Abstract
Autologous stem cell transplantation has greatly improved the prognosis of systemic recurrent non-Hodgkin’s lymphoma. However, no prospective data are available concerning the feasibility and efficacy of this strategy for systemic lymphoma relapsing in the central nervous system. We, therefore, we performed an international multicenter retrospective study of patients with a central nervous system recurrence of systemic lymphoma to assess the outcome of these patients in the era of stem cell transplantation. We collected clinical and treatment data on patients with a first central nervous system recurrence of systemic lymphoma treated between 2000 and 2010 in one of five centers in four countries. Patient- and treatment-related factors were analyzed and compared descriptively. Primary outcome measures were overall survival and percentage of patients transplanted. We identified 92 patients, with a median age of 59 years and a median Eastern Cooperative Oncology Group/World Health Organization performance status of 2, of whom 76% had diffuse large B-cell histology. The majority (79%) of these patients were treated with systemic chemotherapy with or without intravenous rituximab. Twenty-seven patients (29%) were transplanted; age and insufficient response to induction chemotherapy were the main reasons for not being transplanted in the remaining 65 patients. The median overall survival was 7 months (95% confidence interval 2.6–11.4), being 8 months (95% confidence interval 3.8–5.2) for patients ≤ 65 years old. The 1-year survival rate was 34.8%; of the 27 transplanted patients 62% survived more than 1 year. The Memorial Sloan Kettering Prognostic Index for primary central nervous system lymphoma was prognostic for both undergoing transplantation and survival. In conclusion, despite the availability of autologous stem cell transplantation for patients with central nervous system progression or relapse of systemic lymphoma, prognosis is still poor. Long-term survival is, however, possible and more likely in patients able to undergo stem cell transplantation.
Introduction
Patients with a relapsed systemic non-Hodgkin’s lymphoma (NHL) with central nervous system (CNS) localization have a poor prognosis with a median survival of 2–4 months and a 1-year survival rate of less than 10% after various conventional treatments.1–3 In relapsed systemic lymphoma without CNS localization, survival is much improved by myeloablative therapy followed by autologous stem cell transplantation (ASCT), especially when combined with rituximab.4–7 Patients with relapsed lymphoma with a CNS localization have always been excluded from protocols studying ASCT and no prospective data are available concerning the feasibility and efficacy of this treatment in such patients. Retrospective studies of transplanted patients have shown that treatment with high-dose chemotherapy and stem cell transplantation can result in long-term survival in selected patients.8–11 However, there are virtually no data concerning the whole population of patients with a CNS localization of a recurrent systemic lymphoma. The prognosis of these patients is, therefore, unknown. Likewise, it is not known what proportion of these patients actually reach transplantation or which patient- or treatment related factors influence prognosis.
We, therefore, aimed to assess outcome in patients with a first recurrence of systemic NHL in the CNS in the era of stem cell transplantation in a retrospective multicenter study. The International Primary CNS Lymphoma Collaborative Group (IPCG) is an international multidisciplinary group from Europe, North America and Australasia set up to advance knowledge on CNS lymphoma. Given the rarity of CNS recurrence of systemic lymphoma the platform of the IPCG was used to gather information on a larger number of patients than would be possible in a single center or national setting.
Design and Methods
From collaborating centers, patients with a CNS recurrence or progression of systemic lymphoma were selected from local databases. Patients were included if they had been diagnosed with systemic NHL and had a first recurrence in the cerebrospinal fluid (CSF), brain parenchyma or both and were treated for their recurrence between January 2000 and January 2010. Concurrent systemic lymphoma was allowed but not required. For these patients data were collected on baseline characteristics, prognostic factors such as age, performance status, and International Prognostic Index (IPI) score as well as on treatment of both the primary disease and the recurrence or progression. With regard to the treatment of the recurrence or progression, the information recorded was the result and toxicity of the treatment, and survival. Data were rendered anonymous locally and collected in a central database. Independent review board approval was obtained for all participating centers, according to local regulations in the various countries.
Primary outcome measures were overall survival and percentage of patients transplanted. The secondary outcome was toxicity. Patient- and treatment-related factors were analyzed and compared descriptively.
Kaplan-Meier survival estimates were used to predict overall survival. Log-rank statistics to compare times to event across stratification variables were used to describe potential trends. Univariate Cox regression analysis was performed for age at relapse, both as a continuous variable and divided into age up to and over 65 years, and up to and over 50 years. These age limits were chosen since 65 is a commonly used age limit for stem cell transplantation and 50 has been found to be a prognostic factor in primary CNS lymphoma.12 Additionally, the value of the Memorial Sloan Kettering Cancer Center (MSKCC) prognostic score for primary CNS lymphoma was evaluated in this population of patients with secondary CNS lymphoma.12 There are three prognostic categories in this score: category 1 being age ≤50 years, category 2 being age >50 years and Karnofsky performance status ≥70, and category 3 being age >50 years and Karnofsky performance status <70. Other factors evaluated were Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO) status at relapse; chemotherapy regimen at relapse categorized into regimens with or without methotrexate, with or without cytarabine, with or without both methotrexate and cytarabine; and with or without ifosfamide; treatment with rituximab in the first line therapy; treatment with rituximab at relapse; localization of the recurrence in the brain parenchyma and transplantation. Correlations between baseline characteristics and treatment at recurrence, and ASCT were tested using cross-tabulation and the Pearson χ2 test.
Results
Five centers from four countries included 92 patients (38 women and 54 men) with CNS recurrence or progression of systemic aggressive NHL. The patients’ baseline and treatment characteristics at initial disease presentation are shown in Table 1. The majority of patients (76%) had diffuse large B-cell histology. At initial presentation 39% of the patients had two or more extranodal sites involved; in 22% the bone marrow was involved and in 12% a localization in a sinus, orbit or testis was present. Twenty patients (22%) initially had stage I disease; in three of them this was localized in a sinus, orbit or testis, seven had a breast lymphoma. Sixteen patients (17%) were at high risk according to the IPI score (4 or 5 risk factors) and 16 (17%) had high-intermediate risk (3 factors); the IPI score was not available for 37 patients (39%). Various chemotherapy regimens had been used as primary treatment; most of the patients (74%) had been pretreated with cyclophosphamide, adriamycin, vincristine and prednisone (CHOP) or CHOP-like regimens. Twenty-two percent had received specific CNS-directed therapy such as high-dose methotrexate (2%) or intrathecal chemotherapy (20%). Thirty-nine patients (42%) had received intravenous rituximab.
Table 1.
Baseline characteristics of the patients and their treatment at lymphoma presentation.
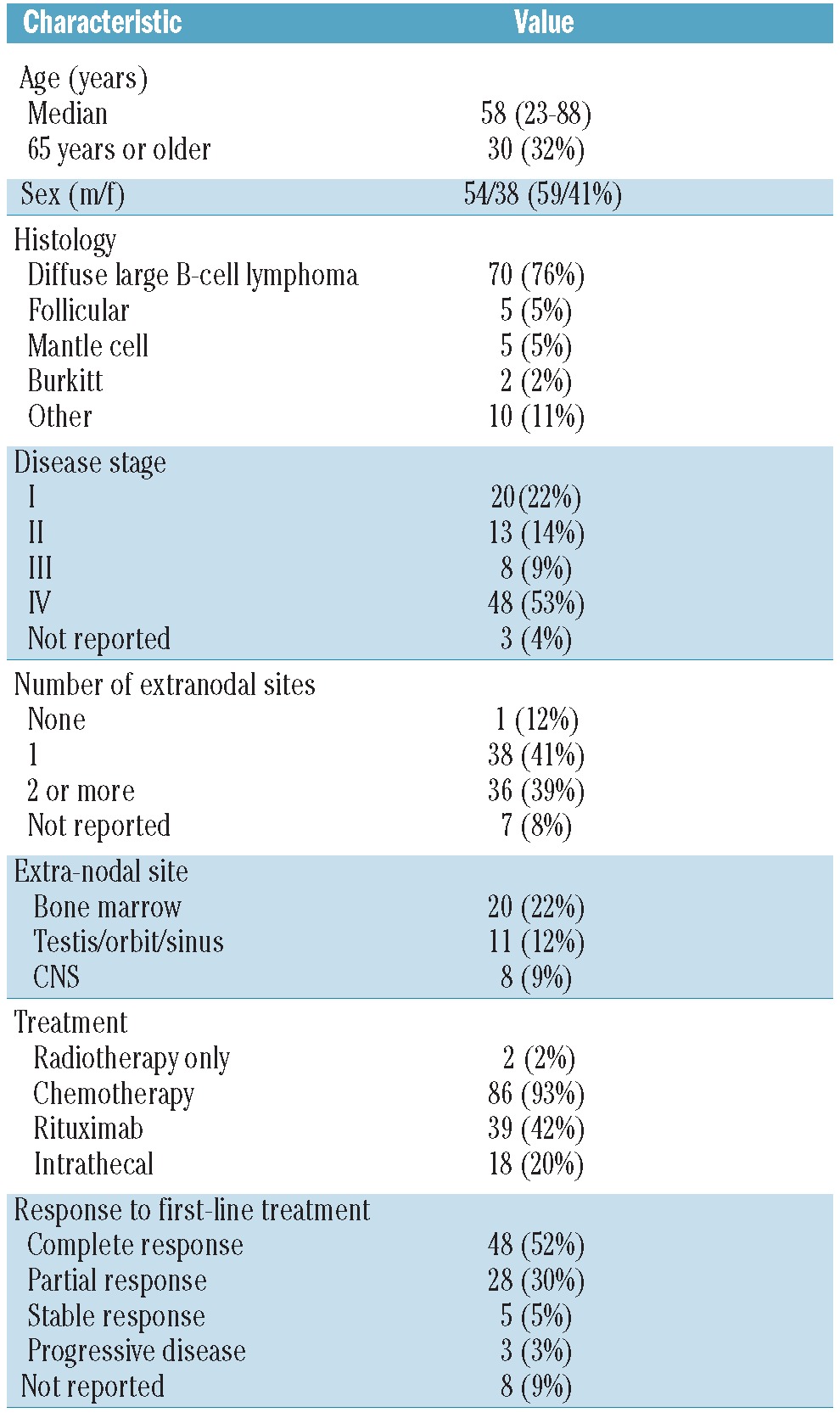
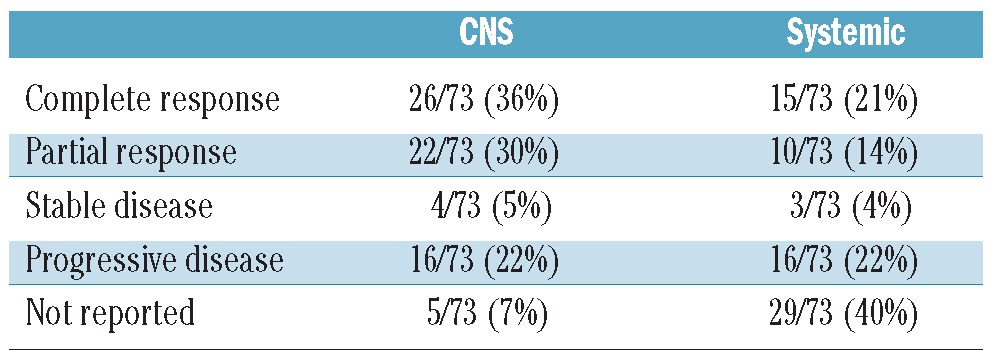
The patients’ clinical features at recurrence are shown in Table 2. Their median age at relapse was 59 years (range, 22–89 years); 32/92 patients were older than 65 years at the time the relapse was diagnosed. The median interval between the primary diagnosis and CNS relapse was 9 months (range, 0–281 months). The median ECOG/WHO performance status was 2. Sixty-five patients presented with symptoms of CNS disease, 12 with symptoms of systemic disease and 15 with symptoms of both. At least 53 patients (58%) had systemic disease activity concurrent with the CNS relapse but for 34 (37%) patients information on the presence or absence of systemic disease activity was not available. Fifty-five percent of patients had cranial nerve deficits or peripheral sensory or motor symptoms; 37% had headache or mental status changes. The CNS recurrence was localized only in the CSF in 37/92 patients (40%). In 47/92 (51%) of patients the recurrence was localized in the brain parenchyma, with or without concurrent CSF involvement; the percentage was similar for patients treated with or without rituximab in the firstline therapy. Seventy-three patients (79%) were treated with systemic chemotherapy for the recurrence. Many different regimens were used: 54 patients (74%) received a regimen containing methotrexate and 30 (41%) a regimen containing cytarabine. Twenty-three patients (25%) were treated with rituximab at relapse (Table 3). Response to induction treatment is shown in Table 4. Data on CNS response were available for 68 of the 73 patients treated with chemotherapy (93%) and on systemic response for 44 (60%). Although some data on response are missing, at least 48/73 (66%) were responsive to induction treatment in the CNS (complete or partial response) and 25/73 (34%) systemically. Response rates were similar for patients with CSF localization only and those with brain parenchymal disease. Twenty-seven patients (29%) underwent ASCT and 65 (71%) did not. The reason patients were not transplanted was age over 65 years (n=17, 18%), poor performance status or co-morbidities (n=14, 15%), complications of induction treatment (n=5, 5%), inadequate harvest (n=3, 3%) and insufficient response to induction treatment (n=13, 14%). Transplantation was not considered for four patients and for nine patients the reason for not performing a transplant was not reported or unknown.
Table 2.
Clinical features of the patients at relapse.
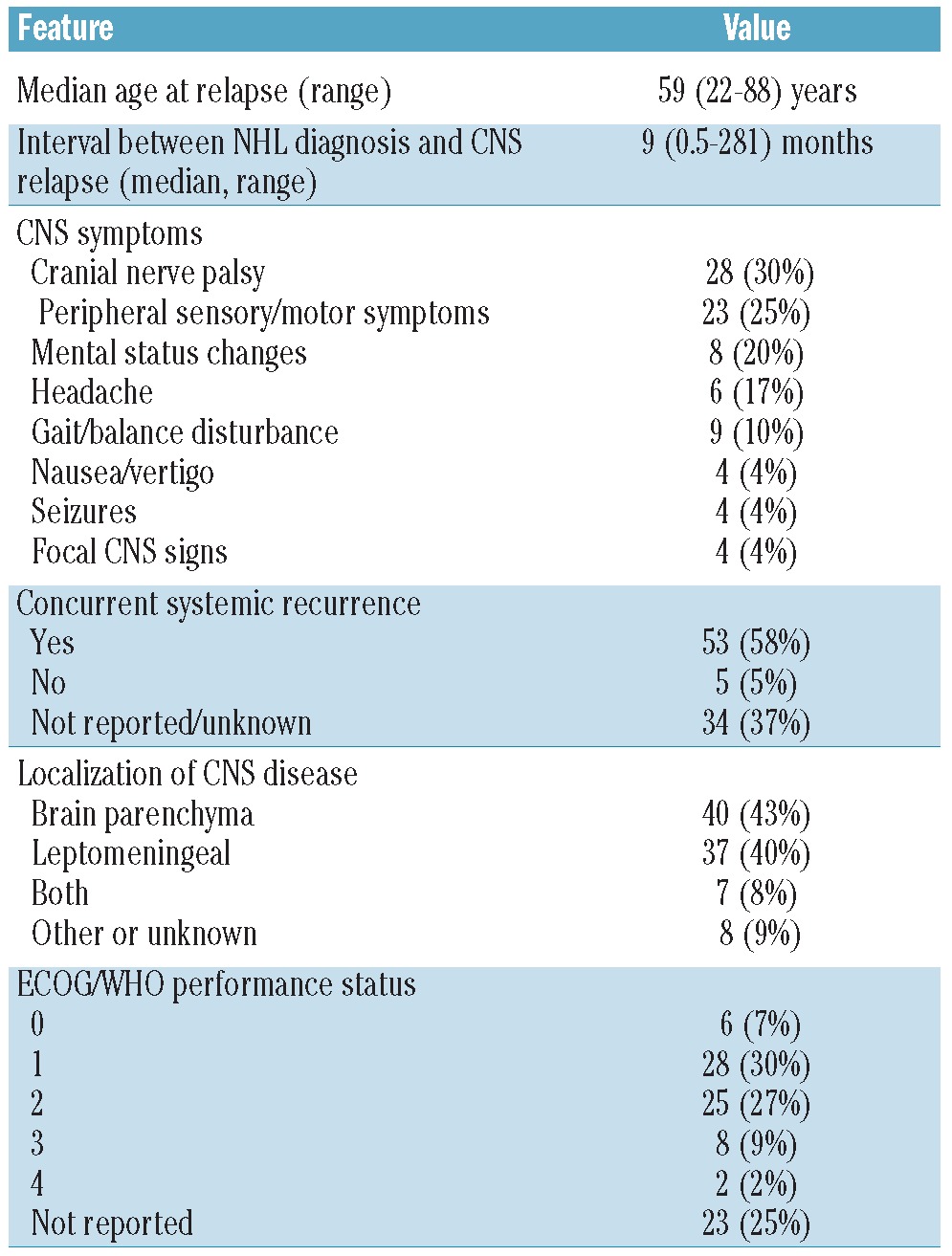
Table 3.
Treatment of relapse.
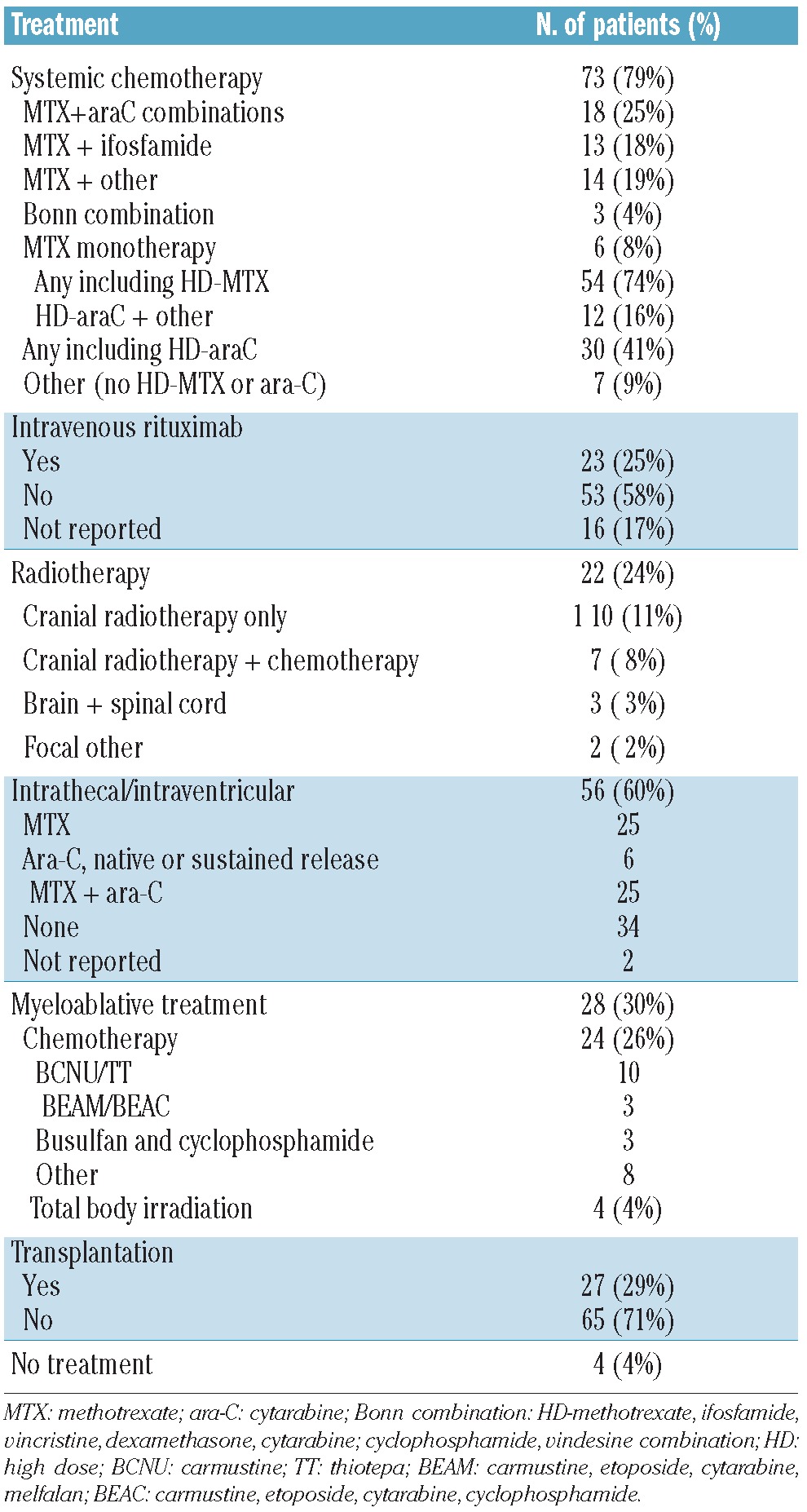
Table 4.
Response to induction chemotherapy at recurrence.
The median overall survival was 7 months (95% CI: 2.6–11.4) for the whole population of patients, 8 months (95% CI: 3.8–12.2) for patients ≤65 years of age, and 3 months (95% CI: 0.7–5.3) for patients aged >65 years. The difference between patients up to the age of 65 and those older was not statistically significant (log rank P=0.25). The overall survival rate was 35% at 1 year and 22% at 3 years. The median overall survival for transplanted patients was not reached. The overall survival rates in patients who had undergone ASCT were 62%, 54% and 42% at 1 year, 2 years and 3 years, respectively. The corresponding rates for patients who were not transplanted were 25%, 17% and 14% at 1, 2 and 3 years. At the end of the follow-up 66 patients (72%) had died; the median follow-up for patients still alive was 51 months (range, 2–147 months), while that for transplanted patients was 56 months (range, 5–147 months). The cause of death was lymphoma in 38 patients (41%); in 15% this was the systemic lymphoma, in 17% the CNS lymphoma and in 9% both. Twenty-one patients (23%) died of sepsis with or without other metabolic factors, and seven (8%) died of neurotoxicity (n=1) and unknown causes.
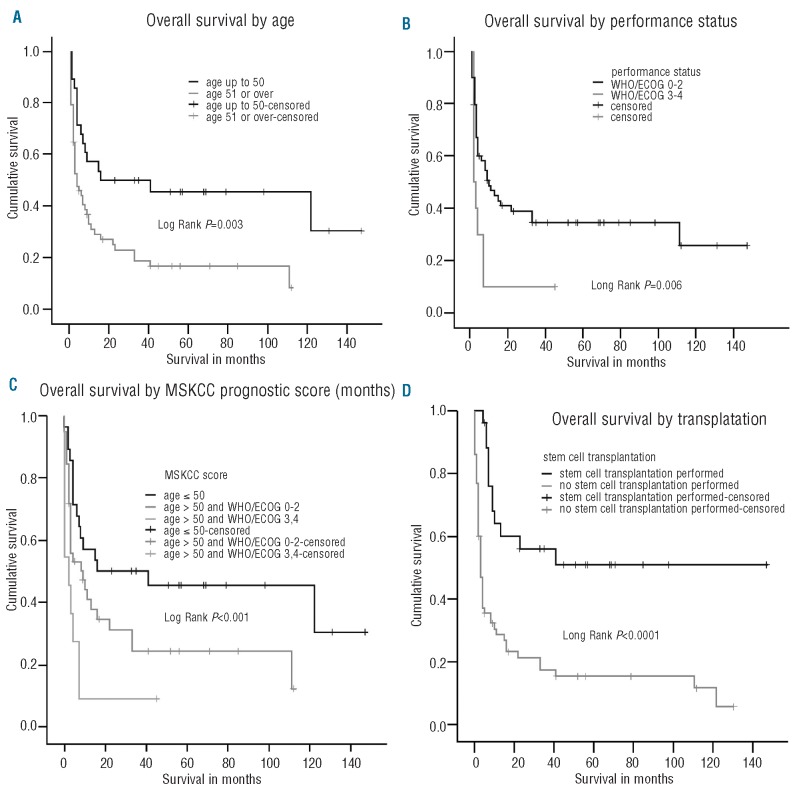
At univariate analysis prognostic factors for overall survival were age at relapse (P=0.003, Figure 1A), ECOG/WHO status at relapse (P=0.006, Figure 1B), and the combination of these as summarized in the MSKCC prognostic score (P=0.001, Figure 1C). The median overall survival in patients with a MSKCC prognostic score of 1 (age ≤50 years) was 16 months, and differed significantly from the 9 months in patients with a MSKCC score of 2 (age >50 years and ECOG/WHO score 0–2) and 2 months in patients with a MSKCC score of 3 (age >50 years and ECOG/WHO score >2) (P=0.001). No treatment factors other than transplantation (P<0.0001) were statistically significant: of the 27 transplanted patients, 62% survived more than 1 year, compared with 25% of patients who were not transplanted. (Figure 1D) Survival was not influenced by the interval between primary diagnosis and relapse, by chemotherapy regimen at relapse, by localization of the CNS recurrence in the CSF or the brain parenchyma, or by rituximab treatment in first-line therapy or at recurrence. At multivariate analysis both MSKCC prognostic score (P=0.007) and transplantation (P=0.002) remained statistically significant. Both age under 50 years at recurrence (P=0.001) and the MSKCC prognostic score (P=0.004) were also predictive of undergoing ASCT.
Figure 1.
(A) Overall survival (in months) by age. (B) Overall survival (in months) by performance status. (C) Overall survival (in months) by MSKCC prognostic score. (D) Overall survival (in months) by stem cell transplantation.
Discussion
We performed a retrospective, multicenter, international study to document outcome in patients with recurrent systemic lymphoma and secondary CNS disease in the era of stem cell transplantation. We included 92 patients in whom the clinical features at presentation of the relapse were compatible with those of prior publications: a median age of 59 years, median ECOG/WHO performance status of 2 and a majority (72%) of patients presenting with symptoms of CNS disease.13,14 We found a median survival of 7 months with 35% 1-year and 22% 3-year survival rates. No prior data are available on survival in unselected populations with CNS recurrence of systemic lymphoma in the era of stem cell transplantation. Previous series covered long periods of time in which transplantation was not available for a majority of patients. Only one study has been published on patients treated after 2002 but in this series only 8/20 patients had recurrent systemic lymphoma; the others had relapsed primary CNS lymphoma.15 Although our study is retrospective, it comprises the only series of patients diagnosed with CNS recurrence of systemic lymphoma in the CSF and/or brain parenchyma treated in the last 10 years. Our 3-year survival rate of 22% seems better than that previously found in CNS recurrent aggressive lymphoma before stem cell transplantation became available.14,16 However, it is considerably worse than the survival of patients with recurrent systemic diffuse large B-cell lymphoma, in whom the 3-year overall survival rate was reported to be 49–55%.7,17 In patients with recurrent primary CNS lymphoma the median survival is reported to be 5–18 months, the 18 months having been reported in a prospective phase II study investigating ASCT.18–21 Thus, although our retrospective and unselected series is not comparable with prospective studies in which all patients had to conform to strict inclusion criteria, from these data the prognosis does appear to be worse in patients with CNS recurrence of systemic lymphoma than in patients with primary CNS lymphoma or with systemic disease only.
As expected, age and performance status were prognostic for survival; furthermore, the MSKCC prognostic index for primary CNS lymphoma appears to be valid also in recurrent secondary CNS lymphoma. Contrary to the findings in isolated brain parenchyma relapse of NHL, the use of methotrexate was not a prognostic factor in our patients, possibly because few patients in our series were treated without methotrexate.22 Of the treatment factors analyzed, only transplantation was a significant prognostic factor for survival (log-rank P<0.001). However, only patients with acceptable performance status responding to induction chemotherapy were eligible for this treatment and few comparable patients not treated with ASCT are available in this study.
Patients in our series who actually underwent transplantation had a fair chance of long-term survival, with 42% of these patients being alive at 3 years. However, only a minority (29%) of patients were actually treated with ASCT despite the fact that all patients were accrued from centers performing this procedure. A major reason for not being transplanted was age over 65 years (32 patients). Excluding these patients, 42% (25/60) of patients were transplanted, which is similar to the 49–63% of patients transplanted in prospective trials investigating ASCT in recurrent systemic diffuse large B-cell lymphoma.4,7,17,23 Other than age and performance status, both factors which cannot be influenced, insufficient response to reinduction treatment was the main reason ASCT was not performed in our series.
The efficacy of re-induction chemotherapy is a major determinant of the outcome after ASCT.10,11,24 Sixty-six percent of our patients had an objective CNS response to chemotherapy and at least 35% had a systemic response; data, especially regarding systemic responses, were unfortunately missing for a substantial number of patients. However, the proportion of patients dying from CNS lymphoma (17%) was very similar to that of patients dying of systemic lymphoma (15%), with progressive disease in 9% of both. The efficacy of re-induction therapy does, therefore, seem similar in systemic disease and in the CNS.
In 41% of patients the lymphoma was the main cause of death. Sepsis with or without other metabolic factors was described as the main cause of death in 24% and other or unknown causes in 6.5% of patients. Thus although infectious complications occurred frequently, especially in older patients, the main determinant of the poor prognosis was the disease itself, reflecting insufficient efficacy of the treatment employed, despite significant toxicity.
We observed a relatively large proportion of patients with brain parenchymal disease. In past series of patients, most patients with CNS localization of systemic lymphoma were diagnosed with leptomeningeal disease, with brain parenchymal disease reported in only 20–25% of patients.13,16,25,26 The major change in the treatment of systemic NHL in the last decade has been the addition of rituximab. We, therefore, investigated whether treatment with rituximab influenced the localization of the CNS disease, but that was not the case in our series. Other series in which this issue was addressed have yielded conflicting results and a definitive conclusion cannot be made.1,27 In any case, outcome did not differ between patients with or without brain parenchymal disease.
Naturally, our study has its limitations. As described previously it is retrospective, with patients’ data collected from dedicated centers, and there may have been bias in recognizing patients and confounding from missing data. However, being multicenter and multinational the results will be broadly applicable and similar data, describing a relatively large population of patients with this rare disease entity exclusively in the era of ASCT, are not yet available. It is clear that improved treatments are dearly needed; several systematic and prospective studies are currently underway and will, it is to be hoped, yield better results.
In conclusion, despite the availability of ASCT as consolidation treatment for patients with CNS progression or relapse of systemic NHL, these patients’ prognosis is still poor, for the most part because of insufficient efficacy of treatment. However, long-term survival is possible and more likely in patients able to undergo ASCT. The MSKCC prognostic index for primary CNS lymphoma was found to be prognostic both for undergoing ASCT and for survival in these patients with secondary CNS lymphoma, but no specific treatment variable could predict outcome. Until the results of prospective studies currently underway become available, the data obtained might aid clinicians in deciding whether to aim for long-term survival or even a cure in a given patient by treating aggressively and aiming for ASCT or whether the potential toxicity and limited chances of efficacy support a more palliative treatment.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOV-ER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113(17):3896–902 [DOI] [PubMed] [Google Scholar]
- 2.Bollen EL, Brouwer RE, Hamers S, Hermans J, Kluin M, Sankatsing SU, et al. Central nervous system relapse in non-Hodgkin lymphoma. A single-center study of 532 patients. Arch Neurol. 1997;54(7): 854–9 [DOI] [PubMed] [Google Scholar]
- 3.Bjorkholm M, Hagberg H, Holte H, Kvaloy S, Teerenhovi L, Anderson H, et al. Central nervous system occurrence in elderly patients with aggressive lymphoma and a long-term follow-up. Ann Oncol. 2007; 18(6):1085–9 [DOI] [PubMed] [Google Scholar]
- 4.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995; 333(23):1540–5 [DOI] [PubMed] [Google Scholar]
- 5.Gribben JG, Goldstone AH, Linch DC, Taghipour G, Mcmillan AK, Souhami RL, et al. Effectiveness of high-dose combination chemotherapy and autologous bone marrow transplantation for patients with non-Hodgkin’s lymphomas who are still responsive to conventional-dose therapy. J Clin Oncol. 1989;7(11):1621–9 [DOI] [PubMed] [Google Scholar]
- 6.Freedman AS, Takvorian T, Anderson KC, Mauch P, Rabinowe SN, Blake K, et al. Autologous bone marrow transplantation in B-cell non-Hodgkin’s lymphoma: very low treatment-related mortality in 100 patients in sensitive relapse. J Clin Oncol. 1990;8(5):784–91 [DOI] [PubMed] [Google Scholar]
- 7.Vellenga E, van Putten WL, van der Veer M, Zijlstra JM, Fibbe WE, van Oers MH, et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+ NHL: a prospective randomized HOVON trial. Blood. 2008;111(2):537–43 [DOI] [PubMed] [Google Scholar]
- 8.Alvarnas JC, Negrin RS, Horning SJ, Hu WW, Long GD, Schriber JR, et al. High-dose therapy with hematopoietic cell transplantation for patients with central nervous system involvement by non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2000;6(3A):352–8 [DOI] [PubMed] [Google Scholar]
- 9.Kasamon YL, Jones RJ, Piantadosi S, Ambinder RF, Abrams RA, Borowitz MJ, et al. High-dose therapy and blood or marrow transplantation for non-Hodgkin lymphoma with central nervous system involvement. Biol Blood Marrow Transplant. 2005;11(2):93–100 [DOI] [PubMed] [Google Scholar]
- 10.Williams CD, Pearce R, Taghipour G, Green ES, Philip T, Goldstone AH. Autologous bone marrow transplantation for patients with non-Hodgkin’s lymphoma and CNS involvement: those transplanted with active CNS disease have a poor outcome--a report by the European Bone Marrow Transplant Lymphoma Registry. J Clin Oncol. 1994;12(11):2415–22 [DOI] [PubMed] [Google Scholar]
- 11.van Besien K, Przepiorka D, Mehra R, Giralt S, Khouri I, Gajewski J, et al. Impact of preexisting CNS involvement on the outcome of bone marrow transplantation in adult hematologic malignancies. J Clin Oncol. 1996;14(11):3036–42 [DOI] [PubMed] [Google Scholar]
- 12.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5 [DOI] [PubMed] [Google Scholar]
- 13.Hollender A, Kvaloy S, Lote K, Nome O, Holte H. Prognostic factors in 140 adult patients with non-Hodgkin’s lymphoma with systemic central nervous system (CNS) involvement. A single centre analysis. Eur J Cancer. 2000;36(14):1762–8 [DOI] [PubMed] [Google Scholar]
- 14.Paulus JA, Bos GM, Lowenberg B, van den Bent MJ. [Treatment results and the prognosis in patients with localization of non-Hodgkins-lymphoma in the central nervous system]. Ned Tijdschr Geneeskd. 1998;142(40):2196–200 [PubMed] [Google Scholar]
- 15.Fischer L, Korfel A, Kiewe P, Neumann M, Jahnke K, Thiel E. Systemic high-dose methotrexate plus ifosfamide is highly effective for central nervous system (CNS) involvement of lymphoma. Ann Hematol. 2009;88(2):133–9 [DOI] [PubMed] [Google Scholar]
- 16.Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol. 2009;27(1):114–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisselbrecht C, Glass B, Mounier N, Singh GD, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27): 4184–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26(15):2512–8 [DOI] [PubMed] [Google Scholar]
- 19.Reni M, Ferreri AJ, Villa E. Second-line treatment for primary central nervous system lymphoma. Br J Cancer. 1999;79:530–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahnke K, Thiel E, Martus P, Herrlinger U, Weller M, Fischer L, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159–65 [DOI] [PubMed] [Google Scholar]
- 21.Hottinger AF, Deangelis LM, Yahalom J, Abrey LE. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69(11):1178–82 [DOI] [PubMed] [Google Scholar]
- 22.Doolittle ND, Abrey LE, Shenkier TN, Tali S, Bromberg JE, Neuwelt EA, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111(3):1085–93 [DOI] [PubMed] [Google Scholar]
- 23.Crump M, Baetz T, Couban S, Belch A, Marellus D, Howson-Jan K, et al. Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG). Cancer. 2004;101(8):1835–42 [DOI] [PubMed] [Google Scholar]
- 24.Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103 (10):3684–8 [DOI] [PubMed] [Google Scholar]
- 25.Jahnke K, Thiel E, Martus P, Schwartz S, Korfel A. Retrospective study of prognostic factors in non-Hodgkin lymphoma secondarily involving the central nervous system. Ann Hematol. 2006;85(1):45–50 [DOI] [PubMed] [Google Scholar]
- 26.van Besien K, Ha CS, Murphy S, McLaughlin P, Rodriguez A, Amin K, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91(4):1178–84 [PubMed] [Google Scholar]
- 27.Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21(5):1046–52 [DOI] [PubMed] [Google Scholar]