Abstract
The vasculature of the eye and the heart share several common characteristics. The easily accessible vessels of the eye are therefore—to some extent—a window to the heart. There is interplay between cardiovascular functions and risk factors and the occurrence and progression of many eye diseases. In particular, arteriovenous nipping, narrowing of retinal arteries, and the dilatation of retinal veins are important signs of increased cardiovascular risk. The pressure in the dilated veins is often markedly increased due to a dysregulation of venous outflow from the eye. Besides such morphological criteria, functional alterations might be even more relevant and may play an important role in future diagnostics. Via neurovascular coupling, flickering light dilates capillaries and small arterioles, thus inducing endothelium-dependent, flow-mediated dilation of larger retinal vessels. Risk factors for arteriosclerosis, such as dyslipidaemia, diabetes, or systemic hypertension, are also risk factors for eye diseases such as retinal arterial or retinal vein occlusions, cataracts, age-related macular degeneration, and increases in intraocular pressure (IOP). Functional alterations of blood flow are particularly relevant to the eye. The primary vascular dysregulation syndrome (PVD), which often includes systemic hypotension, is associated with disturbed autoregulation of ocular blood flow (OBF). Fluctuation of IOP on a high level or blood pressure on a low level leads to instable OBF and oxygen supply and therefore to oxidative stress, which is particularly involved in the pathogenesis of glaucomatous neuropathy. Vascular dysregulation also leads to a barrier dysfunction and thereby to small retinal haemorrhages.
Keywords: Retinal vessels, Cardiovascular risk, Vascular dysregulation, Endothelial function, Systemic hypertension, Systemic hypotension, Retinal venous pressure, Retinal vein occlusion, Glaucoma
Introduction
The heart and the eye, two organs at first sight not linked to each other, have more in common than one would expect. The vasculature of the eye, although some peculiarities do exist, shares many features with the vasculature of the heart and is often exposed to the same intrinsic and environmental influences. Thus, the eye, with its easily accessible vasculature, may indeed be a window to the heart, but knowledge about some unique vascular features is necessary. It is the aim of this review (i) to describe the basic characteristics of the vasculature of the eye, (ii) to spark interest for the eye as a ‘vascular’ organ and the inherent advantages of depicting the microvasculature directly, and (iii) to make cardiologists aware of ophthalmologists' concerns about systemic conditions potentially aggravating eye diseases.
Vasculature of the eye
Blood supply to the eye faces the following challenges: (i) the retina has the highest oxygen consumption per volume in the body, (ii) the very exposed eye needs constant temperature to function, and (iii) the blood supply should not hinder the optical function. Nature has solved these needs in the following ways: (i) transparent parts such as the cornea and lens are supplied by a transparent aqueous humour; (ii) within the retina, oxygen transport is facilitated by intracellular haemoglobin; (iii) the translucent retina has only a few blood vessels and the photoreceptors receive their oxygen and nutrition from the choroid, which, in turn, has the highest blood flow (BF) per volume in the body; and (iv) the eye has no lymphatic vessels and it possesses an immune privilege.
Anatomy of ocular circulation
The circulation of the eye essentially comprises four parts: (i) the circulation of the anterior part of the eye, particularly the ciliary body that produces the aqueous humour; (ii) a retinal circulation similar to brain circulation but lacks autonomic innervation; (iii) a choroidal vasculature with fenestrated capillaries and the greatest density of autonomic innervations known in the body; and (iv) the optic nerve head (ONH);1 (Figure 1).
Figure 1.
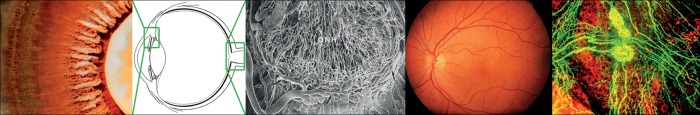
The ciliary body is highly perfused and produces the aqueous humour (left: photo taken from the back of the eye). The optic nerve head has a very dense network of long capillaries (middle). The retinal circulation is similar to brain circulation but without autonomic innervation. In contrast, the vasculature of the choroid is densely innervated (right).
Regulation of ocular blood flow
The retinal BF is auto-regulated2 and therefore—within a certain range—is independent of perfusion pressure (PP). The main regulators are the vascular endothelium cells and the neural and glial cells.3 A simplified function of neurovascular coupling (NVC) is depicted in Figure 2. If flickering light is projected onto the retina, both the arteries and veins dilate via a process mediated mainly by nitric oxide (NO). The visual stimulation of the retina primarily dilates capillaries and very small arterioles, thereby inducing a flow-mediated dilation of the larger retinal vessels, as observed with a retinal vessel analyser.4 Therefore, these tests also provide hints regarding the function of the vascular endothelium and may thus be particularly interesting for the cardiologist, as endothelial dysfunction is associated with most, if not all, cardiovascular risk factors.5 The densely innervated choroid (Figure 1) reacts to physical and psychological stressors as well as to temperature. If a cold airstream blows towards the eye, cold receptors in the sclera induce an increase in choroid BF.6
Figure 2.
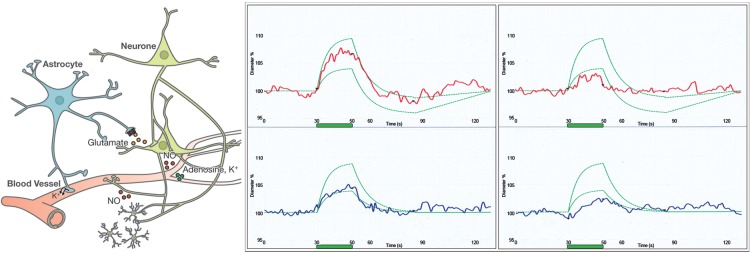
The size of the retinal vessels is influenced by neural and glial cells (neurovascular coupling), shown in a simplified view on the left. Flickering light (green bar) leads to vasodilation of arteries (red) and veins (blue) in healthy subjects (middle) and to a lesser extent in subjects with vascular dysregulation (right). The green curves indicate the normal range. (Modified after Flammer J, Mozaffarieh M, Bebie H. Basic Sciences in Ophthalmology–Physics and Chemistry. Springer Publications, in print, with permission.)
The ONH BF is influenced by the NVC but also by circulating molecules diffusing from the choroid into the ONH.
Measurement of ocular blood flow
A number of different methods are available to determine ocular blood flow (OBF), depending on the vessels of interest.7 Retroocular vessels are measured by colour Doppler imaging (Figure 3), while intraocular vessels can be observed directly by ophthalmoscopy or visualized with the help of fluorescence or indocyanine green angiography (Figure 4) and BF velocity can be quantified by Laser Doppler velocimetry. The BF in a capillary bed such as the ONH can be quantified by laser-flowmetry or laser-speckling. The bulk flow to the eye can be estimated by thermography8 (Figure 4). The dynamic changes over time can be observed with a retinal vessel analyser (Figure 2).
Figure 3.
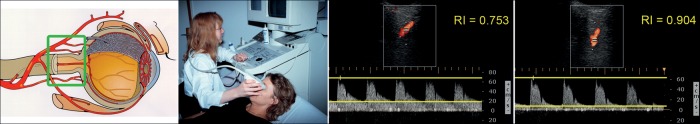
The vessels behind the eye (ophthalmic artery, central retinal artery, and the ciliary arteries) can be visualized and its flow quantified by colour Doppler imaging. Shown is the outcome from the ophthalmic artery of a healthy subject with normal resistivity (middle) and of a glaucoma patient with high resistivity (right). (Modified after Flammer J, Mozaffarieh M, Bebie H. Basic Sciences in Ophthalmology–Physics and Chemistry. Springer Publications, in print, with permission.)
Figure 4.
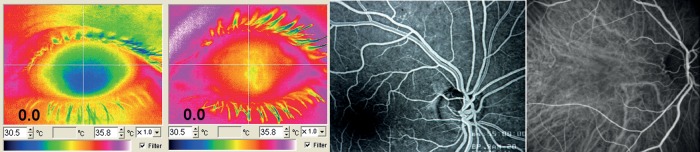
The bulk flow can be quantified with the help of thermography. Left: A relatively cool eye of a subject with vascular dysregulation in relation to a normal control (middle left). The retinal circulation is visualized with fluorescence angiography (middle right) and choroid circulation with the indocyanine green angiography (right).
Defective ocular blood flow
As in all vascularized tissues, a marked reduction in OBF leads to an infarction, such as retinal infarction or ischaemic anterior optic neuropathy (Figure 5). The main causes are arteriosclerosis and emboli (originating from the carotid artery and the heart) or vasculitis such as giant cell arteritis. Arteriosclerosis frequently involves the retroocular vessels at early stages,9 probably due to the mechanical strain imposed by the rotating eye. In contrast, intraocular vessels may show some hyalinosis but not arteriosclerosis.
Figure 5.
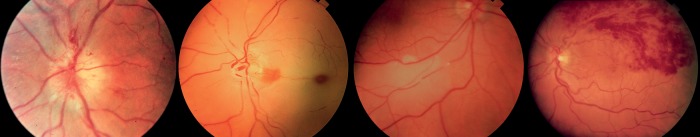
Classical ocular blood flow dysfunctions: (i) Anterior ischaemic neuropathy. (ii) Central retinal arterial occlusion. (iii) Embolus in a retinal artery. (iv) Retinal branch vein occlusion.
Are retinal vessels a window to the heart? The cardiologist's perspective
The retina is a unique site where the microcirculation can be imaged directly. Thus, it provides a window for detecting changes in microvasculature relating to the development of cardiovascular diseases such as arterial hypertension or coronary heart disease10 (Figure 6). Analysis of the retinal microvasculature provides information about the structure as well as the function of the vessels and this information can be easily obtained repeatedly over time. However, its clinical application has only recently gained some attention.11
Figure 6.
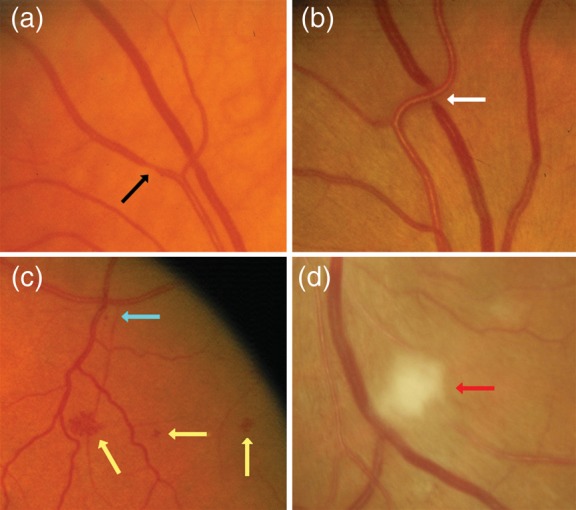
Examples of retinal vascular signs in patients with cardiovascular diseases. Black arrow: focal arteriolar narrowing. White arrow: arterio-venous nicking. Yellow arrow: haemorrhage. Blue arrow: micro-aneurysm. Red arrow: cotton wool spot. (From Liew and Wang,10 reused with permission from the author and the publisher.)
Structural retinal changes
Systemic cardiovascular diseases like arterial hypertension, coronary heart disease, or diabetes mellitus, as well as obesity are all associated with structural vascular changes in the retina. These include narrowing of arterioles, dilatation of veins, and a decrease in the arteriovenous ratio (AVR). According to the classification by Keith, Wagener, and Barker, four grades of retinal changes in hypertensive patients have been proposed: focal or general arteriolar narrowing (grade 1), arterio-venous nipping (grade 2), flame-shaped haemorrhages and exudates (grade 3), and papilledema (grade 4). At present, because of the often early diagnosis and treatment of hypertension, grades 3 and 4 are very rarely seen. In contrast, arteriolar narrowing and arterio-venous nipping are observed much more frequently. However, the clinical and prognostic significance of such mild degrees of retinopathy has been questioned,12–14 because these alterations appear to be largely non-specific arteriolar changes, except in young patients in whom modification from a normal retina should raise doubts. In contrast, grade 3 and 4 retinal changes are associated with an increased risk of cardiovascular events.15,16 Recent selective methodologies for investigating retinal changes in hypertension allow quantification of geometrical and topological properties of the arteriolar and venular tree. Evidence from both cross-sectional and longitudinal studies utilizing these new techniques documented an independent association between narrowed retinal arteriolar diameter and elevated blood pressure and showed that narrow retinal arterioles and smaller AVR may precede arterial hypertension and predict the development of hypertension in initially normotensive individuals.17–19
Structural alterations of peripheral small resistance arteries, as indicated by an increased media-to-lumen ratio (M/L), are frequently associated with several cardiovascular risk factors, including hypertension or diabetes mellitus, and contribute to the development of target organ damage.20 At present, the best methodological approach to detecting M/L in small resistance arteries is wire or pressure micromyography, which allows a demonstration that an increased M/L of subcutaneous small arteries relates to reduced coronary flow reserve and to some indexes of cardiac damage in hypertensive patients.21,22 In addition, the M/Ls of peripheral small arteries are independently associated with the occurrence of cardiovascular events, either in a high-risk population or in patients at low-moderate risk.23,24 Unfortunately, the invasive nature of this measurement, which requires a biopsy of subcutaneous fat from the glutaeal or omental regions, prevents larger-scale application of this method. In order to develop alternative non-invasive approaches for the evaluation of microvascular structure, the interest of many researchers was focused on the retinal vascular district. A recent and promising approach includes a confocal measurement of the external diameter of retinal arterioles and an evaluation of the internal diameter with a laser Doppler technique. From these two measurements, it is possible to calculate the wall-to-lumen (W/L) ratio of retinal arterioles.25 By this new approach, called scanning laser Doppler flowmetry (SLDF), the authors observed an increased W/L in essential hypertensive patients,26 an alteration even more evident in hypertensive patients with previous cardiovascular events.25 In a very recent report, the W/L of retinal arterioles evaluated by SLDF has been compared with the M/L of subcutaneous small arteries, assessed by the micromyographic technique, in the same subjects. A close correlation was observed between M/L and W/L, thus indicating that SLDF may provide similar information regarding microvascular morphology compared with invasive, but prognostically relevant, micromyographic measurements of the M/L of subcutaneous small arteries.27
Other interesting reports evidenced structural retinal changes as an early indicator of the presence28 and severity of coronary artery disease.29 Furthermore, there is a relation with coronary artery calcification and myocardial perfusion.30,31 Recently fractal analysis and quantification of microvascular branching has gained some interest in cardiovascular literature and has been recently demonstrated to predict cardiovascular mortality. Patients with suboptimal branching (very dense or very sparse) have an impaired prognosis.32
Future studies are needed to confirm the usefulness of such a non-invasive retinal microvascular approach to obtain a better stratification of cardiovascular risk and its prognostic relevance. A further advantage of retinal vessel analysis is the possibility of depicting not only arteries but also veins. Similar to arteries, veins are not mere passive vessels, but may also actively adapt to the vascular needs. Contrary to the retinal arteries, dilated venules bear a worse cardiovascular prognosis.33 These retinal veins, however, are often dilated by high retinal venous pressure (RVP) induced by local vasoconstriction at the level of the ONH.
Functional retinal changes
These morphological findings, however, should be supplemented by functional tests. As mentioned above, flicker light-induced vasodilation of the retinal vessel arteries and veins may give important functional information about the vascular endothelium. An impaired endothelial function is characteristic (although not pathognomonic) of atherosclerosis, a process beginning early in life and eventually leading to myocardial infarction, stroke, and other devastating vascular complications. Endothelial dysfunction precedes the development of morphological vascular changes, and thus, the assessment of endothelial function provides important diagnostic and prognostic information, particularly in patients with cardiovascular risk factors.5,34 In the past several years, invasive and non-invasive tools for in vivo assessment of endothelial function have been developed, all with their inherent advantages and disadvantages.35
Flicker light-induced vasodilatation in the retinal artery may be a valuable additional tool in this respect, particularly as it has been shown to be endothelium- and NO-dependent, however, independent from sympathetic innervations. Indeed, NO plays a role not only in the maintenance of retinal arterial and venous tone, but also in hyperaemic responses to flickering light, since the latter was abolished by systemic infusion of a NO-synthase inhibitor.36 Reduced flicker light-induced vasodilatation has already been demonstrated in patients with cardiovascular risk factors, such as diabetes, hypertension, obesity, and dyslipidaemia, and can be improved with the respective therapy.37–39 This was first demonstrated in essential hypertension. The increase in BF velocity in the central retinal artery and retinal capillary flow induced by flickering, as well as their decrease induced by NO-synthase inhibition, both present in healthy subjects, were abolished in young, untreated patients with uncomplicated hypertension.40 Interestingly, 7 days of treatment with an angiotensin receptor blocker can partially restore retinal endothelial function40,41 in parallel to what occurs in other districts.42 At the moment, these promising data are limited by small sample size and cross-sectional design. Future research should focus on the relationship between retinal vascular reactivity and other established techniques for the study of endothelial function, as well as on their possible prognostic significance, since this approach can provide unique insight into cerebral microcirculation, which is a crucial district for atherosclerotic, and in particular hypertensive, organ damage.
Pathophysiology of tissue damage: an ophthalmologic perspective
Cardiologists are concerned about potential consequences of cardiovascular risk factors and whether the eye could serve as a window for morphological and functional changes preceding the changes in the heart. On the other hand, ophthalmologists are concerned about systemic conditions inducing or aggravating eye diseases. For the optimal treatment of the patients, it is of importance for the cardiologist or internist to understand the vascular pathophysiology behind the most common eye diseases. Indeed, many prevalent eye diseases can be considered systemic diseases, e.g. diabetic or hypertensive retinopathy and, to some extent, also glaucoma.
The impact of chronic hypoxia
While acute and severe hypoxia leads to infarction, chronic hypoxia leads to an increase in Hypoxia-inducible factor (HIF)-1alpha (Figure 7) and thereby to an up-regulation of a number of molecules such as endothelin-1 (ET-1) and vascular endothelial growth factor (VEGF). This, in turn, has three potential consequences: stimulation of neovascularisation, weakening of the blood–retina barrier (BRB), and local vasoconstriction of veins.
Figure 7.
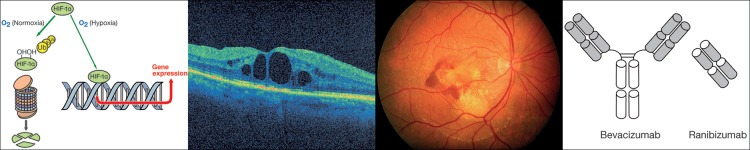
Left: Under hypoxic condition hypoxia-inducible factor-1 alpha (HIF-1α) is increased and enhances expression of genes such as endothelin-1 or vascular endothelial growth factor. (From Flammer J, Mozaffarieh M, Bebie H. Basic Sciences in Ophthalmology–Physics and Chemistry. Springer Publications, in print, with permission.) This leads to weakening of the BRB (an example is the macular oedema, second from left) or to neovascularization (an example is wet age-related macular degeneration, second from right) Right: Antibody or antibody fragment injection into the eye binds VEGF, thereby restoring wet age-related macular degeneration in a dry age-related macular degeneration.
This is best exemplified with age-related macular degeneration (AMD) normally remaining ‘dry’ and only moderately reducing visual acuity. One potential consequence of dry AMD is that hypoxia can induce growth of new vessels from the choroid into the retina thereby turning it to ‘wet’ AMD. One of the main stimuli involved is VEGF. Binding of VEGF by antibodies or fragments of antibodies thereby reduces symptoms relatively quickly (Figure 7). However, note that this treatment does not eliminate the underlying disease of the AMD or the hypoxia, and therefore, the treatment needs to be repeated.
The impact of systemic hypertension
As outlined above, severe arterial hypertension leads to hypertensive retinopathy. Hypertension and all other risk factors for arteriosclerosis,43 however, are also related to other eye diseases such as cataracts, AMD and increased intraocular pressure (IOP).44
The impact of systemic hypotension
Arterial hypotension is also very important for the eye, but far less known. It is a particularly well-established risk factor for glaucomatous optic neuropathy (GON).45,46 As a consequence, blood pressure should not be lowered too rigorously in patients suffering from both systemic arterial hypertension and glaucoma. Spontaneous systemic hypotension [as it occurs particularly in the context of primary vascular dysregulation (PVD)] is very often observed in patients with normal tension glaucoma (NTG). Glaucoma patients with progression of GON despite a normal or normalized IOP may profit from a therapeutical increase in blood pressure, although unfortunately, controlled studies are not yet available.
Besides systemic hypotension, nocturnal over- and non-dipping as well as increased blood pressure (BP) fluctuation are related to progression of GON. Hypotension is related to increased sensitivity to ET-1,47 which further reduces OBF. The relationship between PP or PP-fluctuation and GON-progression is now clearly established.48 Perfusion pressure is defined as arterial pressure minus venous pressure. However, in most of these studies, RVP was not measured but calculated based on the assumption that the venous pressure is equal to IOP, an assumption that is not correct in all cases.
Retinal venous pressure
RVP must be at least as high as the IOP (otherwise the vessels would collapse) and as high as the cerebrospinal fluid pressure, since the central retinal vein leaves the eye via the anterior optic nerve and then crosses the subarachnoid space. Retinal venous pressure is measured using a contact lens dynamometer.49 This pressure is sometimes higher than the IOP even in healthy subjects, but increases are quite often observed in conditions like glaucoma50 and diabetes mellitus, at high altitudes and in subjects with PVD. Retinal venous pressure varies over time and can be markedly influenced by drugs. Consequently, the PP is often smaller than previously assumed and pharmacological reduction of RVP is a promising approach to improving OBF. Whether increased venous pressure is a marker of increased cardiovascular risk is not known yet, but might deserve further evaluation.
Dysregulation of blood flow
In addition to responding to PP and structural changes in ocular blood vessels, OBF is markedly influenced by local regulation.51 Many determining factors for regulation are involved, meaning that different types of dysregulation can occur. We distinguish secondary from primary types of dysregulation.52
Secondary vascular dysregulation
Pathological processes such as inflammations often lead to changes in the circulating blood and this, in turn, can have an effect in remote organs. One frequently encountered alteration is an increase in ET-1 level in circulating blood, and one of the remote tissues most often involved is the ONH. The reason for this is the fact that the blood–brain barrier in the ONH is partly abrogated by the proximity to the fenestrated vessels of the choroid (Figure 8). Increased ET-1 level in the circulating blood is found in patients with multiple sclerosis (MS)53 and transiently during optic neuritis,54 in rheumatoid arthritis55 and fibromyalgia.56 While increased ET-1 levels in the blood have little impact on brain or retinal BF, as long as the barrier is intact, it has a major influence on BF of the choroid and the ONH.57 The ONH, in such cases, sometimes appears slightly pale. In the case of giant cell arteritis, ET-1 is particularly increased in the subgroup of patients in which the eye is involved.58 In addition, in such cases, the ET-receptors are also up-regulated.59 The involvement of the ET system in giant cell arteritis explains why affected patients often indicate symptoms similar to amaurosis fugax, in addition to reporting a reduced feeling of thirst, both preceding the sudden blindness.
Figure 8.
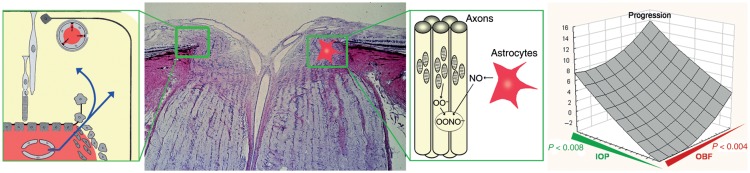
In the optic nerve head (ONH) (second from left), the blood–brain barrier is partly abrogated by the proximity to the fenestrated vessels of the choroid (left). Unstable oxygen supply in glaucoma patients increases superoxide anion (O2−) in the mitochondria of the axons. If neighbouring astrocytes are activated, nitric oxide (NO) diffuses into the axons resulting in the damaging peroxynitrite (ONOO−) (second from right). Indeed, visual field progression in glaucoma patients (right) increases not only with increasing intraocular pressure (green) but also with decreasing ocular blood flow (red). (From Flammer and Mozaffarieh,114 with permission.)
Primary vascular dysregulation
Even more important than the secondary vascular dysregulation is the so-called PVD syndrome.7,60 PVD is a predisposition to react differently to a number of stimuli like coldness61,62 or physical or emotional stress. The most prominent sign is the dysregulation of vessels, which gave the syndrome its name.63 However, PVD encompasses a number of additional signs and symptoms. In terms of blood vessels, vasospasms are the best known. This explains why, in the past, the term vasospastic syndrome64 was often used. We prefer the term PVD, as the syndrome can also include inappropriate vasodilation or barrier dysfunction, among other symptoms.
Primary vascular dysregulation occurs more often in females than in males,65 in thin more than in obese subjects,65–67 in academics more than in blue-collar workers,68 and in Asians more than in Caucasians. Patients tend to be more active both physically and mentally. The main signs are arterial hypotension (particularly when they are young)69 and cold extremities with an increased response to coldness.61 In addition, patients often indicate altered drug sensitivity (partly due to altered expression of ABC-proteins),70 decreased sensations of thirst71 [ET-1 increases the prostaglandin (PG) E2 level in the centre of thirst], and prolonged sleep onset time72 (as we all can only fall asleep after warming up our feet). In terms of ocular perfusion, PVD subjects often have reduced autoregulation,73 increased spatial irregularities of retinal vessels, stiffer vessels (i.e. fast pulse wave propagation), and reduced NVC74,75 (Figure 2).
Interestingly, in PVD subjects, OBF correlates with BF in the extremities,76,77 while such a correlation is absent in non-PVD subjects. Primary vascular dysregulation predisposes patients to certain eye diseases such as retinal arterial78 and vein occlusion79 or central serous chorioretinopathy.80 However, it is a clear risk factor for glaucoma, particularly NTG.81 Furthermore, subjects with PVD have an inverse response pattern regarding choroidal and ONH circulation with respect to blood gas perturbation.82
Primary vascular dysregulation has a particular impact on glaucoma.52 If glaucomatous damage occurs or progresses despite an IOP in the normal range, vascular factors are most often involved.83 Healthy subjects with PVD and glaucoma patients progressing despite a normal IOP have the following shared characteristics: reduced auto-regulation84,85 stiffer retinal vessels,86 reduced NVC,74,75 correlation between OBF and finger BF,87 increased level of ET-1,71 and altered gene expression in circulating lymphocytes.87 In addition, an increased level of DNA breaks,88 silent myocardial ischaemia,89 and nocturnal over-dipping90 occur particularly in glaucoma patients with PVD. Nocturnal hypotension might partly be due to decreased reuptake of sodium in the proximal renal tubuli91 due to stimulation of PGE2 by ET-1. Glaucoma patients have also demonstrated an abnormal ET-1 response to postural changes.92 Although PVD leads to vascular-induced damage in the eye, its impact on the heart, on the coronary microcirculation in particular, needs further study.
Oxidative stress as a consequence of unstable ocular blood flow
Oxidative stress plays a crucial role in many diseases. In case of glaucoma, the role of hypoxia in the pathogenesis of GON has long been debated.93 On the one hand, progression of GON is linked to reductions in OBF85 (Figure 8). On the other hand, hypoxia (as it occurs, for example, in the context of coronary artery disease or MS), while sometimes leading to mild atrophy of ONH, rarely leads to GON. The eye can adapt quite well to mild and stable hypoxia. In contrast, the eye can adapt less well to oxidative stress. Unstable oxygen supply increases oxidative stress, particularly in the mitochondria of the ONH. This, in turn, leads to GON if adjacent astrocytes are simultaneously activated and induced to overexpress NO synthase-2 (Figure 8).
Oxygen supply can be unstable if oxygen saturation fluctuates, as occurs, for example, in sleep apnoea. The more frequent cause is an unstable OBF. The OBF, in turn, is unstable if IOP fluctuates at a high enough level or PP is low enough to exceed the capacity of autoregulation, or if autoregulation itself is disturbed. This is mainly the case in subjects with PVD. The involvement of PVD explains why NTG occurs more often in females than in males,94 but is also more frequent in Asian countries than in Europe or North America.95
Blood–retina barrier
Like the brain, the retina can only properly function if the BRB is intact. The BRB is damaged by inflammation but also by hypoxia.96 Blood flow and barrier dysfunction are therefore linked. Molecules such as ET-1, which are involved in the regulation of the vessel size, also influence the barrier. Macular oedema is one potential manifestation of hypoxia97 (Figure 7).
Retinal haemorrhages
Haemorrhages occur if vessels are ruptured. These bleedings are normally large and can also break into the vitreous. Smaller haemorrhages, however, also occur if the BRB is opened at the level of both the endothelial cells (e.g. by VEGF or ET-1) and the basal membrane [by mettalloproteinase-9 (MMP-9)]98 (Figure 9). Indeed, the number of retinal haemorrhages in diabetes patients is correlated with the MMP-9 concentration in the vitreous.99,100
Figure 9.
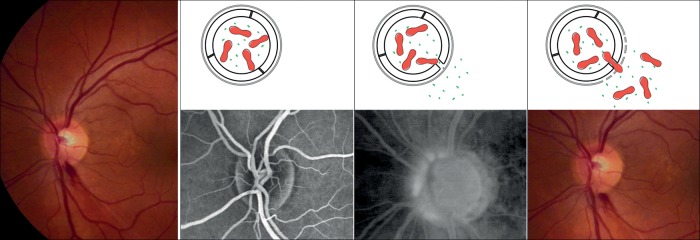
Pathogenesis of optic disc splinter haemorrhages: Under normal conditions, the vessels in and around the optic nerve head are watertight. If the barrier is opened at the level of the endothelial cells, small molecules such as water as well as fluorescein can leak out. If, at the same time, the basal membrane in the same area is also weakened, erythrocytes can also escape. (Modified after Grieshaber and Flammer,115 with permission.)
Splinter haemorrhages at the border of the ONH also occur in the context of glaucoma.101 In these patients, VEGF,102 ET-1,103 and MMP-9104 are indeed increased in the circulation blood, particularly in glaucoma patients with PVD, which explains the higher prevalence of such haemorrhages in NTG patients and in females. As mentioned before, these molecules can diffuse from the choroid into the neighbouring tissue (Figure 8). However, they can also be over-expressed by the local neural tissue in cases of local hypoxia, which explains why the frequency of haemorrhages, to some extent, is reduced after IOP reduction. If the BRB is opened at the level of the endothelial cells, this can allow the escape of water and small molecules such as fluorescein. If, at the same time, the basal membrane is also weakened by MMP-9, erythrocytes can also escape (Figure 9).
Retinal vein occlusion
Retinal vein occlusion (RVO) is often referred to as retinal venous thrombosis. However, increasing evidence now indicates that RVO might occur without thrombosis and that if thrombosis occurs, it might be secondary.105 The risk factors for RVO are similar to those for arterial occlusions, and anticoagulation treatments106 do not protect against RVO. Reduced OBF, glaucoma, PVD,107 and stress increase the risk of RVO and circulating ET-1 levels are increased in nearly all cases.79 In addition, OBF is also very often reduced and RVP increased in the contralateral clinically non-affected eye. Molecules from the circulating blood diffusing into the ONH, or produced locally either by the diseased arteries or by the hypoxic tissue, lead to a local venous constriction and thereby increase RVP.105 This leads to the so-called praestasis syndrome and eventually to a clinical picture of RVO (Figure 10). The weakened BRB further contributes to retinal oedema and haemorrhages. The positive clinical effect of anti-VEGF therapy in humans,108 as well as the positive effect of ET-1 blockers in experimental animals,109 supports this assumption.
Figure 10.
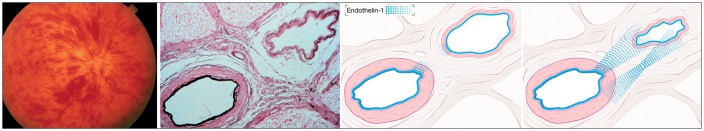
Pathogenesis of retinal vein occlusion: At the lamina cribrosa, the central artery and central vein are topographically very close and share a common adventitia (middle). This enables a molecular cross talk between the two vessels (right). Endothelin-1 (blue), for example, can diffuse from the ailing artery as well as from the adjacent hypoxic tissue to the very sensitive vein, leading to venous constriction. [Modified after Fraenkl SA, Mozaffarieh M, Flammer J. (2010), Figures 1a, 2, 4). With kind permission from Springer Science + Business Media B.V.]
Therapeutic aspects
Treatments of diabetes (e.g. with insulin) or vasculitis (with steroids) and the elimination of risk factors for arteriosclerosis (such as reduction of BP in systemic hypertension) are the gold standards. Other treatment modalities, however, are also on the horizon. A very low BP in a patient with progressing GON should be raised with an increased salt intake91 or, in extreme cases, with a very low dose of fludrocortisone,110 although well-controlled intervention studies are not yet available. Magnesium111 and low doses of calcium antagonists112 improve vascular regulation of arteries and veins in the eye, particularly in patients with PVD. Oxidative stress in the mitochondria can be reduced, for example, by ginkgo biloba.113
Conclusion
Ocular blood flow has many aspects in common with the systemic circulation, but also has some peculiarities. This includes the BRB, autoregulation, NVC, the influence of circulating molecules on BF of the ONH, and the lack of autonomic innervation of retinal vessels. In addition to structural vascular abnormalities, the dysregulation of arteries and veins is also important. Intraretinal haemorrhages are often a consequence of disturbed BRB. Venous dysregulation increases RVP and can lead to RVO. While hypoxia plays a major pathophysiological role in diabetic retinopathy and in wet AMD, an unstable oxygen supply contributes to GON by increasing the oxidative stress. While systemic hypertension increases the risk of infarctions or diabetic retinopathy, systemic hypotension and increased fluctuations in BP are risk factors for GON. Retinal vascular changes also predict, to some extent, cardiovascular events.
Conflict of interest: none declared.
References
- 1.Mozaffarieh M, Flammer J. Ocular Blood Flow and Glaucomatous Optic Neuropathy. 1st ed. Berlin/Heidelberg: Springer; 2009. [Google Scholar]
- 2.Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43:317–321. doi: 10.3129/i08-056. [DOI] [PubMed] [Google Scholar]
- 3.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotliar KE, Mucke B, Vilser W, Schilling R, Lanzl IM. Effect of aging on retinal artery blood column diameter measured along the vessel axis. Invest Ophthalmol Vis Sci. 2008;49:2094–2102. doi: 10.1167/iovs.07-0711. [DOI] [PubMed] [Google Scholar]
- 5.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallar J, Acosta MC, Belmonte C. Activation of scleral cold thermoreceptors by temperature and blood flow changes. Invest Ophthalmol Vis Sci. 2003;44:697–705. doi: 10.1167/iovs.02-0226. [DOI] [PubMed] [Google Scholar]
- 7.Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 8.Gugleta K, Orgul S, Flammer J. Is corneal temperature correlated with blood-flow velocity in the ophthalmic artery? Curr Eye Res. 1999;19:496–501. doi: 10.1076/ceyr.19.6.496.5286. [DOI] [PubMed] [Google Scholar]
- 9.Buchi ER, Schiller P, Felice M, Bunkenburg A, Daicker B. Common histopathological changes in aged human orbital arteries. Int Ophthalmol. 1993;17:37–42. doi: 10.1007/BF00918866. [DOI] [PubMed] [Google Scholar]
- 10.Liew G, Wang JJ. [Retinal vascular signs: a window to the heart?] Rev Esp Cardiol. 2011;64:515–521. doi: 10.1016/j.recesp.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008;1:156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- 12.Cuspidi C, Macca G, Salerno M, Michev L, Fusi V, Severgnini B, Corti C, Meani S, Magrini F, Zanchetti A. Evaluation of target organ damage in arterial hypertension: which role for qualitative funduscopic examination? Ital Heart J. 2001;2:702–706. [PubMed] [Google Scholar]
- 13.Dimmitt SB, West JN, Eames SM, Gibson JM, Gosling P, Littler WA. Usefulness of ophthalmoscopy in mild to moderate hypertension. Lancet. 1989;1:1103–1106. doi: 10.1016/s0140-6736(89)92384-2. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs FD, Maestri MK, Bredemeier M, Cardozo SE, Moreira FC, Wainstein MV, Moreira WD, Moreira LB. Study of the usefulness of optic fundi examination of patients with hypertension in a clinical setting. J Hum Hypertens. 1995;9:547–551. [PubMed] [Google Scholar]
- 15.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. J Am Med Assoc. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 16.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 17.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, de Jong PT, Breteler MM. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66:1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 18.Smith W, Wang JJ, Wong TY, Rochtchina E, Klein R, Leeder SR, Mitchell P. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension. 2004;44:442–447. doi: 10.1161/01.HYP.0000140772.40322.ec. [DOI] [PubMed] [Google Scholar]
- 19.Chew SK, Xie J, Wang JJ. Retinal arteriolar diameter and the prevalence and incidence of hypertension: a systematic review and meta-analysis of their association. Curr Hypertens Rep. 2012;14:144–151. doi: 10.1007/s11906-012-0252-0. [DOI] [PubMed] [Google Scholar]
- 20.Rizzoni D, Agabiti-Rosei E. Structural abnormalities of small resistance arteries in essential hypertension. Intern Emerg Med. 2012;7:205–212. doi: 10.1007/s11739-011-0548-0. [DOI] [PubMed] [Google Scholar]
- 21.Rizzoni D, Palombo C, Porteri E, Muiesan ML, Kozakova M, La Canna G, Nardi M, Guelfi D, Salvetti M, Morizzo C, Vittone F, Rosei EA. Relationships between coronary flow vasodilator capacity and small artery remodelling in hypertensive patients. J Hypertens. 2003;21:625–631. doi: 10.1097/00004872-200303000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Muiesan ML, Rizzoni D, Salvetti M, Porteri E, Monteduro C, Guelfi D, Castellano M, Garavelli G, Agabiti-Rosei E. Structural changes in small resistance arteries and left ventricular geometry in patients with primary and secondary hypertension. J Hypertens. 2002;20:1439–1444. doi: 10.1097/00004872-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 23.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 24.Mathiassen ON, Buus NH, Sihm I, Thybo NK, Morn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany MJ, Christensen KL. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens. 2007;25:1021–1026. doi: 10.1097/HJH.0b013e32805bf8ed. [DOI] [PubMed] [Google Scholar]
- 25.Harazny JM, Ritt M, Baleanu D, Ott C, Heckmann J, Schlaich MP, Michelson G, Schmieder RE. Increased wall: lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension. 2007;50:623–629. doi: 10.1161/HYPERTENSIONAHA.107.090779. [DOI] [PubMed] [Google Scholar]
- 26.Ritt M, Harazny JM, Ott C, Schlaich MP, Schneider MP, Michelson G, Schmieder RE. Analysis of retinal arteriolar structure in never-treated patients with essential hypertension. J Hypertens. 2008;26:1427–1434. doi: 10.1097/HJH.0b013e3282ffdc66. [DOI] [PubMed] [Google Scholar]
- 27.Rizzoni D, Porteri E, Duse S, De Ciuceis C, Rosei CA, La Boria E, Semeraro F, Costagliola C, Sebastiani A, Danzi P, Tiberio GA, Giulini SM, Docchio F, Sansoni G, Sarkar A, Rosei EA. Relationship between media-to-lumen ratio of subcutaneous small arteries and wall-to-lumen ratio of retinal arterioles evaluated noninvasively by scanning laser Doppler flowmetry. J Hypertens. 2012;30:1169–1175. doi: 10.1097/HJH.0b013e328352f81d. [DOI] [PubMed] [Google Scholar]
- 28.Michelson EL, Morganroth J, Nichols CW, MacVaugh H., III Retinal arteriolar changes as an indicator of coronary artery disease. Arch Intern Med. 1979;139:1139–1141. [PubMed] [Google Scholar]
- 29.Tedeschi-Reiner E, Strozzi M, Skoric B, Reiner Z. Relation of atherosclerotic changes in retinal arteries to the extent of coronary artery disease. Am J Cardiol. 2005;96:1107–1109. doi: 10.1016/j.amjcard.2005.05.070. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension. 2008;51:119–126. doi: 10.1161/HYPERTENSIONAHA.107.098343. [DOI] [PubMed] [Google Scholar]
- 31.Wong TY, Cheung N, Islam FM, Klein R, Criqui MH, Cotch MF, Carr JJ, Klein BE, Sharrett AR. Relation of retinopathy to coronary artery calcification: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2008;167:51–58. doi: 10.1093/aje/kwm256. [DOI] [PubMed] [Google Scholar]
- 32.Liew G, Mitchell P, Rochtchina E, Wong TY, Hsu W, Lee ML, Wainwright A, Wang JJ. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur Heart J. 2011;32:422–429. doi: 10.1093/eurheartj/ehq431. [DOI] [PubMed] [Google Scholar]
- 33.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, Cushman M, Duncan BB. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166:2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 34.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 35.Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 36.Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003;285:H631–H636. doi: 10.1152/ajpheart.00111.2003. [DOI] [PubMed] [Google Scholar]
- 37.Kotliar KE, Lanzl IM, Schmidt-Trucksass A, Sitnikova D, Ali M, Blume K, Halle M, Hanssen H. Dynamic retinal vessel response to flicker in obesity: a methodological approach. Microvasc Res. 2011;81:123–128. doi: 10.1016/j.mvr.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Reimann M, Prieur S, Lippold B, Bornstein SR, Reichmann H, Julius U, Ziemssen T. Retinal vessel analysis in hypercholesterolemic patients before and after LDL apheresis. Atheroscler Suppl. 2009;10:39–43. doi: 10.1016/S1567-5688(09)71808-2. [DOI] [PubMed] [Google Scholar]
- 39.Mandecka A, Dawczynski J, Blum M, Muller N, Kloos C, Wolf G, Vilser W, Hoyer H, Muller UA. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007;30:3048–3052. doi: 10.2337/dc07-0927. [DOI] [PubMed] [Google Scholar]
- 40.Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004;35:1289–1293. doi: 10.1161/01.STR.0000126597.11534.3b. [DOI] [PubMed] [Google Scholar]
- 41.Ott C, Schlaich MP, Harazny J, Schmidt BM, Michelson G, Schmieder RE. Effects of angiotensin II type 1-receptor blockade on retinal endothelial function. J Hypertens. 2008;26:516–522. doi: 10.1097/HJH.0b013e3282f3adb0. [DOI] [PubMed] [Google Scholar]
- 42.Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;35(1 Pt 2):501–506. doi: 10.1161/01.hyp.35.1.501. [DOI] [PubMed] [Google Scholar]
- 43.Imai K, Hamaguchi M, Mori K, Takeda N, Fukui M, Kato T, Kawahito Y, Kinoshita S, Kojima T. Metabolic syndrome as a risk factor for high-ocular tension. Int J Obes (Lond) 2010;34:1209–1217. doi: 10.1038/ijo.2010.32. [DOI] [PubMed] [Google Scholar]
- 44.Flammer J, Orgul S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17:267–289. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 45.Kaiser HJ, Flammer J. Systemic hypotension: a risk factor for glaucomatous damage? Ophthalmologica. 1991;203:105–108. doi: 10.1159/000310234. [DOI] [PubMed] [Google Scholar]
- 46.Okumura Y, Yuki K, Tsubota K. Low diastolic blood pressure is associated with the progression of normal-tension glaucoma. Ophthalmologica. 2012;228:36–41. doi: 10.1159/000335978. [DOI] [PubMed] [Google Scholar]
- 47.Gass A, Flammer J, Linder L, Romerio SC, Gasser P, Haefeli WE. Inverse correlation between endothelin-1-induced peripheral microvascular vasoconstriction and blood pressure in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 1997;235:634–638. doi: 10.1007/BF00946939. [DOI] [PubMed] [Google Scholar]
- 48.Sung KR, Lee S, Park SB, Choi J, Kim ST, Yun SC, Kang SY, Cho JW, Kook MS. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci. 2009;50:5266–5274. doi: 10.1167/iovs.09-3716. [DOI] [PubMed] [Google Scholar]
- 49.Stodtmeister R. [The pulsation and the pressure of the central retinal vein and their relation to glaucoma damage and therapy] Klin Monbl Augenheilkd. 2008;225:632–636. doi: 10.1055/s-2008-1027233. [DOI] [PubMed] [Google Scholar]
- 50.Jonas JB. Central retinal artery and vein collapse pressure in eyes with chronic open angle glaucoma. Br J Ophthalmol. 2003;87:949–951. doi: 10.1136/bjo.87.8.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- 53.Haefliger IO, Flammer J. Le syndrome vasospastique, un facteur de risque de la neuropathie glaucomateuse. In: Béchetoille A, editor. Les Glaucomes. France: Japperrenard; 1997. pp. 273–275. [Google Scholar]
- 54.Haufschild T, Shaw SG, Kaiser HJ, Flammer J. Transient raise of endothelin-1 plasma level and reduction of ocular blood flow in a patient with optic neuritis. Ophthalmologica. 2003;217:451–453. doi: 10.1159/000073079. [DOI] [PubMed] [Google Scholar]
- 55.Pache M, Schwarz HA, Kaiser HJ, Wuest P, Kloti M, Dubler B, Flammer J. Elevated plasma endothelin-1 levels and vascular dysregulation in patients with rheumatoid arthritis. Med Sci Monit. 2002;8:CR616–9. [PubMed] [Google Scholar]
- 56.Pache M, Ochs J, Genth E, Mierau R, Kube T, Flammer J. Increased plasma endothelin-1 levels in fibromyalgia syndrome. Rheumatology (Oxford) 2003;42:493–494. doi: 10.1093/rheumatology/keg131. [DOI] [PubMed] [Google Scholar]
- 57.Pache M, Kaiser HJ, Akhalbedashvili N, Lienert C, Dubler B, Kappos L, Flammer J. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur Neurol. 2003;49:164–168. doi: 10.1159/000069085. [DOI] [PubMed] [Google Scholar]
- 58.Pache M, Kaiser HJ, Haufschild T, Lubeck P, Flammer J. Increased endothelin-1 plasma levels in giant cell arteritis: a report on four patients. Am J Ophthalmol. 2002;133:160–162. doi: 10.1016/s0002-9394(01)01202-8. [DOI] [PubMed] [Google Scholar]
- 59.Dimitrijevic I, Andersson C, Rissler P, Edvinsson L. Increased tissue endothelin-1 and endothelin-B receptor expression in temporal arteries from patients with giant cell arteritis. Ophthalmology. 2010;117:628–636. doi: 10.1016/j.ophtha.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 60.Emre M, Orgul S, Gugleta K, Flammer J. Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol. 2004;88:662–666. doi: 10.1136/bjo.2003.032110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saner H, Wurbel H, Mahler F, Flammer J, Gasser P. Microvasculatory evaluation of vasospastic syndromes. Adv Exp Med Biol. 1987;220:215–218. doi: 10.1007/978-1-4613-1927-6_38. [DOI] [PubMed] [Google Scholar]
- 62.Guthauser U, Flammer J, Mahler F. The relationship between digital and ocular vasospasm. Graefes Arch Clin Exp Ophthalmol. 1988;226:224–226. doi: 10.1007/BF02181185. [DOI] [PubMed] [Google Scholar]
- 63.Flammer J. The Concept of Vascular Dysregulation in Glaucoma. In: Haefliger IO, Flammer J, editors. Nitric Oxide and Endothelin in the Pathogenesis of Glaucoma. Philadelphia: Lippincott-Raven; 1998. pp. 14–21. [Google Scholar]
- 64.Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res. 2001;20:319–349. doi: 10.1016/s1350-9462(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 65.Mozaffarieh M, Fontana Gasio P, Schotzau A, Orgul S, Flammer J, Krauchi K. Thermal discomfort with cold extremities in relation to age, gender, and body mass index in a random sample of a Swiss urban population. Popul Health Metr. 2010;8:17. doi: 10.1186/1478-7954-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kavroulaki D, Gugleta K, Kochkorov A, Katamay R, Flammer J, Orgul S. Relation of body mass index and blood pressure to subjective and objective acral temperature. Klin Monbl Augenheilkd. 2009;226:328–331. doi: 10.1055/s-0028-1109290. [DOI] [PubMed] [Google Scholar]
- 67.Gasser P, Stumpfig D, Schotzau A, Ackermann-Liebrich U, Flammer J. Body mass index in glaucoma. J Glaucoma. 1999;8:8–11. [PubMed] [Google Scholar]
- 68.Flammer J. Glaucoma. 3rd ed. Seattle/Toronto/Bern/Göttingen: Hogrefe&Huber; 2006. [Google Scholar]
- 69.Gherghel D, Orgul S, Gugleta K, Flammer J. Retrobulbar blood flow in glaucoma patients with nocturnal over-dipping in systemic blood pressure. Am J Ophthalmol. 2001;132:641–647. doi: 10.1016/s0002-9394(01)01193-x. [DOI] [PubMed] [Google Scholar]
- 70.Wunderlich K, Zimmerman C, Gutmann H, Teuchner B, Flammer J, Drewe J. Vasospastic persons exhibit differential expression of ABC-transport proteins. Mol Vis. 2003;9:756–761. [PubMed] [Google Scholar]
- 71.Teuchner B, Orgul S, Ulmer H, Haufschild T, Flammer J. Reduced thirst in patients with a vasospastic syndrome. Acta Ophthalmol Scand. 2004;82:738–740. doi: 10.1111/j.1600-0420.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 72.Pache M, Krauchi K, Cajochen C, Wirz-Justice A, Dubler B, Flammer J, Kaiser HJ. Cold feet and prolonged sleep-onset latency in vasospastic syndrome. Lancet. 2001;358:125–126. doi: 10.1016/S0140-6736(01)05344-2. [DOI] [PubMed] [Google Scholar]
- 73.Hasler PW, Orgul S, Gugleta K, Vogten H, Zhao X, Gherghel D, Flammer J. Vascular dysregulation in the choroid of subjects with acral vasospasm. Arch Ophthalmol. 2002;120:302–307. doi: 10.1001/archopht.120.3.302. [DOI] [PubMed] [Google Scholar]
- 74.Gugleta K, Zawinka C, Rickenbacher I, Kochkorov A, Katamay R, Flammer J, Orgul S. Analysis of retinal vasodilation after flicker light stimulation in relation to vasospastic propensity. Invest Ophthalmol Vis Sci. 2006;47:4034–4041. doi: 10.1167/iovs.06-0351. [DOI] [PubMed] [Google Scholar]
- 75.Gugleta K, Kochkorov A, Waldmann N, Polunina A, Katamay R, Flammer J, Orgul S. Dynamics of retinal vessel response to flicker light in glaucoma patients and ocular hypertensives. Graefes Arch Clin Exp Ophthalmol. 2012;250:589–594. doi: 10.1007/s00417-011-1842-2. [DOI] [PubMed] [Google Scholar]
- 76.Girardin F, Orgul S, Erb C, Flammer J. Relationship between corneal temperature and finger temperature. Arch Ophthalmol. 1999;117:166–169. doi: 10.1001/archopht.117.2.166. [DOI] [PubMed] [Google Scholar]
- 77.Mozaffarieh M, Osusky R, Schotzau A, Flammer J. Relationship between optic nerve head and finger blood flow. Eur J Ophthalmol. 2010;20:136–141. doi: 10.1177/112067211002000119. [DOI] [PubMed] [Google Scholar]
- 78.Kaiser HJ, Flammer J, Messerli J. Vasospasm - a risk factor for nonarteric anterior ischemic optic neuropathy? Neuro-ophthalmol. 1996;16:6. [Google Scholar]
- 79.Haufschild T, Prunte C, Messerli J, Flammer J. Increased endothelin-1 plasma level in young adults with retinal vascular occlusive diseases. Klin Monbl Augenheilkd. 2004;221:357–359. doi: 10.1055/s-2004-812813. [DOI] [PubMed] [Google Scholar]
- 80.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 81.Gasser P, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am J Ophthalmol. 1991;111:585–588. doi: 10.1016/s0002-9394(14)73703-1. [DOI] [PubMed] [Google Scholar]
- 82.Gugleta K, Orgul S, Hasler P, Flammer J. Circulatory response to blood gas perturbations in vasospasm. Invest Ophthalmol Vis Sci. 2005;46:3288–3294. doi: 10.1167/iovs.05-0158. [DOI] [PubMed] [Google Scholar]
- 83.Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38(Suppl):S3–S6. doi: 10.1016/0039-6257(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 84.Gherghel D, Orgul S, Dubler B, Lubeck P, Gugleta K, Flammer J. Is vascular regulation in the central retinal artery altered in persons with vasospasm? Arch Ophthalmol. 1999;117:1359–1362. doi: 10.1001/archopht.117.10.1359. [DOI] [PubMed] [Google Scholar]
- 85.Gherghel D, Orgul S, Gugleta K, Gekkieva M, Flammer J. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am J Ophthalmol. 2000;130:597–605. doi: 10.1016/s0002-9394(00)00766-2. [DOI] [PubMed] [Google Scholar]
- 86.Oettli A, Gugleta K, Kochkorov A, Katamay R, Flammer J, Orgul S. Rigidity of retinal vessel in untreated eyes of normal tension primary open-angle glaucoma patients. J Glaucoma. 2011;20:303–306. doi: 10.1097/IJG.0b013e3181e666a1. [DOI] [PubMed] [Google Scholar]
- 87.Yeghiazaryan K, Flammer J, Orgul S, Wunderlich K, Golubnitschaja O. Vasospastic individuals demonstrate significant similarity to glaucoma patients as revealed by gene expression profiling in circulating leukocytes. Mol Vis. 2009;15:2339–2348. [PMC free article] [PubMed] [Google Scholar]
- 88.Mozaffarieh M, Schoetzau A, Sauter M, Grieshaber M, Orgul S, Golubnitschaja O, Flammer J. Comet assay analysis of single-stranded DNA breaks in circulating leukocytes of glaucoma patients. Mol Vis. 2008;14:1584–1588. [PMC free article] [PubMed] [Google Scholar]
- 89.Waldmann E, Gasser P, Dubler B, Huber C, Flammer J. Silent myocardial ischemia in glaucoma and cataract patients. Graefes Arch Clin Exp Ophthalmol. 1996;234:595–598. doi: 10.1007/BF00185290. [DOI] [PubMed] [Google Scholar]
- 90.Collignon N, Dewe W, Guillaume S, Collignon-Brach J. Ambulatory blood pressure monitoring in glaucoma patients. The nocturnal systolic dip and its relationship with disease progression. Int Ophthalmol. 1998;22:19–25. doi: 10.1023/a:1006113109864. [DOI] [PubMed] [Google Scholar]
- 91.Pechere-Bertschi A, Sunaric-Megevand G, Haefliger I, Panarello F, Maillard M, Burnier M. Renal sodium handling in patients with normal pressure glaucoma. Clin Sci (Lond) 2007;112:337–344. doi: 10.1042/CS20060082. [DOI] [PubMed] [Google Scholar]
- 92.Kaiser HJ, Flammer J, Wenk M, Luscher T. Endothelin-1 plasma levels in normal-tension glaucoma: abnormal response to postural changes. Graefes Arch Clin Exp Ophthalmol. 1995;233:484–488. doi: 10.1007/BF00183429. [DOI] [PubMed] [Google Scholar]
- 93.Kaiser HJ, Schoetzau A, Stumpfig D, Flammer J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am J Ophthalmol. 1997;123:320–327. doi: 10.1016/s0002-9394(14)70127-8. [DOI] [PubMed] [Google Scholar]
- 94.Orgül S, Flammer J, Gasser P. Female preponderance in normal-tension glaucoma. Ann Ophthalmol. 1995;27:5. [Google Scholar]
- 95.Pekmezci M, Vo B, Lim AK, Hirabayashi DR, Tanaka GH, Weinreb RN, Lin SC. The characteristics of glaucoma in Japanese Americans. Arch Ophthalmol. 2009;127:167–171. doi: 10.1001/archophthalmol.2008.593. [DOI] [PubMed] [Google Scholar]
- 96.Kaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol. 2008;2:879–889. doi: 10.2147/opth.s3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol. 2008;2:919–930. doi: 10.2147/opth.s4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- 99.Jin M, Kashiwagi K, Iizuka Y, Tanaka Y, Imai M, Tsukahara S. Matrix metalloproteinases in human diabetic and nondiabetic vitreous. Retina. 2001;21:28–33. doi: 10.1097/00006982-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 100.Descamps FJ, Martens E, Kangave D, Struyf S, Geboes K, Van Damme J, Opdenakker G, Abu El-Asrar AM. The activated form of gelatinase B/matrix metalloproteinase-9 is associated with diabetic vitreous hemorrhage. Exp Eye Res. 2006;83:401–407. doi: 10.1016/j.exer.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 101.Drance SM, Fairclough M, Butler DM, Kottler MS. The importance of disc hemorrhage in the prognosis of chronic open angle glaucoma. Arch Ophthalmol. 1977;95:226–228. doi: 10.1001/archopht.1977.04450020028004. [DOI] [PubMed] [Google Scholar]
- 102.Lip PL, Felmeden DC, Blann AD, Matheou N, Thakur S, Cunliffe IA, Lip GY. Plasma vascular endothelial growth factor, soluble VEGF receptor FLT-1, and von Willebrand factor in glaucoma. Br J Ophthalmol. 2002;86:1299–1302. doi: 10.1136/bjo.86.11.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Emre M, Orgul S, Haufschild T, Shaw SG, Flammer J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol. 2005;89:60–63. doi: 10.1136/bjo.2004.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Golubnitschaja-Labudova O, Liu R, Decker C, Zhu P, Haefliger IO, Flammer J. Altered gene expression in lymphocytes of patients with normal-tension glaucoma. Curr Eye Res. 2000;21:867–876. doi: 10.1076/ceyr.21.5.867.5534. [DOI] [PubMed] [Google Scholar]
- 105.Fraenkl SA, Mozaffarieh M, Flammer J. Retinal vein occlusions: the potential impact of a dysregulation of the retinal veins. EPMA J. 2010;1:253–261. doi: 10.1007/s13167-010-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Browning DJ, Fraser CM. Retinal vein occlusions in patients taking warfarin. Ophthalmology. 2004;111:1196–1200. doi: 10.1016/j.ophtha.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 107.Messerli J, Flammer J. [Central vein thrombosis in younger patients] Klin Monbl Augenheilkd. 1996;208:303–305. doi: 10.1055/s-2008-1035222. [DOI] [PubMed] [Google Scholar]
- 108.Yunoki T, Miyakoshi A, Nakamura T, Fujita K, Fuchizawa C, Hayashi A. Treatment of macular edema due to branch retinal vein occlusion with single or multiple intravitreal injections of bevacizumab. Jpn J Ophthalmol. 2012;56:159–164. doi: 10.1007/s10384-011-0114-3. [DOI] [PubMed] [Google Scholar]
- 109.Stangos AN, Petropoulos IK, Pournaras JA, Mendrinos E, Pournaras CJ. The vasodilatory effect of juxta-arteriolar microinjection of endothelinA receptor inhibitor in healthy and acute branch retinal vein occlusion minipig retinas. Invest Ophthalmol Vis Sci. 2010;51:2185–2190. doi: 10.1167/iovs.09-3735. [DOI] [PubMed] [Google Scholar]
- 110.Gugleta K, Orgul S, Stumpfig D, Dubler B, Flammer J. Fludrocortisone in the treatment of systemic hypotension in primary open-angle glaucoma patients. Int Ophthalmol. 1999;23:25–30. doi: 10.1023/a:1006434231844. [DOI] [PubMed] [Google Scholar]
- 111.Gaspar AZ, Gasser P, Flammer J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Ophthalmologica. 1995;209:11–13. doi: 10.1159/000310566. [DOI] [PubMed] [Google Scholar]
- 112.Mozaffarieh M, Konieczka K, Flammer J. Calcium channel blockers: their use in normal tension glaucoma. Expert Rev Ophthalmol. 2010;5:9. [Google Scholar]
- 113.Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis. 2012;18:390–402. [PMC free article] [PubMed] [Google Scholar]
- 114.Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007;52(Suppl 2):S162–S173. doi: 10.1016/j.survophthal.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 115.Grieshaber MC, Flammer J. Does the blood-brain barrier play a role in Glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S115–S121. doi: 10.1016/j.survophthal.2007.08.005. [DOI] [PubMed] [Google Scholar]


