Abstract
Agroecology and industrial ecology can be viewed as complementary means for reducing the environmental footprint of animal farming systems: agroecology mainly by stimulating natural processes to reduce inputs, and industrial ecology by closing system loops, thereby reducing demand for raw materials, lowering pollution and saving on waste treatment. Surprisingly, animal farming systems have so far been ignored in most agroecological thinking. On the basis of a study by Altieri, who identified the key ecological processes to be optimized, we propose five principles for the design of sustainable animal production systems: (i) adopting management practices aiming to improve animal health, (ii) decreasing the inputs needed for production, (iii) decreasing pollution by optimizing the metabolic functioning of farming systems, (iv) enhancing diversity within animal production systems to strengthen their resilience and (v) preserving biological diversity in agroecosystems by adapting management practices. We then discuss how these different principles combine to generate environmental, social and economic performance in six animal production systems (ruminants, pigs, rabbits and aquaculture) covering a long gradient of intensification. The two principles concerning economy of inputs and reduction of pollution emerged in nearly all the case studies, a finding that can be explained by the economic and regulatory constraints affecting animal production. Integrated management of animal health was seldom mobilized, as alternatives to chemical drugs have only recently been investigated, and the results are not yet transferable to farming practices. A number of ecological functions and ecosystem services (recycling of nutrients, forage yield, pollination, resistance to weed invasion, etc.) are closely linked to biodiversity, and their persistence depends largely on maintaining biological diversity in agroecosystems. We conclude that the development of such ecology-based alternatives for animal production implies changes in the positions adopted by technicians and extension services, researchers and policymakers. Animal production systems should not only be considered holistically, but also in the diversity of their local and regional conditions. The ability of farmers to make their own decisions on the basis of the close monitoring of system performance is most important to ensure system sustainability.
Keywords: aquaculture, environmental footprint, farming systems, inputs, livestock
Implications
Although animal farming systems undeniably contribute to improving human condition by providing proteins for nutrition and income for rural population, they can also be regarded as a major cause of world's most pressing environmental problems. Animal production is and will increasingly be constrained by competition for natural resources, changing sociocultural values and by the need to operate in a carbon-constrained economy. Agroecology and industrial ecology can help to design sustainable faming systems adapted to this context. Promoting systemic analyses and elaborating on the diversity of local and regional conditions are key elements of these promising approaches, the implementation of which will require changing the position and methods adopted by farmers, extension services, researchers and policymakers.
Introduction
Recent decades have seen interesting turns to alternative farming systems in response to growing ecological concern over industrial agriculture. These numerous alternatives for re-greening agriculture (Rockström and Karlberg, 2010; Koohafkan et al., 2012) span notions or concepts such as ecological or sustainable intensification of agriculture, high nature value farming, eco-agriculture, biological farming, green agriculture, organic farming and more. They promote ways to reconcile natural resource management, food production and ecosystem services in the long term and under climate uncertainty. They can also be seen as responses to both farmer and consumer dissatisfaction with the negative impacts of industrial farming. In this ‘re-greening landscape’, the future for sustainable animal production is a subject of debate. Steinfeld and Wassenaar (2007) argued that future expansion of the livestock sector will rely on intensive livestock systems, whereas Herrero et al. (2010) concluded that more extensive integrated crop–livestock systems could make a more significant contribution to food security. Godfray et al. (2010) contended that it would be possible to design livestock farming systems to reduce net greenhouse gas emissions, whereas Gill et al. (2010) pointed out that the evidence base connecting policy interventions and their consequences on livestock emissions still remains weak.
Despite this limited scientific consensus, it is clear that the overall functioning of ecosystems is closely intertwined with animal production for two main reasons. First, the livestock sector is currently a major driver of land use. From 1961 to 2001, more than 60% of the world maize and barley harvest went to livestock feed (Food and Agriculture Organization (FAO), 2006). Second, the type and amount of animal proteins in human dietary patterns are important drivers of agricultural expansion (Wirsenius et al., 2010). The place of animal farming systems in the re-greening process demands recognition of their positive and negative contributions to agroecosystem processes. It is also important to take into account their diversity, as they cover long gradients of intensification (ranging from grassland-based to large-scale intensive systems) and biogeographical conditions. Given this diversity, we believe that there is no single avenue for reintegrating animal production into ecological thinking. A dual perspective is needed, grounded in the principles of industrial ecology and agroecology as complementary frameworks for exploring how to switch the net effects of animal production from stress to benefits (Janzen, 2011).
Agroecology emerged in the United States during the 1980s as a scientific discipline that applies ecological theory to the design and management of sustainable agroecosystems (Altieri, 1987; Gliessman, 1997; Wezel and Soldat, 2009). Agroecological systems are expected to be productive, to need few chemical inputs and to be resource conserving. In the early 2000s, Francis et al. (2003) redefined agroecology away from this rather narrow field scale and toward a wider food system scale, as ‘the integrative study of the ecology of the entire food system, encompassing ecological, economic and social dimensions’. Despite the recent surge in academic literature on agroecology, animal production systems have been so far ignored in most agroecological thinking (Gliessman, 2006). Processes such as land-use change, greenhouse gas emissions, increased demands on water, pollution and biodiversity losses have all put livestock farming in a bad light (FAO, 2006). However, as underlined by Gliessman (2006), the problem lies not so much with the animals themselves but rather with how they are incorporated into agroecosystems and food systems. Their disconnectedness from the land is probably the main problem threatening the sustainability of animal farming systems. In the second edition of his book on agroecology, Gliessman (2006) devoted a chapter to the beneficial roles animals play in agroecosystems: producing protein-rich food for humans from inedible resources (e.g. crop residues, by-products, grasslands), providing ecosystem services (e.g. carbon sequestration, biodiversity), recycling plant nutrients and providing social benefits.
Industrial ecology also emerged in the United States during the 1980s (Frosch and Gallopoulos, 1989; Frosch, 1992). As a scientific discipline, industrial ecology was defined as the study of material and energy flows through industrial systems. It can also be seen as a new approach to environmental management where technology is used to mitigate the effect of these flows on the environment (Diemer and Labrune, 2007). In this framework, raw material and energy consumption is optimized and wastes are reused as inputs for another production process. Industrial ecology is being increasingly applied to livestock farming, in particular for manure management in large-scale intensive systems (Holm Nielsen, 2010). Industrial ecology and agroecology can thus offer a broad range of options that need to be pursued simultaneously. On the one hand, the application of industrial ecology to animal production can provide solutions to curb the growing competition for land, water and energy, while adding quantitatively to food production. It will also offer solutions for the reduction of waste and its negative impacts on the environment. On the other hand, agroecology targets a very substantial proportion of grassland-based livestock systems and plays an important role in biodiversity conservation (Veen et al., 2009). By considering biodiversity as both a resource and an output in livestock systems, agroecology puts food and ecosystem integrity at the same level of priority; it can thus provide alternative dual-benefit solutions through stimulating natural processes for input cost reduction and income gain.
The aim of this work is to explore potential routes for the development of ecology-based alternatives for animal production. The first section proposes five principles for the design of sustainable animal production systems. These principles are based on key ecological processes proposed by Altieri (2002) to be optimized for sustaining yields, while minimizing the negative environmental impact of animal production systems. They are illustrated with examples taken from a wide range of systems. The second section examines six case studies covering a long gradient of intensification, where we highlight how the different principles can combine to generate environmental, social and economic benefits. In the last section, we discuss how the founding principles of agroecology and industrial ecology can be mobilized in animal production systems, and conclude on perspectives for promoting such ecology-based systems.
Five principles for the development of ecology-based alternatives for animal production
Ecology-based management of animal production systems requires a deep understanding of the processes by which agroecosystems can produce food, fiber, etc. more sustainably, using fewer external inputs (Altieri, 2002). Altieri (2002) proposed a set of five ecological processes to be optimized: (i) strengthening of the ‘immune system’ of agricultural operations (nurturing proper functioning of natural pest control), (ii) decreasing toxicity in the environment through reduction or elimination of agrochemicals, (iii) optimizing metabolic functioning of soils (organic matter decomposition and nutrient cycling), (iv) balancing regulatory systems (nutrient cycles, water balance, energy flows, population regulation, etc.) and (v) enhancing conservation and regeneration of soil and water resources and biodiversity. To extend ecological thinking to animal production systems, we propose five principles that are based on the application of these ecological processes, and illustrate them in a wide range of systems.
Adopting management practices aiming to improve animal health
Applying agroecology to the question of animal health implies to focus on the causes of animal diseases in order to reduce their occurrence. The use of chemical drugs needs to be minimized as the dumping of medicine residues in the environment and the spread of resistance to antibiotics represent public health and environment issues. Major attention will therefore be given to choosing animals adapted to harsh environments and using a set of breeding practices that favor animal adaptations and strengthen their immune systems. Adaptation of animals to hot climates include small body size (and thus a high surface/volume ratio to evacuate body heat), thin skin with little subcutaneous fat, little or no hair or feathers and behavioral adaptations, such as night feeding (Mandonnet et al., 2011). Goats are well adapted to harsh environments, as they exploit a wide range of plant species, decrease their metabolic requirements and recycle urea in response to severe undernutrition and are able to concentrate urine under drought conditions (Silakinove, 2000). In cattle, Bos indicus genotypes faced with food scarcity reacted to long-term food fluctuations by mobilizing and restoring body fat reserves, and were less sensitive than B. indicus × Holstein cross-breds to metabolic disorders and diseases in the common food scarcity conditions of the tropics (Jenet et al., 2006). Local species or breeds that have been selected in tropical environments are more resistant to trypanosomes, gastrointestinal parasites and ticks (Mandonnet et al., 2011). Nematode resistance in sheep can be selected by a classical quantitative approach (Sechi et al., 2009), which suggests that selection programs could extend the benefits of using adapted genetic resources.
Adaptation to harsh environments also requires farmers to adopt management practices that make the best possible use of livestock adaptations. For example, it is possible to choose the frequency and seasonality of reproduction in relation to period and intensity of nutritional stress (Mandonnet et al., 2011). Management of flock health in organic sheep farming systems benefits from rotational grazing, as nematode larvae decline in temporarily ungrazed plots. Rotationally grazed or newly sown pastures should thus be dedicated to lambs, which are more vulnerable than dry ewes (Cabaret, 2007). As parasites usually differ between livestock species, alternate grazing with cattle reduces parasite burden in sheep (Mahieu and Aumont, 2009). Consumption of tannin-rich plants including sulla (Hedysarum coronarium), sainfoin and trefoil also reduces infestation by parasitic nematodes (Hoste et al., 2006). In the tropics, feeding lambs with wilted cassava foliage or banana foliage decreased nematode fecundity while providing suitable energy intake (Marie-Magdeleine et al., 2010). These plants have been shown to improve clinical status (diarrhea indices, etc.) and reduce mortality under parasitic challenge, while limiting the need for chemical drugs (Paolini et al., 2005; Kidane et al., 2010). In addition to a direct antiparasitic activity, they might also have some indirect effects by increasing host resistance. Observations that sick ruminants were able to consume substances that were not part of their normal diet and contained active ingredients capable of improving their health support the hypothesis that animals can self-medicate (Gradé et al., 2009). In pen studies, lambs experiencing negative internal states preferentially consumed foods containing a specific compound known to rectify their state of discomfort (Villalba et al., 2006). Parasitized lambs also slightly increased intake of a tannin-containing food when experiencing a parasite burden (Villalba et al., 2010). Self-selection of plant secondary metabolites could thus hold a place in the quest for alternatives to chemical drugs in pastoral systems.
Farming practices combining diet, housing and strain choice to simulate host defenses are crucial for pigs, poultry and rabbits, which are usually housed at high densities in intensive systems. Although maintaining animals in a clean, dry environment is essential to prevent the spread of microbes, close to sterile conditions may also hold back the development of their immune system. At birth, the digestive tract is sterile and progressively colonized by flora of the mother and of the environment, which exerts a barrier effect against exogenous pathogenic bacteria, and stimulates the immune system (Rhee et al., 2004). Stringent hygienic conditions altered the development of digestive microflora and stimulated inflammatory response genes in pigs (Mulder et al., 2009). Conversely, the adoption at 1 day of age of renewal rabbit females by reproductive females permitted the early implantation of a functional, diverse symbiote, which increased rabbit resistance to pathogens. Dietary fiber stimulated the activity of cecal microflora and provided an appropriate supplementation to ensure digestive comfort (Gidenne et al., 2010). In poultry, susceptibility to dietary stress was genetic strain dependent (Hangalapura et al., 2005), further emphasizing the importance of choosing genotypes adapted to particular production objectives. Breeding practices should also limit social stress. Mixing animals has been shown to suppress the immune response to a viral vaccine in pigs (De Groot et al., 2001) and to impair growth in finishing bulls (Mounier et al., 2006). Appropriate breeding practices that maintain stable dominant relationships are likely to increase social tolerance, and thereby limit both feed inputs and the use of chemical drugs in intensive systems.
In aquaculture, controlling water quality is pivotal for health management. In intensive systems, an alternative to antibiotics is the use of probiotics and prebiotics administered via the feed or directly in water to benefit fish through direct or indirect modulation of gut microbial balance, additional sources of nutrients and direct effects on water quality (Balcázar et al., 2006). It can improve fish health status, resistance to diseases, growth performance and body composition (Merrifield et al., 2010): feeding turbot larvae (Scophthalmus maximus) with rotifers enriched in lactic acid bacteria (Lactobacillus and Carnobacterium sp.) provided protection against a pathogenic Vibrio, and increased their mean weight and survival rate compared with control larvae (Gatesoupe, 1994).
Decreasing the inputs needed for production
A high proportion of arable land is devoted to soybeans and corn for animal feeds, which require chemical fertilizers and large quantities of water for irrigation. Thus, a major challenge is reducing the inputs required for production. This can be done by either increasing the efficiency of feed utilization by animals, or feeding them on cheap or natural resources that do not compete with human food supply. Strategies for input reduction can also depend on the natural preservation of supporting services in grasslands or ponds.
Improving the efficiency of nutrient utilization by the animals can help reducing the import of nutrients from outside the farm. Research initially focused on pigs and poultry, as these species compete directly with human food supply. Low digestibility of phosphorous (P) in pig feeds was partly alleviated by the diet supplementation with natural microbial phytase (Dourmad et al., 2009). Benefits of improving the efficiency of feed utilization can be extended by appropriate feeding practices: in laying hens, sequential feeding of wheat grain and protein–mineral concentrate improved feed conversion, and could facilitate the use of local feedstuffs introduced as whole grains, thus reducing feeding costs (Faruk et al., 2010). In organic egg production systems, stimulating the hens to exercise natural foraging behavior reduced the import of nutrients into the system: high-producing layers were able to forage on crops consisting of grass/clover, pea/vetch/oats, lupin and quinoa without negative effects on health or performance (egg weight and BW; Horsted and Hermansen, 2007). Geese that grazed unfertilized grass growing between tree rows in a walnut plantation increased walnut production by 26% and tree growth by 6% (Dubois et al., 2008). There was no microbial contamination (Escherichia coli) of the fruits if geese were removed at least 2 months before harvesting.
Feeding systems based on natural resources and agricultural by-products enable to spare resources for human food supply. Permanent pastures and rangelands are cheap and natural resources. Major limitations of rangeland-based feeding systems are the large areas required to compensate for low forage productivity, which increases farm work (fences or shepherding), and the seasonal and year-to-year variability in the amount and quality of forages (Jouven et al., 2010). Alternative feed resources such as millet, wheat, oat and barley straws are other cheap resources that serve as supplemental feed for ruminants, horses and donkeys in many agroecosystems around the world. In California, crops leave residues such as culled Brussels sprouts, waste tomatoes and carrot pulp after juice extraction, which are used to supplement grazing animals or forages (Gliessman, 2006). Various tropical forages make a viable alternative to soybean meal in diets for lambs (Archimède et al., 2010) or growing pigs (Rodríguez et al., 2006). Draught animal power for land preparation and transport reduces energy use in tropical farming systems. Because of competing demands on water for drinking, hygiene and energy, it is also urgent to improve water management in aquaculture and for crop forage irrigation. In aquaculture, a variety of technologies have been developed to offer solutions to limited water resources and degradation of water quality: these are recirculating aquaculture systems (RAS; Martins et al., 2010), and integrated intensive aquaculture installations that can take place in coastal waters, offshore environments or in ponds, and are adaptable for several combinations of fish, shrimp, shellfish, sea urchin and seaweeds (Neori et al., 2004; Ren et al., 2012). These systems would decrease some of the inputs needed for production (e.g. water, nutrients and land) but they are energy demanding. As pointed out by Martins et al. (2009), a small water exchange rate in RAS can also create problems resulting from the accumulation of growth-inhibiting factors coming from fishes (e.g. cortisol), bacteria (metabolites) and feed (metals). Maize has been widely cultivated to produce forages with a high-energy density, but requires large quantities of water during summer in temperate areas. Sorghum, with similar nutritional characteristics but greater resistance to water stress, could be used for animal feeding (Selle et al., 2010).
As an alternative to mineral fertilization, plant production can be stimulated by taking advantage of synergies between plant species. In grassland-based production systems, grass–legume mixtures can achieve high-forage yields as a result of symbiotic nitrogen (N) fixation in legume nodules. Altering the composition of forage mixtures did not affect dry matter intake, milk production or blood metabolite profiles of lactating Holstein cows (Soder et al., 2006). Functional diversity enhanced the resistance of temperate grasslands to weed invasion in both extensively and intensively managed swards (Frankow-Lindberg et al., 2009). Including forages in the crop rotation also led to higher yields in the grain crops planted after the forage, because of less soil disturbance, increased soil organic matter and weed control (Gliessman, 2006). In aquaculture systems, pond productivity can be increased by introducing submerged substrates in water to naturally stimulate fish productivity. This principle is based on traditional fishing methods used in Africa (Acadjas; Bene and Obirih-Opareh, 2009) and Asia (Samarahs and Katha fisheries; Shankar et al., 1998), where the periphyton – a complex assemblage of all sessile biota attached to the substratum, including associated detritus and micro-organisms – grows and can constitute a natural food for fishes. Submerged substrates also offer them shelter, while their associated microfauna helps to improve water quality through the trapping of suspended solids, organic matter breakdown and enhanced nitrification. The control of C : N ratio in-pond water through carbohydrate addition offers another alternative to enhance microbial development, protein recycling and biomass production. According to Bosma and Verdegem (2011), manipulating C : N ratio doubled protein input efficiency in ponds, while substrate addition enabled a twofold to threefold increase in production.
Decreasing pollution by optimizing the metabolic functioning of farming systems
N and P excretion and greenhouse gas emission per animal can be manipulated through diet (Martin et al., 2010 for mitigating CH4 emission in ruminants) or appropriate feeding practices (phase feeding for reducing N and P excretion in pigs; Dourmad et al., 2009), but further improvements can be expected using the agroecology concepts. The main synergy derived from mixing crops and animals results from animal manures becoming a resource instead of a nuisance, because they are rich in nutrients and provide soil micro-organisms with a key source of energy.
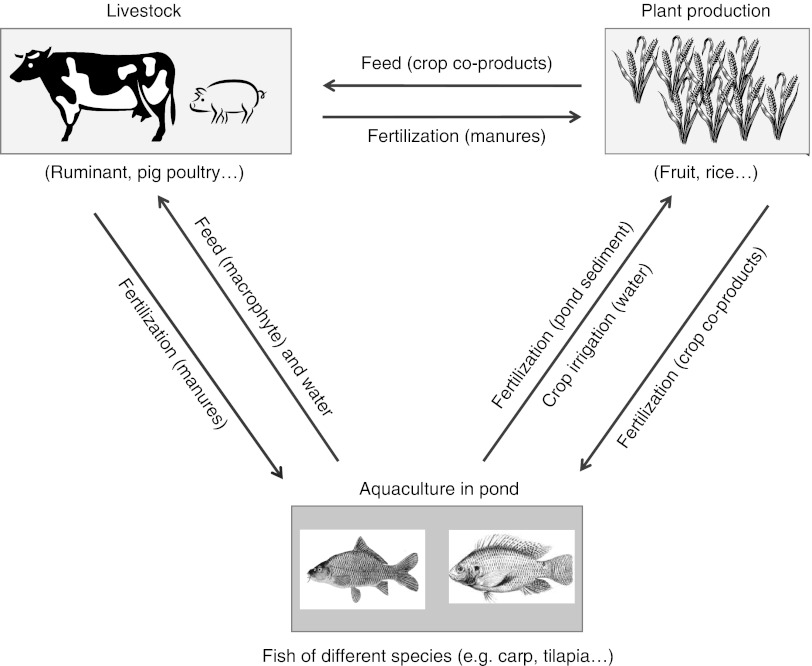
An integrated farm is one in which livestock are incorporated into farm operations specifically to capture positive synergies among farm units (i.e. to perform tasks and supply services to other farm units) and not just as a marketable commodity (Gliessman, 2006). Integration of cropping with livestock systems allows better regulation of biogeochemical cycles and environmental fluxes to the atmosphere and hydrosphere through spatial and temporal interactions among different farm units. In self-sufficient low-input dairy farms in Brittany, a part of the arable crops is used for homegrown feeds, and grass–legume mixtures are integrated in crop rotation with legumes allowing a reduction in fertilizer inputs (Alard et al., 2002). The longer the ley duration within a rotation, the greater is the potential for soil organic C sequestration and mitigation of N losses to the environment (Franzluebbers, 2007). In permanent pastures, grassland diversity may also reduce risks of nitrate leaching because of increased complementarity between species in N uptake and water uptake (De Deyn et al., 2009). Pig farming systems need to optimize organic N and P recycling and minimize nutrient leaching. It should be possible to reach close to 100% efficiency for P retention in the system. System efficiency is much lower for N (about 50%) because of gaseous emissions of NH3, N2 and N2O, but it can be improved in different ways by reducing gaseous emissions from the excreta, improving crop N fertilization, and including legume forages in crop rotations (Rigolot et al., 2010). In Cuba, horses grazing grass–legume mixtures in citrus inter-rows provided efficient weed control (saving fuel, herbicides and labor) in an integrated system where higher fruit quality, due to organic fertilization by horse manure, and diversification increased farm productivity and led to better economic results (González-García et al., 2012). In intensively managed wetlands of southeastern Asia, farmers are adding an aquaculture component to an already integrated crop–livestock system. These integrated agriculture–aquaculture (IAA) systems are based on the recycling of nutrients between farm components (Prein, 2002): livestock manure and other farm wastes fertilize fish ponds, pond sediments fertilize crops and crop co-products feed livestock (Figure 1). When animals are integrated into agrosystems in this way, more of the ecosystem processes operating in natural systems can be incorporated into the functioning of the system, increasing its stability and sustainability. Excreta from one species can even be directly used as components of formulated diets for another species: for example, West African dwarf goats could be sustained with poultry excreta for better live-weight gain, feed conversion ratio, carcass yield and better economic returns to the farmers (Alikwe et al., 2011).
Figure 1.
Simplified flow diagram of the interactions within integrated agriculture–aquaculture systems.
In RAS, water quality can deteriorate as a result of fish catabolism and uneaten feed, an effect that is modulated according to fish species, stocking density and feed characteristics. Thus, water becomes enriched in nitrogenous compounds, P and dissolved organic matter and depleted in dissolved oxygen. Maintaining water quality, despite very limited water exchange, requires efficient mechanical and biological water treatments. To improve water treatment efficiency by optimizing the metabolic functioning of farming systems, some recent approaches aim to combine RAS with an Integrated Multi-Trophic Aquaculture (IMTA). This approach is based on the cultivation of aquaculture species (principally finfish) with other extractive aquaculture species (principally seaweeds, and suspension and deposit feeders such as mussels, oysters and shrimps). The objective is to recapture some of the nutrients and energy that are lost in finfish monocultures, and transforming them into additional crops with commercial value (Neori et al., 2004; Ren et al., 2012).
Enhancing diversity within animal production systems to strengthen their resilience
Agricultural intensification has drastically reduced diversity, that is, the variety of both plant and animal species and the variety of management practices and production factors. Recent empirical evidence suggests that we have underestimated the potential for diversity in animal production systems (Tichit et al., 2011). Diversity is an essential property, as it is expected to strengthen the resilience of animal production systems through mechanisms operating at different levels.
At herd level, diversity in both animal species and management practices secures pastoral systems (Mace and Houston, 1989; Tichit et al., 2004). Rearing different animal species offers a risk-spreading strategy against droughts, disease outbreaks and market price fluctuations. Adapting management practices to the biological characteristics of each species is also a key lever to ensure resilience (e.g. by modulating breeding practices according to female longevity and climate sensitivity). Combining several herbivore species at grazing enables higher overall vegetation capture and live-weight gain (Nolan and Connolly, 1989). The principle of these systems is the use of multiple spatial niches and food resources that also apply for aquaculture. Indeed, in the popular rohu (Labeo rohita) and carp (Cyprinus carpio) combination in south Asia, carp browsing the sediment for food oxidize the pond bottom and suspend nutrients accumulated in sediments, leading to up to 40% higher rohu production and almost doubling pond production (Rahman et al., 2006). Within a monospecific ruminant herd, diversity of lifetime performance is suggested to act as a buffer by stabilizing overall herd production. Managing diversity over time becomes a central issue in large herds where management strategies targeted at different herd segments are expected to increase overall performance (Lee et al., 2009). Diversity in lifetime performance emerged from complex interactions between herd management practices and individual biological responses (Puillet et al., 2010). These interactions generated contrasting groups of females with different production level and feed efficiency. The relative size of these groups in the herd was thus a key determinant of overall performance.
A diversity of forage resources also helps secure the feeding system against seasonal and long-term climatic variability. Grazing animals take advantage of resource diversity to maintain daily intake (Agreil et al., 2005) and performance (Dumont et al., 2007a), with contrasting effects of selective grazing according to breed morphological and physiological traits. In late season, Salers beef cows with a relatively high milk yield potential maintained daily milk yield at the expense of body condition, whereas Charolais cows, which have less milk potential reduced milk yield but lost less live weight (Farruggia et al., 2008). In agro-pastoral systems, the feeding system is based on complementarities between cultivated grasslands, which are used to secure animal performance in crucial periods such as mating or lactation, and rangelands, which are mostly grazed at times when the animals have low nutrient requirements (Jouven et al., 2010). When the availability of feed resources is low or unpredictable, defining seasonal priorities between animals with high requirements or key production objectives (e.g. improving body condition), which will need to be given priority access to the best resources, and animals with low requirements or secondary production objectives, also helps in the design of efficient feeding systems. The diversity of grassland types within a farm has been shown to improve farm self-sufficiency for forage in both dairy (Andrieu et al., 2007) and suckler farms (Martin, 2009). Recent work has also emphasized that a diversity of grazing management practices, that is, in terms of stocking rate and periods, can enhance ability to overcome drought events (Sabatier et al. 2012).
Preserving biological diversity in agroecosystems by adapting management practices
In the past decade, concern over biodiversity erosion has spread to domestic biodiversity (e.g. animal genetic resources and local breeds; Taberlet et al., 2011). Higher performance of commercial breeds means that local breeds tend to be replaced by more productive ones, or at least outcrossed. Loss of genetic diversity also occurs in commercial breeds via the development of artificial insemination, with only a few males being involved in reproduction schemes. Although the narrow differences in diet selection between local and commercial breeds in grassland-based systems may prevent any clear management implications being identified (Dumont et al., 2007b), local breeds have greater abilities to survive, produce and maintain reproduction levels in harsh environments. Using local breeds is thus well suited to economically marginal conditions, owing to reduced veterinary intervention, ease of breeding and lower feedstuff costs. Animal products from traditional breeds with strong local identity can fetch premium prices, as consumers perceive them as being of superior sensory or nutritional quality, or are attracted by the image of a particular region or tradition (Casabianca and Matassino, 2006); this process could help preserve resistance or adaptation traits that would otherwise be rapidly lost and difficult to rescue.
Agricultural intensification and homogenization are important driving forces of flora and fauna diversity erosion in temperate agroecosystems. At field scale, grassland management for production purposes usually conflicts with grassland management for conservation purposes (Plantureux et al., 2005). Biodiversity in grasslands thus tends to increase as grass utilization decreases (Klimek et al., 2007; Dumont et al., 2009), but this compels farmers to underutilize their grasslands, leading to production losses per unit area. This stresses the need to test for practices able to preserve biodiversity while still ensuring good economic returns. Late grazing (Sjödin, 2007), preserving legume-rich grasslands (Goulson et al., 2005) and introducing sown margin strips at the edge of arable fields (Marshall et al., 2006), favored pollinator abundance and species richness as a result of positive trophic interactions. Farruggia et al. (2012) recently showed that temporarily excluding cattle from pastures during flowering peak can offer an opportunity to double grassland butterfly populations without decreasing stocking rate. In southern Sweden, Franzén and Nilsson (2008) concluded that 20% to 50% of semi-natural farmland left ungrazed in May to July to provide abundant nectar, and pollen resources could compensate for intense grazing applied on parts of the remaining farm area. The choice of these temporarily ungrazed plots should take into account not only the biodiversity ‘potential’ of each plot, but also their location, so that they can act as dispersal sources or ecological corridors (Öckinger and Smith, 2007). Manipulating the timing and intensity of grazing throughout spring is also an alternative management tool for grassland bird conservation that does away with recommendations based on late grazing or grazing exclusion (Durant et al., 2008).
At farm level, maintaining contrasting management practices is crucial for plant and animal species that share different habitat requirements. At this scale, production and conservation objectives are not necessarily in conflict, because both can benefit from a management strategy on the basis of allocating specific functions to grassland plots in relation to vegetation type. Jouven and Baumont (2008) modeled grassland-based beef systems and found that meat production could be maintained by deploying biodiversity-friendly practices on up to 40% of farm area; the practices resulting in the optimal production–biodiversity equilibrium depended on farming context, that is, type of grassland, overall stocking rate and herd management. Combining different management practices (in terms of grazing intensity and cutting frequency) was found to improve production/biodiversity trade-off, but this improvement was less costly for extensive than for intensive farms (Tichit et al., 2011). Benefits of management practice diversity are even greater at landscape scale. Recent work has demonstrated that the proportion of management practices (grazing v. cutting) and their spatial arrangement can affect the long-term dynamics of bird populations in agro-landscapes. Converting certain intensive practices into extensive ones affected production; however, acting on the spatial arrangement of practices to increase landscape heterogeneity helped to reconcile production and biodiversity (Sabatier et al., 2010).
Some landscape features can exert multiple functions and thus play a role in biodiversity conservation. A typical example is extensive fishponds, which form food production ecosystems, attractive landscape features and a habitat for wild bird species. In fishponds with controlled fish biomass (400 kg/ha), the presence of aquatic vegetation over 10% to 15% of the total area improved water quality, benefited fish reproduction and offered a refuge and nesting habitat for waders (Bernard, 2008). However, the interactions between the biotic and abiotic compartments of fishponds are complex, and depend on practices and regional conditions.
Case studies
Agroecology implies considering agroecosystems as a whole, in their biological, technical and social dimensions. It goes further than adjusting practices in current agroecosystems; it integrates interactions among all agroecosystem components and recognizes the complex dynamics of ecological processes. We therefore present six case studies covering a long gradient of production types, intensification levels and biogeographical conditions. They relate to either agroecology or industrial ecology. The first case study is emblematic of linkages between farming sub-systems; it mainly concerns Asia and South America, and has not yet spread to other parts of the world. The second and third case studies illustrate grassland or rangeland-based systems with either sheep or dairy cattle. Although our two examples are French, such systems are found in many parts of the world (United States of America, New Zealand, Australia) where they represent sustainable alternatives to large-scale intensive systems. The fourth case study focuses on rabbit organic farming; though still marginal, this system is of interest as it explores alternatives to confinement and aims to reconnect animal production with the land. The fifth case study deals with a pig farming system in which waste management is achieved through the principles of industrial ecology. This kind of system is under development in Northern and Western Europe, where the negative environmental impacts of industrialized farming are worsened by the spatial aggregation of industrial farms. The sixth and last case study is emblematic of a policy to reduce water use, pollution and inputs in intensive aquaculture systems. This technology was developed during the late 1980s, mainly in the Netherlands and Denmark. Through these case studies, we examine how our five principles combine in each system (Table 1), and we look at system performance and environmental impact on the basis of available indicators.
Table 1.
The main agroecological principles that apply in six case studies ranging from grassland-based systems to large-scale intensive systems
| Case studies in the text | ||||||
|---|---|---|---|---|---|---|
| Principle | IAA systems | Dairy cows | Pastoral sheep | Organic rabbits | Pigs | RAS fish |
| Integrated management of animal health | ** | * | ** | |||
| Reduce inputs needed for production | *** | *** | ** | *** | *** | ** |
| Reduce pollutions | ** | *** | * | *** | *** | |
| Take advantage of system diversity | ** | ** | *** | ** | * | |
| Preserve biological diversity | * | ** | * | |||
| Agroecology | X | X | X | X | ||
| Industrial ecology | X | X | ||||
IAA = integrated agriculture–aquaculture; RAS = recirculating aquaculture systems.
***Major importance; **Important; *Marginal.
IAA systems
IAA systems promote nutrient linkages between two or more farming activities, one of which is aquaculture (Figure 1). To increase in-pond nutrient supply, fertilization pathways start from plant waste or manure entering the water, followed by decomposition by pond micro-organisms and in-pond growth of natural fish food such as phytoplankton, zooplankton, benthic organisms and detritus. While diversifying production, IAA systems also reduce their environmental impact via nutrient recycling.
In the Mekong Delta, a first category of IAA is represented by low-input fish farming with intensive fruit production (>5 t/ha per year of fruit with fertilizer input ⩾100 kg N/ha per year) and a minor rice farming activity. A series of narrow trenches within the orchards supplies water for crop irrigation and to extract nutrient-rich mud as crop fertilizer. Different fish species are commonly reared, but with low yields of 0.5 to 2.0 t/ha per year (Nhan et al., 2006; Phong et al., 2010). A second category is dominated by rice production with less-intensive fruit production (<2 t/ha per year with low fertilizer input ⩽50 kg N/ha per year). Fish production is in ponds or in rice fields with fish yields between 2 and 10 t/ha per year with moderate input of on-farm seasonal nutrient resources. Not just fish yield but also livestock growth performance, biomass production relative to inputs (Kumaresan et al., 2009) and economic benefits can all be substantially increased. Introducing tilapia into existing integrated farming systems increased gross margins from US$50–150 to 300/household in peri-urban areas of Bangladesh (Karim et al., 2011). Life-cycle assessment has been used to assess the environmental impact of IAA systems in the Mekong Delta (Phong et al., 2011). Energy requirements per kcal were twice lower in rice-based medium-input fish farming (14.2 kJ/kcal) than in the orchard-based low-input fish farming system (on average 27.1 kJ/kcal). On an average, environmental impacts (global warming potential, land use, etc.) were 35% to 45% lower per kg of fish protein than per kg of pig or poultry protein. However, fish grown under waste-fed conditions can become contaminated with human or animal excreta-related pathogens, antibiotics or antibiotic-resistant bacteria (Sapkota et al., 2008). Wastewater and excreta can also contain numerous heavy metals and organic chemicals.
Self-sufficient low-input dairy systems in a sustainable agriculture network
The basic principle of French RAD (réseaux d'agriculture durable) dairy systems is to sustain the level of added value derived from dairy production without necessarily maximizing outputs per animal or per unit area (Alard et al., 2002). A key component consists in maximizing the use of grazed herbage at the expense of maize silage and reducing the use of concentrate feeds. Grasslands comprise a high proportion of grass–legume mixtures, and grazing season is extended in summer, autumn and winter. Herd management is tailored to adapt animal requirements to resources by grouping calving periods.
In Brittany, RAD farms involved in the ‘low-input fodder system’ agri-environmental scheme demonstrated significant environmental improvements (Le Rohellec et al., 2009): total N pressure (i.e. excreta, mineral and organic fertilizers and manure) was 33% lower (121 v. 161 kg N/ha for conventional farms). Frequency index of pesticide applications dropped from 0.66 to 0.21 3 years after the scheme was adopted, because of the increase in grassland area and thresholds imposed for maize and wheat. RAD farms recorded 33% less energy consumption per 1000 l of milk than conventional farms because of less mineral fertilization and, to a lesser extent, savings on concentrate feeds and fuel for mechanization. RAD dairy farms had a lower milk sale and a lower land area per work unit than conventional farms (RAD, 2010). Milk quota was comparable in the two networks, but was not met in the RAD farms, where the priority was to feed the herd at lowest cost. Thus, RAD farms achieved a higher added value (€57k v. €44k in 2009), largely explained by their cost-cutting strategy. They also reached a higher net income per work unit (€19k v. €7k in 2009). The moderate decrease in productivity (−200 l of milk/ha main fodder area in RAD v. conventional farms) was largely compensated for by overall reduction in input costs (feed costs nearly halved at €68 v. €121/1000 l milk and lower mechanization expenses at €−65/ha). Such low-input dairy systems open up new options for existing farming systems, because they display good economic results while limiting pollution risks. At watershed level, the expansion of such systems would result in a significant decrease in N fluxes (Moreau et al., 2012). Their limited dependence on nonrenewable energy offers a potential advantage when faced with higher energy prices, but they could be sensitive to climate change, in particular inter-annual variability in grass growth.
Agro-pastoral sheep farming systems
In pastoral farming systems, performance can be improved by organizing the animal production cycle according to forage diversity and seasonal dynamics. In a meat-sheep farm on the dry Larzac plateau, 280 highly prolific rustic ewes were reared fully outdoors on 260 ha of native vegetation (2 t DM/hayear) plus 18 ha of fertilized vegetation (4t DM/hayear), 10.2 ha hay fields and 2.8 ha cereals (Molénat et al., 2005). Ewes were first mated at 19 months, and all lambings were concentrated in spring. Thus, the ewes had high nutritional requirements in the period of high grass availability on fertilized then on native vegetation. Mating and rebuilding of body reserves in autumn and early winter was secured by grazing regrowths in fertilized plots. Replacement lambs and nonlactating ewes grazed native vegetation, which was divided into paddocks to ensure long periods for vegetation recovery. The system thus benefited from replacement ewe–lambs learning to exploit rangelands at a young age and mobilized their ability to express compensatory growth.
Animal productivity was high at 1.8 lambs per female older than 1 year. Of the lambs, 20% were sold ‘lean’ at weaning and the remaining 80% were sold fattened at 3 to 4 months. Up to 68% of feed consumed by the flock was provided by rangeland, and 93% of flock requirements were supplied by feed produced on-farm. Gross profit margin (€97/ewe) was higher than that simultaneously recorded in 18 meat-sheep farms of central France (€58/ewe; M. Benoit and G. Laignel, personal communication) because of high animal productivity and very low system inputs. The net income (€24.6k/work unit) was also higher than the 18 meat-sheep farm average (€15.0k/work unit). This system thus contributed to a sustainable utilization of natural resources and required little nonrenewable energy (55.8 MJ/kg lamb carcass based on life-cycle assessment) for a moderate net emission of greenhouse gases (18.8 kg eq-CO2/kg carcass). By limiting the risk of shrub encroachment, sheep grazing also preserved native Mediterranean species in this highly diverse vegetation community. Two major limitations to its implementation on wider scales could be, first, the marked seasonality of production, which concentrates workload and is out of line with market requirements, and second, the management of grazing and manure distribution, which requires close monitoring of vegetation dynamics and animal behaviour.
Organic rabbit systems
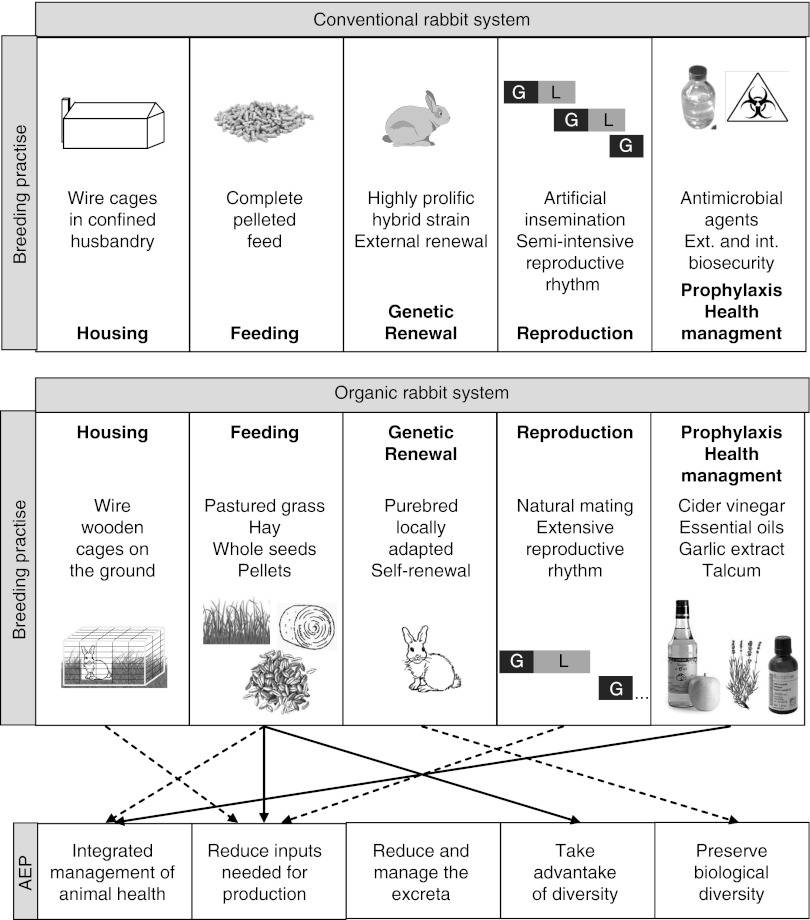
Organic rabbit production systems meet most agroecological principles (Figure 2). In north-western France, 1200 organic rabbits are currently produced every year from 70 females in a meat-sheep farm. Purebred animals are raised outdoors in wire and wooden cages placed on the ground, which limits housing requirements. Cages are moved every day in 3 ha of unfertilized permanent grass–clover pastures, which helps fight against coccidiosis, the main threat in organic rabbit production (Licois and Marlier, 2008). The rabbits also have access to locally grown hay, alfalfa, straw, seed mixtures and pellets of commercial origin. Straw is laid on the top of cages to provide dietary fiber and shade on sunny days. Rabbits frequently sort the different seeds, and therefore their refusals are offered to sheep. Antimicrobial agents have only been used once in the last 7 years. Animal health is managed using essential oils, garlic extract and cider vinegar (Benguesmia et al., 2011). This small-scale organic system is autonomous, but much less productive than the conventional system, in which prolific, fast-growing hybrid strains are fed complete pelleted feed (21 v. 51 rabbits/female per year). Both prolificacy (6 v. 8 weaned rabbits) and growth rate (25 v. 32 g/day) were lower than in the conventional system. Mortality was quite high (24% v. 10% between 2 weeks and slaughter), raising animal welfare issues. In addition, outdoor housing did not satisfy internal and external biosecurity, despite this being a priority for the integrated management of animal health. Conversely, use of local resources and grazing reduced feeding costs. The economic sustainability of the system was reached by on-farm sales, as carcass price was 7.5 times higher (€12.5 v. €1.67/kg) than in conventional systems. This resulted in gross margin minus feeding costs being 2.5 times higher than in conventional farms (€281 v. €110/female per year). This system is strongly based on local resources and independent to chemical antibiotics to manage animal health. Owing to low structural costs and high sale prices, it enabled one person to live on the farm from organic rabbit production, despite poor animal performance.
Figure 2.
Schematic representation of conventional and organic rabbit production systems. Major (solid lines) and more marginal (dashed lines) contributions to agroecological principles (AEP) in the organic system (G = gestation; L = lactation).
Toward an ecologically sound, efficient pig farming system
Most of the environmental impacts of pig farming systems are associated with the production of feed ingredients, animal housing and manure storage. A farm in central France optimizes the metabolic functioning of the system by using manure from 180 sows to produce biogas for heating and, after treatment, to fertilize 252 ha of cereals, oilseeds and peas. All the crops are used to produce pig feeds, except sunflower, millet and buckwheat, which are sold. A digester produces 368.000 m3 of biogas from liquid and solid pig manure, as this proves the most effective way to avoid environmental losses of CH4 from liquid manure while also reducing the biological activity of drug residues (Petersen et al., 2007). The biogas produced 880 electric mWh, the annual electricity consumption of 250 houses, and a heat production of 847 thermal mWh. The total volume of biomass recycled every year through the digester was 5300 t, of which 84% came from the farm via pig manure and silage of intercrops. Rapeseed was pressed to extract oil for the digester motor, and the pulp was recycled back into the pig diet. Animals were raised in heated housing, exploiting heat produced from the digester, thus cutting back energy costs and greenhouse gas emission (Rigolot et al., 2010). Six different diets were produced on the farm to meet pig nutritional requirements. Growth performance was similar or slightly better than in conventional French pig farms (gain-to-feed ratio 2.7 and mortality 4.6% in growing pigs v. 2.65 and 6%), but reproductive performance was slightly lower, at 10.5 (v. 11.2) weaned piglets per litter and 86% (v. 89%) fertility at first mating. Mortality before weaning was slightly lower (18% v. ⩾20%) than in conventional farms, which, together with 75% of feed ingredients for sows, growing and finishing pigs being produced on the farm, led to better economic results. Marked annual variations in gross margin per sow were strongly buffered by sales of crops produced on the farm. This system benefited employment, as 8.5 work units are needed for production, meat processing, on-farm sale and waste recycling; however, it also required a major initial investment, as the digester installation cost €793k, of which 55% was own funds.
Intensive fish farming in RAS
African catfish (Clarias gariepinus) occupies second place in Dutch fish production, and is produced exclusively in RAS, which basically consists of two growth phases. The first phase grows 10 g fingerlings to an average weight of 100 to 150 g at fish densities ranging between 100 and 300 kg/m3. During the second phase, fishes are grown to a market size of 1500 g at densities of 200 to 500 kg/m3. Within the size and density ranges used, fish welfare was not impacted negatively by increasing density (van de Nieuwegiessen et al., 2009). In addition to opportunities for water economies and improved waste management, a high degree of production traceability was feasible in RAS. Life-cycle assessment revealed that feed had the strongest environmental impact in RAS (Roque d'Orbcastel et al., 2009), which calls for further research to minimize feed conversion ratio and select appropriate feed ingredients; these authors estimated an energy use of 16 kWh/kg fish in RAS, this value being 1.4 to 1.8 higher in RAS than in traditional flow through system. Energy consumption per kg of trout or sea bass produced in RAS was 15 to 20 kWh/kg (Roque d'Orbcastel et al., 2009), which is in the range of the amount of energy needed to fish 1 kg of cod (5 to 21 kWh/kg; Ziegler, 2006). In intensive RAS tilapia farms, water requirement was estimated at 238 l/kg fish (Eding et al., 2009). What limits the development of RAS technology is first the high initial capital investment (Badiola et al., 2012), which requires high stocking densities and outputs to cover investment costs, and second the complexity of the system and its dynamic properties, which require highly skilled management (Wik et al., 2009). Some technological innovation could be developed in order to improve the profitability of RAS. The overall fish production cost in a RAS equipped with an ion-exchange resin was indeed fairly low, estimated at US$0.8/kg fish assuming a feed conversion ratio of 1.8 kg feed per kg fish (Gendel and Lahav, 2012). The efficiency of waste removal on the basis of new processes such as denitrification, nanofiltration or reverse osmosis (Martins et al., 2010), or recovering wastes for other productions (Metaxa et al., 2006) would have an impact on both system performance and environmental footprint (Wik et al., 2009).
Discussion
Agroecology and industrial ecology act jointly to reinstate animal production in ecological thinking. We have reformulated the ecological processes proposed by Altieri (2002) and propose the following five principles for animal production systems: (i) adopting management practices aiming to improve animal health, (ii) decreasing the inputs needed for production, (iii) decreasing pollution by optimizing the metabolic functioning of farming systems, (iv) enhancing diversity within animal production systems to strengthen their resilience and (v) preserving biological diversity in agroecosystems by adapting management practices. Owing to the large amounts of resources needed to produce animal proteins, we thus considered reduction of not only agrochemicals but all types of inputs needed for production. Animal genetic resources and local breeds offer opportunities to adapt livestock to constraining feeding environments, and hence their preservation is highly relevant for the development of ecology-based alternatives for animal production. Focusing on Altieri's definition of agroecology, we paid little attention to the food system component, because as animal scientists we place our specific contribution at the interface between animal sciences and ecology. However, most case studies presented illustrate this food system dimension in terms of market and/or economic performance of the systems.
Differential mobilization of the principles of agroecology in animal production systems
On the basis of examples taken from six existing systems, we show that the five principles could apply to all types of animal production systems, but that they were not mobilized equally (Table 1). Several principles were mobilized in each case study, consistent with the systemic vision inherent to agroecology. The two principles concerning the economy of inputs and the reduction of pollution emerged in nearly all the case studies. This could be explained by the economic and regulatory constraints affecting animal production. Numerous standards have been imposed in the last 10 to 15 years to fight pollution, while inputs are expensive in animal production systems, so that any savings can improve system performance. Therefore, R&D efforts have tended to focus on these factors, followed by recommendations for good farming practices. The principle of integrated management of animal health has seldom been mobilized. Several hypotheses can be advanced to account for this finding: (i) the regulations (except those for organic-label productions) still allow the use of chemical drugs and therefore do not incentivize work on alternatives, (ii) in several systems, particularly grassland-based systems, health risks are relatively low and (iii) alternatives to chemical drugs have only recently been investigated, and thus are not yet ready for transfer into practice. Finally, the preservation of biodiversity was a secondary objective that only applied in grassland-based systems. However, a number of ecological functions and ecosystem services (recycling of nutrients, forage yield, pollination, resistance to weed invasion, etc.) are closely linked with biodiversity, and their persistence depends largely on maintaining biological diversity in agroecosystems (Altieri and Nicholls, 2005).
The extent to which the five principles were mobilized differed according to whether the systems fitted agroecology or industrial ecology. In particular, the principle of enhancing resource diversity seemed specific to agroecology systems, whereas pollution reduction objectives were more marked in industrial ecology systems. Diversity is a fundamental characteristic of weakly artificialized systems tied to the physical environment and faced with uncontrolled events. The purpose of agroecology is to recognize this diversity instead of merely enduring it; it aims at using diversity in a programmed way to strengthen the adaptive capacity and resilience of farming systems (Altieri et al., 2012). By contrast, systems based on industrial ecology have a highly controlled composition and a much looser link to the land. Industrial ecology explores possible interactions between different types of activities or enterprises (Frosch, 1992). In this way, the outputs from one activity can become inputs to another, so that these systems make it possible to treat and make productive use of waste from other agricultural or non-agricultural systems.
Agroecology and industrial ecology are thus probably part of a continuum: first, because both approaches are grounded on the ecosystem concept, and offer two contrasting alternatives to minimize the environmental footprint of animal production systems; and second, because they will probably have to be coupled to meet natural resource preservation, while helping to feed the world's population. Systems based on agroecology will use the diversity of natural resources not directly utilizable by humans to produce meat and milk, while simultaneously preserving vast areas with high biodiversity potential. Industrial ecology will add quantitatively to production, while at the same time reducing pollution and saving scarce natural resources. Behind this overall scheme, there remains the question of the place given to these different systems, in particular those that break with the dominant models in each sector. It remains unclear whether agroecological systems and products can expand in today's supply chains or whether specific new supply chains should be tailored. An important issue for the scaling up of agroecology approaches is the development of farmer-to-farmer networks, of institutional articulation and the identification of niche markets compatible with the characteristics of agroecological products (Altieri et al., 2001). The implementation of agroecology- or industrial ecology-based farming systems will depend on their consistency with the local contexts: land occupation, urbanization, present state of agricultural systems, etc. The process by which one alternative could be favored over the other will also depend on the allocation of financial investments in research and extension services, and on the ability of public intervention to influence lock-in situations in agricultural research (Vanloqueren and Baret, 2009).
Agroecology: a way to secure income while limiting the environmental footprint of farming systems
The innovative animal production systems described in this review show that the advantages of systems involving agroecology over ‘conventional’ systems are strongly dependent not only on the evaluation basis (animal, farm, etc.), but also on farming context (type of production, market, etc.). In most of the case studies considered, a major issue when applying the principles of agroecology was to secure the farmer's income by adjusting the output-to-input ratio at production-unit scale. For comparable levels of production, the decrease in inputs resulting from the search for maximum food self-sufficiency led to an increase in the added value created on the farm (e.g. in the low-input dairy system) and a greater stability of income against market fluctuations (e.g. in the pig system). The gain relative to conventional systems was even more marked when output increased (e.g. IAA systems, where production can be increased 100-fold). However, the ultimate economic result remains to be adjusted according to (i) agricultural policy context and (ii) labor requirements. The application of agroecology principles is usually accompanied by better environmental performance, which could give rise to payment for environmental services (PES). These financial resources could help make up possible production losses. From different experiences across the world, it is expected that PES could provide economic incentives for agroecology scale-up. This would require appropriate mechanisms to transfer resources from beneficiaries at multiple scales back to the providers (Farley et al., 2012).
Ecology-based animal production systems mobilize specific skills and require a great deal of time to supervise and observe the system or time spent on crop production in search of self-sufficiency. Labor productivity and productivity per unit area are therefore often lower than in conventional systems. Income is ensured by higher-value products (e.g. organic rabbits), sometimes with on-farm processing (e.g. pigs). Further development of these systems for pig and poultry production channels that have lost their link with the land will need to integrate territorial distribution as a factor. Industrial ecology mainly aims to reduce pollution by mobilizing advanced technology in animal production systems, which often entails buying in special capital-intensive equipment. Owing to high investment and fixed costs, it is likely that this can only be developed in large-size farms. The development of these systems thus depends on taking a financial risk, notwithstanding possible public aid. However, this risk may be offset by proximity to large urban centers where consumers are concentrated, but which are highly intolerant of pollution.
One issue inherent to the agroecology approach is the need to limit the negative impact of animal production systems by adjusting the relations between productivity and ecosystem services, and between resources required for production and emissions of farm wastes. To incentivize farmers to opt in and make changes, it will be necessary to quantify the input reduction that can be obtained by enhancing diversity within the system and stimulating its internal regulations. Such input reduction should be balanced against reductions of farm wastes and possible production losses. With a view to limiting the environmental footprint of animal production, it will make sense to express animal outputs according to limiting resources, which may be water, arable land area, P or energy, for which animal production is in direct competition with other human activities. According to local context, the main risk of pollution will depend on the nature of farm wastes, their geographical concentration and seasonal emission patterns. These considerations make it difficult to propose a single simple framework that can compare different animal production systems on a same core set of criteria. There is a clear urgency to define thresholds in the use of limiting factors to keep within planetary boundaries (Rockström and Karlberg, 2010), but each threshold would need to be qualified according to type of production and local context.
Perspectives for encouraging ecology-based animal production systems
The strengths of ecology-based animal production systems lie in their self-sufficiency, which through interacting with their environment can produce part of the resources needed for production, and recycle on-farm wastes. Food self-sufficiency breaks dependency on erratic market prices, but is often associated with dependency on pedoclimatic conditions. Although agroecology-based systems are a priori, more tightly constrained and more weather sensitive than conventional systems, they mobilize other production factors such as the use of local breeds that have greater abilities to produce in harsh environments, and may also be better aligned to meeting consumer expectations (Casabianca and Matassino, 2006). Working on medium-term rather than year-based animal production objectives is a key challenge, as short-term performance usually conflicts with long-term performance (Weiner, 2003). Extending time scale allows flexibility in the management of herds and plots, and offers another way to cope with these uncertainties instead of merely enduring them.
The possible long-term increase in production costs (e.g. fuel, mineral fertilizers and concentrate feeds) and future regulations limiting the use of some of the inputs required for animal production could lead to a deintensification of animal production systems for economic reasons in a context of market price fluctuation. Organic farming offers a valuable platform for simulating what could happen in such a context of increasing input costs coupled with limited possibilities for intervention. For instance, an intensive reproduction pattern with three lambings in 2 years consistently led to the highest net income in conventional meat-sheep production systems over the last 20 years, but did not improve economic results on organic meat-sheep farms, where it even proved riskier and more difficult to manage (Benoit et al., 2009). Highly feed self-sufficient agroecological systems, in which animals are integrated in a way that increases the stability of the system, could thus become even more sustainable in the future, if relations between productivity, input costs and ecosystem services drastically change.
Labor is a key determinant of the acceptability of agroecological practices to farmers. As underlined by Tripp (2008), agroecological practices are not necessarily more labor intensive than conventional ones. However, the success of agroecological management is often dependent on the efficient organization of labor supply, and it usually requires some additional time for learning and adaptation for the establishment of an agroecological system (Tripp, 2008). Moreover, the labor component is more than just a number of hours invested per hectare; labor is also qualitatively different from that in conventional systems with different types of knowledge, skills and farmer capacities for monitoring system performance. Set procedures or generic solutions can no longer be applied, but instead decisions have to be made on the basis of local knowledge and skills obtained through detailed observation of how the system works (Nhan et al., 2006; González-García et al., 2012). Two approaches could contribute to improving work productivity: (i) using new technologies that facilitate the collection and processing of information on the system, and that reduce the time-consuming handling of animals; and (ii) proposing indicators or other evaluation tools to help farmers acquire specific knowledge and skills relevant to an agroecology-based management of their system for added efficiency in real-time decision making.
We conclude that ecology-based alternatives for animal production in turn imply changes in the positions adopted by the different stakeholders:
-
•
Technicians and extension services are no longer providers of ready-made solutions, but instead become expert contacts who stimulate the ability of farmers to learn and strengthen their decisional self-reliance.
-
•
Researchers cannot simply propose generic solutions and tools, but must consider animal production systems both holistically (on different scales from the animal to the landscape) and in their diversity, associating biology with economics and sociology.
-
•
Policymakers cannot base their decisions on simple generic indicators, but must take into account the diversity of local and regional situations, supported by appropriate evaluation tools for animal production systems.
Acknowledgments
This work was funded by a grant from the INRA Animal Physiology and Livestock Systems division. The authors thank Marc Benoit, Jean-Baptiste Coulon, Benoit Dedieu, Jean-Yves Dourmad and Eliel González-García for stimulating discussions and helpful comments on the manuscript.
References
- Agreil C, Fritz H, Meuret M 2005. Maintenance of daily intake through bite mass diversity adjustment in sheep grazing on heterogeneous and variable vegetation. Applied Animal Behaviour Science 91, 35–56 [Google Scholar]
- Alard V, Béranger C, Journet M 2002. A la recherche d'une agriculture durable. Etude de systèmes herbagers économes en Bretagne. INRA Editions, Paris [Google Scholar]
- Alikwe PCN, Faremi AY, Fajemisin AN, Akinsoyinu AO 2011. Performances and nitrogen utilization of West African dwarf goats fed soybean and dried poultry waste-based concentrates as supplements to Cynodon nlemfuensis basal diet. Journal of Applied Sciences in Environmental Sanitation 6, 181–189 [Google Scholar]
- Altieri MA 1987. Agroecology: the scientific basis of alternative agriculture. Westview Press, Boulder, CO, USA [Google Scholar]
- Altieri MA 2002. Agroecological principles and strategies for sustainable agriculture In Agroecological innovations: increasing food production with participatory development (ed. NT Uphoff), pp. 40–46 Earthscan Publication Ltd, London, UK [Google Scholar]
- Altieri MA, Nicholls CI 2005. Agroecology and the search for a truly sustainable agriculture. Basic Textbooks for Environmental Training, United Nations Environment Program, 291pp.
- Altieri MA, Buckles D, Bunch R, Carter S, Casanova A, Engel P, Figueroa R, Venegas C 2001. Scaling up successful agroecological initiatives in Latin America and the Caribbean, Retrieved on May 1, 2001, from nature.berkeley.edu/~agroeco3/sane/
- Altieri MA, Funes-Monzote FR, Petersen P 2012. Agroecologically efficient agricultural systems for smallholder farmers: contributions to food sovereignty. Agronomy for Sustainable Development 32, 1–13 [Google Scholar]
- Andrieu N, Poix C, Josien E, Duru M 2007. Simulation of forage management strategies considering farm-level land diversity: example of dairy farms in the Auvergne. Computers and Electronics in Agriculture 55, 36–48 [Google Scholar]
- Archimède H, González-García E, Despois P, Etienne T, Alexandre G 2010. Substitution of corn and soyabean with green banana fruits and Gliricidia sepium forage in sheep fed hay-based diets. Effects on intake, digestion and growth. Journal of Animal Physiology and Animal Nutrition 94, 118–128 [DOI] [PubMed] [Google Scholar]
- Badiola M, Mendiola D, Bostock J 2012. Recirculating Aquaculture Systems (RAS) analysis: main issues on management and future challenges. Aquaculture Engineering 51, 26–35 [Google Scholar]
- Balcázar JL, de Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Músquiz JL 2006. The role of probiotics in aquaculture. Veterinary Microbiology 114, 173–186 [DOI] [PubMed] [Google Scholar]
- Bene C, Obirih-Opareh N 2009. Social and economic impacts of agricultural productivity intensification: the case of brush park fisheries in Lake Volta. Agricultural Systems 102, 1–10 [Google Scholar]
- Benguesmia M, Niepceron A, Boucher S, Cortet J, Chaumeil T, Cabaret J 2011. Assessing the use of cider vinegar on parasitism and biological growth in organic farming rabbits. Actes des 14èmes Journées de la Recherche Cunicole, 22–23 Novembre 2011, Le Mans, France, pp. 9–12.
- Benoit M, Tournadre H, Dulphy Laignel G, Prache S, Cabaret J 2009. Is intensification of reproduction rhythm sustainable in an organic sheep production system? A 4-year interdisciplinary study. Animal 3, 753–763 [DOI] [PubMed] [Google Scholar]
- Bernard S 2008. L’étang, l'homme et l'oiseau. Incidences des modes de gestion des étangs piscicoles sur les ceintures de végétation et l'avifaune nicheuse en Sologne, Brenne, Bresse, Territoire de Belfort et Champagne humide. PhD, ENS Lyon, France.
- Bosma RH, Verdegem MCJ 2011. Sustainable aquaculture in ponds: principles, practices and limits. Livestock Science 139, 58–68 [Google Scholar]
- Cabaret J 2007. Practical recommendations on the control of helminth parasites in organic sheep production systems. In CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2(019), 1–6.
- Casabianca F, Matassino D 2006. Local resources and typical animal products In Livestock farming systems – product quality based on local resources leading to improved sustainability (ed. R Rubino, L Sepe, A Dimitriadou and A Gibon), pp. 9–26 EAAP publication no. 118. Wageningen Academic Publishers, The Netherlands [Google Scholar]
- De Deyn GB, Quirk H, Yi Z, Oakley S, Ostle NJ, Bardgett RD 2009. Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. Journal of Ecology 97, 864–875 [Google Scholar]
- De Groot J, Ruis MAW, Scholten JW, Koolhaas JM, Boersma WJA 2001. Long-term effects of social stress on antiviral immunity in pigs. Physiology and Behavior 73, 145–158 [DOI] [PubMed] [Google Scholar]
- Diemer A, Labrune S 2007. L’écologie industrielle: quand l’écosystème industriel devient un vecteur du développement durable. Développement durable et territoires. Retrieved August 30, 2007, from http://developpementdurable.revues.org/4121
- Dourmad JY, Rigolot C, Jondreville C 2009. Influence de la nutrition sur l'excrétion d'azote, de phosphore, de cuivre et de zinc des porcs, et sur les émissions d'ammoniac, de gaz à effet de serre et d'odeurs. INRA Productions Animales 22, 41–48 [Google Scholar]
- Dubois JP, Bijja M, Auvergne A, Lavigne F, Fernandez X, Babilé R 2008. Qualité des parcours de palmipèdes: choix des espèces végétales, rendement et résistance au piétinement. Actes des 8èmes Journées de la Recherche sur les Palmipèdes à Foie Gras. 30–31 Octobre 2008, Arcachon, France.
- Dumont B, Farruggia A, Garel JP, Bachelard P, Boitier E, Frain M 2009. How does grazing intensity influence the diversity of plants and insects in a species-rich upland grassland on basalt soils? Grass and Forage Science 64, 92–105 [Google Scholar]
- Dumont B, Garel JP, Ginane C, Decuq F, Farruggia A, Pradel P, Rigolot C, Petit M 2007. a. Effect of cattle grazing a species-rich mountain pasture under different stocking rates on the dynamics of diet selection and sward structure. Animal 1, 1042–1052 [DOI] [PubMed] [Google Scholar]
- Dumont B, Rook AJ, Coran C, Röver KU 2007. b. Effects of livestock breed and grazing intensity on biodiversity and production in grazing systems. 2. Diet selection. Grass and Forage Science 62, 159–171 [Google Scholar]
- Durant D, Tichit M, Kernéïs E, Fritz H 2008. Management of agricultural grasslands for breeding waders: integrating ecological and livestock system perspectives – a review. Biodiversity and Conservation 17, 2275–2295 [Google Scholar]
- Eding E, Verdegem M, Martins C, Schlaman G, Heinsbroek L, Laarhoven B, Ende S, Verreth J, Aartsen F, Bierbooms V 2009. Tilapia farming using recirculating aquaculture systems (RAS) – case study in the Netherlands. Handbook for Sustainable Aquaculture. Project no. COLL-CT-2006-030384. Retrieved June 22, 2009, from http://www.sustainaqua.org/
- Farley J, Schmitt Filho A, Alvez J, Ribeiro de Freitas N Jr 2012. How valuing nature can transform agriculture. Solutions 2(6), 64–73 [Google Scholar]
- Farruggia A, Dumont B, D'hour P, Egal D 2008. How does protein supplementation affect the selectivity and performance of Charolais cows on extensively grazed pastures in late autumn? Grass and Forage Science 63, 314–323 [Google Scholar]
- Farruggia A, Dumont B, Scohier A, Leroy T, Pradel P, Garel JP 2012. An alternative rotational grazing management designed to favour butterflies in permanent grasslands. Grass and Forage Science 67, 136–149 [Google Scholar]
- Faruk MU, Bouvarel I, Meme N, Rideau N, Roffidal L, Tukur HM, Bastianelli D, Nys Y, Lescoat P 2010. Sequential feeding using whole wheat and a separate protein–mineral concentrate improved feed efficiency in laying hens. Poultry Science 89, 785–796 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization (FAO) 2006. Livestock's long shadow: environmental issues and options. Publishing Management Service, FAO, Rome, Italy [Google Scholar]
- Francis C, Lieblein G, Gliessman S, Breland TA, Creamer N, Harwood R, Salomonsson L, Helenius J, Rickerl D, Salvador R, Wiedenhoeft M, Simmons S, Allen P, Altieri M, Flora C, Poincelot R 2003. Agroecology: the ecology of food systems. Journal of Sustainable Agriculture 22, 99–118 [Google Scholar]
- Frankow-Lindberg BE, Brophy C, Collins RP, Connolly J 2009. Biodiversity effects on yield and unsown species invasion in a temperate forage ecosystem. Annals of Botany 103, 913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén M, Nilsson SG 2008. How can we preserve and restore species richness of pollinating insects on agricultural land? Ecography 31, 698–708 [Google Scholar]
- Franzluebbers AJ 2007. Integrated crop–livestock systems in the southeastern USA. Agronomy Journal 99, 361–372 [Google Scholar]
- Frosch RA 1992. Industrial ecology: a philosophical introduction. Proceedings of the National Academy of Sciences of the USA 89, 800–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch RA, Gallopoulos NE 1989. Strategies for manufacturing. Scientific American 261, 144–152 [Google Scholar]
- Gatesoupe FJ 1994. Lactic acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic Vibrio . Aquatic Living Resources 7, 277–282 [Google Scholar]
- Gendel Y, Lahav O 2012. A novel approach for ammonia removal from fresh-water recirculated aquaculture systems, comprising ion exchange and electrochemical regeneration. Aquaculture Engineering, doi:10.1016/j.aqueng.2012.07.005, Published online by Elsevier September 1, 2012. [Google Scholar]
- Gidenne T, Garcia J, Lebas F, Licois D 2010. Nutrition and feeding strategy: interactions with pathology In Nutrition of the rabbit (ed. C De Blas and J Wiseman), pp. 179–199 CABI Publishing, Wallingford, Uk [Google Scholar]
- Gill M, Smith P, Wilkinson JM 2010. Mitigating climate change: the role of domestic livestock. Animal 4, 323–333 [DOI] [PubMed] [Google Scholar]
- Gliessman SR 1997. Agroecology: ecological processes in sustainable agriculture. CRC Press, Boca Raton, FL, USA [Google Scholar]
- Gliessman SR 2006. Animals in agroecosystems In Agroecology: the ecology of sustainable food systems, 2nd edition, pp. 269–285 CRC Press, Boca Raton, FL, USA [Google Scholar]
- Godfray HJC, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C 2010. Food security: the challenge of feeding 9 billion people. Science 327, 812–818 [DOI] [PubMed] [Google Scholar]
- González-García E, Gourdine JL, Alexandre G, Archimède H, Vaarst M 2012. The complex nature of mixed farming systems requires multidimensional actions supported by integrative research and development efforts. Animal 6, 763–777 [DOI] [PubMed] [Google Scholar]
- Goulson D, Hanley ME, Darvill B, Ellis JS, Knight ME 2005. Causes of rarity in bumblebees. Biological Conservation 122, 1–8 [Google Scholar]
- Gradé JT, Tabuti JRS, Van Damme P 2009. Four footed pharmacists: indications of self-medicating livestock in Karamoja, Uganda. Economic Botany 63, 29–42 [Google Scholar]
- Hangalapura BN, Nieuwland MGB, Reilingh GD, Buyse J, Van Den Brand H, Kemp B, Parmentier HK 2005. Severe feed restriction enhances innate immunity but suppresses cellular immunity in chicken lines divergently selected for antibody responses. Poultry Science 84, 1520–1529 [DOI] [PubMed] [Google Scholar]
- Herrero M, Thornton PK, Notenbaert AM, Wood S, Msangi S, Freeman HA, Bossio D, Dixon J, Peters M, van de Steeg J, Lynam J, Rao PP, Macmillan S, Gerard B, McDermott J, Sere C, Rosegrant M 2010. Smart investments in sustainable food production: revisiting mixed crop–livestock systems. Science 327, 822–825 [DOI] [PubMed] [Google Scholar]
- Holm Nielsen JB 2010. Bioenergy has a key role to play! Bioenergy in a European context: potentials, politics, developments and barriers. In Proceedings of the 6th International Bioenergy Conference, Scientific Engineering Centre “Biomass”. September 13–15, 2010, Kiev, Ukraine, pp. 1–36.
- Horsted K, Hermansen JE 2007. Whole wheat versus mixed layer diet as supplementary feed to layers foraging a sequence of different forage crops. Animal 1, 575–585 [DOI] [PubMed] [Google Scholar]
- Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO 2006. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends in Parasitology 22, 253–261 [DOI] [PubMed] [Google Scholar]
- Janzen HH 2011. What place for livestock on a re-greening earth? Animal Feed Science and Technology 166–167, 783–796 [Google Scholar]
- Jenet A, Fernandez-Rivera S, Tegegne A, Wettstein HR, Senn M, Saurer M, Langhans W, Kreuzer M 2006. Evidence for different nutrient partitioning in Boran (Bos indicus) and Boran × Holstein cows when re-allocated from low to high or from high to low feeding level. Journal of Veterinary Medicine, Series A 53, 383–393 [DOI] [PubMed] [Google Scholar]
- Jouven M, Baumont R 2008. Simulating grassland utilization in beef suckler systems to investigate trade-offs between production and floristic diversity. Agricultural Systems 96, 260–272 [Google Scholar]
- Jouven M, Lapeyronie P, Moulin CH, Bocquier F 2010. Rangeland utilization in Mediterranean farming systems. Animal 4, 1746–1757 [DOI] [PubMed] [Google Scholar]
- Karim M, Little DC, Shamshul Kabir MD, Verdegem MJC, Telfer T, Wahab MDA 2011. Enhancing benefits from polycultures including Tilapia (Oreochromis niloticus) within integrated pond-dike systems: a participatory trial with households of varying socio-economic level in rural and peri-urban areas of Bangladesh. Aquaculture 314, 225–235 [Google Scholar]
- Kidane A, Houdijk JGM, Athanasiadou S, Tolkamp BJ, Kyriazakis I 2010. Effects of maternal protein nutrition and subsequent grazing on chicory (Chicorium intybus) on parasitism and performance of lambs. Journal of Animal Science 88, 1513–1521 [DOI] [PubMed] [Google Scholar]
- Klimek S, Richter gen. Kemmermann A, Hofmann M, Isselstein J 2007. Plant species richness and composition in managed grasslands: the relative importance of field management and environmental factors. Biological Conservation 134, 559–570 [Google Scholar]
- Koohafkan P, Altieri MA, Holt-Gimenez E 2012. Green Agriculture: foundations for biodiverse, resilient and productive agricultural systems. International Journal of Agricultural Sustainability 10, 61–75 [Google Scholar]
- Kumaresan A, Pathak KA, Bujarbaruah KM, Vinod K 2009. Analysis of integrated animal–fish production system under subtropical hill agro ecosystem in India: growth performance of animals, total biomass production and monetary benefit. Tropical Animal Health Production 41, 385–391 [DOI] [PubMed] [Google Scholar]
- Lee GJ, Atkins KD, Sladek MA 2009. Heterogeneity of lifetime reproductive performance, its components and associations with wool production and liveweight of Merino ewes. Animal Production Science 49, 624–629 [Google Scholar]
- Le Rohellec C, Falaise D, Mouchet C, Boutin M, Thiebot J 2009. Analyse de l'efficacité environnementale et énergétique de la mesure agri-environnementale « Système fourrager économe en intrants » (SFEI), à partir de l'analyse de pratiques de quarante-quatre signataires. Campagne culturale 2006/2007. Rencontres Recherches Ruminants 16, 109–112 [Google Scholar]
- Licois D, Marlier D 2008. Pathologies infectieuses du lapin en élevage rationnel. INRA Productions Animales 21, 257–267 [Google Scholar]
- Mace R, Houston A 1989. Pastoralist strategies for survival in unpredictable environments: a model of herd composition that maximizes household viability. Agricultural Systems 31, 185–204 [Google Scholar]
- Mahieu M, Aumont G 2009. Effects of sheep and cattle alternate grazing on sheep parasitism and production. Tropical Animal Health Production 41, 229–239 [DOI] [PubMed] [Google Scholar]
- Mandonnet N, Tillard E, Faye B, Collin A, Gourdine JL, Naves M, Bastianelli D, Tixier-Boichard M, Renaudeau D 2011. Adaptation des animaux d’élevage aux multiples contraintes de régions chaudes. INRA Productions Animales 24, 41–64 [Google Scholar]
- Marie-Magdeleine C, Mahieu M, Philibert L, Despois P, Archimède H 2010. Effect of cassava (Manihot esculenta) foliage on nutrition, parasite infection and growth of lambs. Small Ruminant Research 93, 10–18 [Google Scholar]
- Marshall EJP, West TM, Kleijn D 2006. Impacts of an agri-environmental field margin prescription on the flora and fauna of arable farmland in different landscapes. Agriculture, Ecosystems & Environment 113, 36–44 [Google Scholar]
- Martin C, Morgavi DP, Doreau M 2010. Methane mitigation in ruminants: from microbe to the farm scale. Animal 4, 351–365 [DOI] [PubMed] [Google Scholar]
- Martin G 2009. Analyse et conception de systèmes fourragers flexibles par modélisation systémique et simulation dynamique. PhD, Toulouse University, France.
- Martins CIM, Pistrin MG, Ende SSW, Eding EH, Verreth JAJ 2009. The accumulation of substances in recirculating aquaculture systems (RAS) affects embryonic and larval development in common carp Cyprinus carpio . Aquaculture 291, 65–73 [Google Scholar]
- Martins CIM, Eding EH, Verdegem MCJ, Heinsbroek LTN, Schneider O, Blancheton JP, Roque d'Orbcastel E, Verreth JAJ 2010. New developments in recirculating aquaculture systems in Europe: a perspective on environmental sustainability. Aquacultural Engineering 43, 83–93 [Google Scholar]
- Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E 2010. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302, 1–18 [Google Scholar]
- Metaxa E, Deviller G, Pagand P, Alliaume C, Casellas C, Blancheton JP 2006. High rate algal pond treatment for water reuse in a marine fish recirculation system. Water purification and fish health. Aquaculture 252, 92–101 [Google Scholar]
- Molénat G, Foulquié D, Autran P, Bouix J, Hubert D, Jacquin M, Bocquier F, Bibe B 2005. Pour un élevage ovin allaitant performant et durable sur parcours: un système expérimental sur le Causse du Larzac. INRA Productions Animales 18, 323–338 [Google Scholar]
- Moreau P, Ruiza L, Mabon F, Raimbault T, Durand P, Delaby L, Devienne S, Vertès F 2012. Reconciling technical, economic and environmental efficiency of farming systems in vulnerable areas. Agriculture Ecosystems & Environment 147, 89–99 [Google Scholar]
- Mounier L, Colson S, Roux M, Dubroeucq H, Boissy A, Ingrand S, Veissier I 2006. Links between specialization in the finishing of bulls, mixing, farmers’ attitudes towards animals and the production of finishing bulls: a survey on French farms. Animal Science 82, 561–568 [Google Scholar]
- Mulder IE, Schmidt B, Stokes CR, Lewis M, Bailey M, Aminov RI, Prosser JI, Gill BP, Pluske JR, Mayer CD, Musk CC, Kelly D 2009. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biology 7, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C 2004. Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231, 361–391 [Google Scholar]
- Nhan DK, Milstein A, Verdegem MCJ, Verreth JAV 2006. Food inputs, water quality and nutrient accumulation in integrated pond systems: a multivariate approach. Aquaculture 261, 160–173 [Google Scholar]
- Nolan T, Connolly J 1989. Mixed v. monograzing by steers and sheep. Animal Production 48, 519–533 [Google Scholar]
- Öckinger E, Smith HG 2007. Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. Journal of Applied Ecology 44, 50–59 [Google Scholar]
- Paolini V, De la Farge F, Prevot F, Dorchies P, Hoste H 2005. Effects of the repeated distribution of sainfoin hay on the resistance and the resilience of goats naturally infected with gastrointestinal nematodes. Veterinary Parasitology 127, 277–283 [DOI] [PubMed] [Google Scholar]
- Petersen SO, Sommer SG, Béline F, Burton C, Dach J, Dourmad JY, Leip A, Misselbrook T, Nicholson F, Poulsen HD, Provolo G, Sorensen P, Vinnerås B, Weiske A, Bernal MP, Böhm R, Juhász C, Mihelic R 2007. Recycling of livestock manure in a whole-farm perspective. Livestock Science 112, 180–191 [Google Scholar]
- Phong LT, van Dam AA, Udo HMJ, van Mensvoort MEF, Tri LQ, Steenstra FA, van der Zijpp AJ 2010. An agro-ecological evaluation of aquaculture integration into farming systems of the Mekong Delta. Agriculture, Ecosystems & Environment 138, 232–241 [Google Scholar]
- Phong LT, de Boer IJM, Udo HMJ 2011. Life cycle assessment of food production in integrated agriculture–aquaculture systems of the Mekong Delta. Livestock Science 139, 80–90 [Google Scholar]
- Plantureux S, Peeters A, McCracken D 2005. Biodiversity in intensive grasslands: effect of management, improvement and challenges. Grassland Science in Europe 10, 417–426 [Google Scholar]
- Prein M 2002. Integration of aquaculture into crop–animal systems in Asia. Agricultural Systems 71, 127–146 [Google Scholar]
- Puillet L, Martin O, Sauvant D, Tichit M 2010. An individual-based model simulating goat response variability and long term herd performance. Animal 4, 2084–2098 [DOI] [PubMed] [Google Scholar]
- RAD 2010. Résultats de l'observatoire technico-économique du RAD. Synthèse 2010. Les Essentiels du réseau agriculture durable, Cesson-Sévigné, France.
- Rahman MM, Verdegem MCJ, Nagelkerke LAJ, Wahab MA, Milstein A, Verreth JAJ 2006. Growth, production and food preference of rohu Labeo rohita (H.) in monoculture and in polyculture with common carp Cyprinus carpio (L.) under fed and non-fed ponds. Aquaculture 257, 359–372 [Google Scholar]
- Ren JS, Stenton-Dozey J, Plew DR, Fang J, Gall M 2012. An ecosystem model for optimizing production in integrated multitrophic aquaculture systems. Ecological Modelling 246, 34–46 [Google Scholar]
- Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL 2004. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. Journal of Immunology 172, 1118–1124 [DOI] [PubMed] [Google Scholar]
- Rigolot C, Espagnol S, Robin P, Hassouna M, Béline F, Paillat JM, Dourmad JY 2010. Modelling of manure production by pigs and NH3, N2O and CH4 emissions. Part II: effect of animal housing, manure storage and treatment practices. Animal 4, 1413–1424 [DOI] [PubMed] [Google Scholar]
- Rockström J, Karlberg L 2010. The quadruple squeeze: defining the safe operating space for freshwater use to achieve a triply green revolution in the anthropocene. Ambio 39, 257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez L, Lopez DJ, Preston TR, Peters K 2006. New Cocoyam (Xanthosoma sagittifolium) leaves as partial replacement for soya bean meal in sugar cane juice diets for growing pigs. Livestock Research for Rural Development 18(7) Article 91. [Google Scholar]
- Roque d'Orbcastel E, Blancheton JP, Aubin J 2009. Towards environmentally sustainable aquaculture: comparison between two trout farming systems using life cycle assessment. Aquaculture Engineering 40, 113–119 [Google Scholar]
- Sabatier R, Doyen L, Tichit M 2010. Modelling trade-offs between livestock grazing and wader conservation in a grassland agroecosystem. Ecological Modelling 221, 1292–1300 [Google Scholar]
- Sabatier R, Doyen L, Tichit M 2012. Action versus result-oriented schemes in a grassland agroecosystem: a dynamic modelling approach. PLoS One 7, e33257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, Lawrence R 2008. Aquaculture practices and potential human health risks: current knowledge and future priorities. Environment International 34, 1215–1226 [DOI] [PubMed] [Google Scholar]
- Sechi S, Salaris S, Scala A, Rupp R, Moreno C, Bishop SC, Casu S 2009. Estimation of (co)variance components of nematode parasites resistance and somatic cell count in dairy sheep. Italian Journal of Animal Science 8, 156–158 [Google Scholar]
- Selle PH, Cadogan DJ, Li X, Bryden WL 2010. Implications of sorghum in broiler chicken nutrition. Animal Feed Science and Technology 156, 57–74 [Google Scholar]
- Shankar KM, Mohan CV, Nandeesha MC 1998. Promotion of substrate based microbial biofilm in ponds – a low cost technology to boost fish production. Naga 1, 18–22 [Google Scholar]
- Silakinove N 2000. The physiological basis of adaptation in goats to harsh environments. Small Ruminant Research 35, 181–193 [Google Scholar]
- Sjödin NE 2007. Pollinator behavioural responses to grazing intensity. Biodiversity Conservation 16, 2103–2121 [Google Scholar]
- Soder KJ, Sanderson MA, Stack JL, Muller LD 2006. Intake and performance of lactating cows grazing diverse forage mixtures. Journal of Dairy Science 89, 2158–2167 [DOI] [PubMed] [Google Scholar]
- Steinfeld H, Wassenaar T 2007. The role of livestock production in carbon and nitrogen cycles. Annual Review of Environment and Resources 32, 271–294 [Google Scholar]
- Taberlet P, Coissac E, Pansu J, Pompanon F 2011. Conservation genetics of cattle, sheep and goats. Comptes Rendus Biologie 334, 247–254 [DOI] [PubMed] [Google Scholar]
- Tichit M, Hubert B, Doyen L, Genin D 2004. A viability model to assess the sustainability of mixed herds under climatic uncertainty. Animal Research 53, 405–417 [Google Scholar]
- Tichit M, Puillet L, Sabatier R, Teillard F 2011. Multicriteria performance and sustainability in livestock farming systems: functional diversity matters. Livestock Science 139, 161–171 [Google Scholar]
- Tripp R 2008. Agricultural change and low-input technology In Agricultural systems: agroecology and rural innovation for development (ed. S Snapp and B Pound), pp. 129–160 Elsevier Academic Press Publication, Amsterdam, The Netherlands [Google Scholar]
- Van de Nieuwegiessen PG, Olwo J, Khong S, Verreth JAJ, Schrama JW 2009. Effects of age and stocking density on the welfare of African catfish, Clarias gariepinus Burchell. Aquaculture 288, 69–75 [Google Scholar]
- Vanloqueren G, Baret P 2009. How agricultural research systems shape a technological regime that develops genetic engineering but locks out agroecological innovations. Research Policy 38, 971–983 [Google Scholar]
- Veen P, Jefferson R, de Smidt J, van der Straaten J 2009. Grasslands in Europe of high nature value. KNNV Uitgeverij, NHBS Distribution, Totnes, Devon, UK [Google Scholar]
- Villalba JJ, Provenza FD, Shaw R 2006. Sheep self-medicate when challenged with illness-inducing foods. Animal Behaviour 71, 1131–1139 [Google Scholar]
- Villalba JJ, Provenza FD, Hall JO, Lisonbee LD 2010. Selection of tannins by sheep in response to gastrointestinal nematode infection. Journal of Animal Science 88, 2189–2198 [DOI] [PubMed] [Google Scholar]
- Weiner J 2003. Ecology – the science of agriculture in the 21st century. Journal of Agricultural Science 141, 371–377 [Google Scholar]
- Wezel A, Soldat V 2009. A quantitative and qualitative historical analysis of the scientific discipline agroecology. International Journal of Agricultural Sustainability 7, 3–18 [Google Scholar]
- Wik TEI, Lindén BT, Wramner PI 2009. Integrated dynamic aquaculture and wastewater treatment modelling for recirculating aquaculture systems. Aquaculture 287, 361–370 [Google Scholar]
- Wirsenius S, Azat C, Berndes G 2010. How much land is needed for global food production under scenario of dietary changes and livestock productivity increases in 2030? Agricultural Systems 103, 621–638 [Google Scholar]
- Ziegler F 2006. Environmental life cycle assessment of seafood products from capture fisheries. PhD, Göteborg University, Sweden.