion of central nervous system localization. In this situation, magnetic resonance imaging (MRI) of the brain and cytological examination of cerebrospinal fluid should be performed. Finally, PET scan might not be the ideal imaging modality to detect isolated allograft localization in heart or kidney transplant recipients due to its high physiological background uptake and renal clearance. Besides excellent sensitivity, the high positive predictive value indicates that FDG-PET has, at least in experienced hands, an excellent ability to differentiate PTLD from other FDG-avid conditions, mostly infectious or inflammatory disorders.
Our data show intense FDG uptake in all visualized lesions on PET scan, with a median mean SUV of 9.0 (range 2.0–18.6) and a median maximum SUV of 17.4 (range 2.6–26.4). These values are more in favor of aggressive lymphomas, although no clear cut-off values have been reported.21–25 FDG uptake intensity was high in all WHO subtypes, although the numbers of especially polymorphic and Hodgkin-like PTLD were too small to draw conclusions about intensity in these lesions. An additional confounding factor in interpretation of SUV is the fact that patients may have more than one histological subtype in different lesions simultaneously.
In conclusion, our study shows a high accuracy when using PET scan in the diagnosis and staging of PTLD following solid organ and hematopoietic stem cell transplantation. Despite some methodological limitations, including its retrospective nature and the lack of well-defined and general accepted diagnostic scoring systems, our analysis confirms the promising role of PET scan in diagnosis and staging of this rare but important disorder. An additional advantage, though this has not been studied yet, may be the use of PET scan to guide biopsy to the most suspicious and easily accessible lesions and to monitor therapy response.
Footnotes
Funding
This work was supported by the FWO-Vlaanderen (GO81411N to GV and TT). GV is holder of the International Roche Chair in Hematology.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow SH, Webber SA, Chadburn A, Ferry JA. Post-transplant lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (eds). WHO classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008;343–9 [Google Scholar]
- 2.Blaes AH, Morrison VA. Post-transplant lymphoproliferative disorders following solid-organ transplantation. Expert Rev Hematol. 2010;3(1):35–44 [DOI] [PubMed] [Google Scholar]
- 3.Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113(20):4992–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29(14):1844–54 [DOI] [PubMed] [Google Scholar]
- 5.Dierickx D, De Rycke A, Vandenberghe P, Janssens A, Lerut E, De Wolf-Peeters C, et al. Recipient-derived chronic lymphocytic leukaemia diagnosed shortly after kidney transplantation on protocol biopsy. Nephrol Dial Transplant. 2009;24(12):3886–90 [DOI] [PubMed] [Google Scholar]
- 6.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005; 80(2 Suppl):S254–64 [DOI] [PubMed] [Google Scholar]
- 7.Guttierez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients. Drugs. 2007; 67(8):1167–98 [DOI] [PubMed] [Google Scholar]
- 8.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31(11): 1860–1 [PubMed] [Google Scholar]
- 9.Gaston RS. Chronic calcineurin inhibitor nephrotoxicity: reflections on an evolving paradigm. Clin J Am Soc Nephrol. 2009;4(12): 2029–34 [DOI] [PubMed] [Google Scholar]
- 10.Juweid ME. FDG-PET/CT in lymphoma. Methods Mol Biol. 2011;727:1–19 [DOI] [PubMed] [Google Scholar]
- 11.Marom EM, McAdams HP, Butnor KJ, Coleman RE. Positron emission tomography with fluoro-2-deoxy-D-glucose (FDG-PET) in the staging of post transplant lymphoproliferative disorder in lung transplant recipients. J Thorac Imaging. 2004;19(2):74–8 [DOI] [PubMed] [Google Scholar]
- 12.O’Conner AR, Franc BL. FDG PET imaging in the evaluation of post-transplant lymphoproliferative disorder following renal transplantation. Nucl Med Commun. 2005;26 (12):1107–11 [DOI] [PubMed] [Google Scholar]
- 13.Bakker NA, Pruim J, de Graaf W, van Son WJ, van der Jagt EJ, van Imhoff GW. PTLD visualisation by FDG-PET: improved detection of extranodal localizations. Am J Transplant. 2006;6(8):1984–5 [DOI] [PubMed] [Google Scholar]
- 14.Von Falck C, Maecker B, Schirg E, Boerner AR, Knapp WH, Klein C, et al. Post transplant lymphoproliferative disease in pediatric solid organ transplant patients: a possible role for [18F]-FDG-PET(/CT) in initial staging and therapy monitoring. Eur J Radiol. 2007;63 (3):427–35 [DOI] [PubMed] [Google Scholar]
- 15.Vianchi E, Pascual M, Nicod M, Delaloye AB, Duchosal MA. Clinical usefulness of FDG-PET/CT scan imaging in the management of posttransplant lymphoproliferative disease. Transplantation. 2008;85(5):707–12 [DOI] [PubMed] [Google Scholar]
- 16.Noraini AR, Gay E, Ferrara C, Ravelli E, Mancini V, Morra E, et al. PET-CT as an effective imaging modality in the staging and follow-up of post-transplant lymphoproliferative disorder following solid organ transplantation. Singapore Med J. 2009;50(12):1189–95 [PubMed] [Google Scholar]
- 17.Blaes AH, Cioc AM, Froelich JW, Peterson BA, Dunitz JM. Positron emission tomography scanning in the setting of post-transplant lymphoproliferative disorders. Clin Transplant. 2009;23(6):794–9 [DOI] [PubMed] [Google Scholar]
- 18.Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne AS, Carrasquillo JA, et al. The role of tumor histogenesis, FDG-PET, and short course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15): 3017–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerusalem G, Beguin Y, Najjar F, Hustinx R, Fassotte MF, Rigo P, et al. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-Hodgkin’s lymphoma (NHL). Ann Oncol. 2001;12(6):825–30 [DOI] [PubMed] [Google Scholar]
- 20.Hartmann EL, Wu C. The evolving challenge of evaluating older renal transplant candidates. Adv Chronic Kidney Dis. 2010;17(4): 358–67 [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez M, Rehn S, Ahlström H, Sundström C, Glimelius B. Predicting malignancy grade with PET in non-Hodgkin’s lymphoma. J Nucl Med. 1995;36(10):1790–6 [PubMed] [Google Scholar]
- 22.Lapela M, Leskinen S, Minn HR, Lindholm P, Klemi PJ, Söderström O, et al. Increased glucose metabolism in untreated non-Hodgkin’s lymphoma: a study with positron emission tomography and fluorine-18-fluorodeoxyglucose. Blood. 1995;86(9): 3522–7 [PubMed] [Google Scholar]
- 23.Schöder H, Noy A, Gönen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(21):4643–51 [DOI] [PubMed] [Google Scholar]
- 24.Phongkitkarun S, Varavithya V, Kazama T, Faria SC, Mar MV, Podoloff DA, et al. Lymphomatous involvement of gastrointestinal tract: evaluation by positron emission tomography with (18)F-fluorodeoxyglucose. World J Gastroenterol. 2005;11(46):7284–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papajik T, Myslivecek M, Sedova Z, Buriankova E, Prochazka V, Koranda P, et al. Standardised uptake value of 18F-FDG on staging PET/CT in newly diagnosed patients with different subtypes of non-Hodgkin’s lymphoma. Eur J Haematol. 2011;86(1):32–7 [DOI] [PubMed] [Google Scholar]


