Abstract
Mammalian germ cells divide mitotically and form nests of associated cells just prior to entering meiosis. At least some nests contain germline cysts that arise by synchronous, incomplete mitotic divisions, but others may form by aggregation. To systematically investigate early murine germ cell development, we lineage marked the progeny of individual, newly arrived primordial germ cells in the E10.5 gonad. All the marked germ cells initially develop into clones containing two, four or eight cells, indicating cyst formation. Surprisingly, growing cysts in both sexes partially fragment into smaller cysts prior to completion and associate with cysts from unrelated progenitors. At the time divisions cease, female clones comprise five cysts on average that eventually give rise to about six primordial follicles. Male cyst cells break apart and probably become spermatogonial stem cells. Thus, cysts are invariant units of mouse germ cell development and cyst fragmentation provides insight into the amplification of spermatogonial stem cells and the origin of primordial follicles.
Keywords: Lineage labeling, Germ cell, Cyst
INTRODUCTION
Germ cell proliferation just prior to meiotic entry in diverse organisms follows a special program of synchronous, incomplete divisions that generates interconnected cell groups known as cysts (Gondos, 1973; Matova and Cooley, 2001). In Drosophila, cyst progenitors undergo four rounds of synchronous divisions without cytokinesis (de Cuevas et al., 1997). The resulting 16-cell cysts are completed just prior to meiotic entry and form the cellular scaffold for follicle formation or spermatogenesis. In mammals, however, the regular occurrence of a cyst stage has remained unclear. Murine primordial germ cells (PGCs) colonize the gonads at around embryonic day (E) 10.5, divide rapidly, associate with somatic cells and cluster into nests (McLaren, 1984; Tam and Snow, 1981) (Fig. 1A). Mitotic divisions cease at E14.5 and female germ cells enter meiosis while male germ cells arrest (Bowles et al., 2006; Koubova et al., 2006). About 1 week later, around the time of birth, female nests break apart, most germ cells undergo apoptosis and the remainder form primordial follicles. Many premeiotic murine germ cells divide synchronously and display intercellular bridges, indicating that cysts contribute to germ cell nests (Greenbaum et al., 2006; Greenbaum et al., 2009; Pepling and Spradling, 1998). However, studies of fetal germ cells in vitro and of mosaic ovaries in vivo have suggested that nests also form by cell aggregation (Bendel-Stenzel et al., 2000; Gomperts et al., 1994; Mork et al., 2012).
Fig. 1.
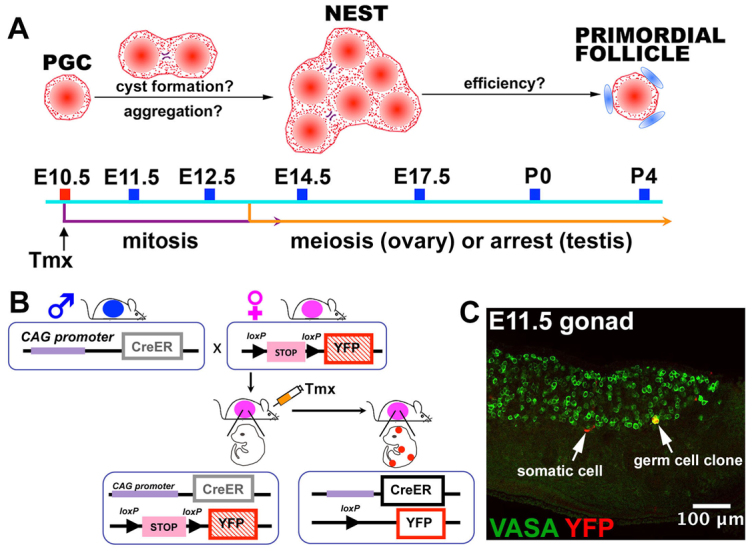
Lineage tracing of individual mouse fetal germ cells. (A,B) Timecourse of fetal germ cell development (A), showing mitotic germline cyst-forming divisions, meiosis (or arrest in testis), and primordial follicle formation. Tamoxifen (Tmx) was given to mice of the genotype illustrated in B on E10.5 at a level sufficient to label only about one primordial germ cell (PGC) per gonad. Germ cell clones were analyzed at E11.5, E12.5, E14.5, E17.5, P0, P4 and at 4 weeks. (C) An E11.5 mouse fetal gonad showing a single clone of lineage-labeled germ cells (yellow, arrow) recognized by expression of both YFP (pseudo-colored red) and VASA (pseudo-colored green). A labeled somatic cell (red, arrow) is also shown.
Cysts may be functionally important in several respects. They can serve as reservoirs of undifferentiated germ cells, including stem cells (Brawley and Matunis, 2004; Cheng et al., 2008; Kai and Spradling, 2004; Nakagawa et al., 2007; Nakagawa et al., 2010). Drosophila cysts are essential for fertility in both sexes and germ cells do not divide synchronously unless interconnected (de Cuevas et al., 1997). Each female cyst gives rise to a single oocyte as well as to sister cells that serve as nurse cells. Mitochondria and other organelles move through the intercellular bridges to form the Balbiani body or ‘mitochondrial cloud’ of the oocyte (Cox and Spradling, 2003). Less is known about the structure and function of murine cysts, but organelles also move through cyst bridges (Pepling and Spradling, 2001) and a Balbiani body is present in young primordial follicles (Pepling et al., 2007). Mice deficient for the intercellular bridge protein TEX14 contain nested germ cells that no longer seem to be interconnected, but only males are sterile (Greenbaum et al., 2006; Greenbaum et al., 2011).
Here, we characterize in detail the behavior of cysts during fetal development in both males and females. We show that all PGCs initially develop into cysts that undergo a novel process of fragmentation into smaller cysts prior to meiotic entry. The programmed breakdown of fetal male germline cysts provides a model for studying spermatogonial stem cell replenishment by cyst fragmentation in adults. The number of female cysts at the time of meiotic entry might determine the number of primordial follicles that are produced shortly after birth.
MATERIALS AND METHODS
Glossary
We use the following terms in a precise manner to aid the discussion of germ cell behavior.
Cluster or nest: germ cells that clump together morphologically; the interconnected nature of such cells cannot be determined from morphological observation.
Clone: germ cells that derive from a single lineage-marked germ cell, regardless of whether they remain clustered or connected by intercellular bridges.
Germline cyst: a cluster of interconnected germ cells generated by mitotic divisions with incomplete cytokinesis.
Intercellular bridges: the arrested cytokinesis furrows that join the cytoplasm of individual germ cells within a cyst.
Cyst fragments: groups of interconnected cells released from a germline cyst by breakage of one or more of its intercellular bridges; unless single, the cells in each cyst fragment will still have the properties of a cyst.
Germ cell aggregate: two or more germ cells that appear clustered together in a nest but which did not form entirely by incomplete cytokinesis so that some cells are not cytoplasmically joined to others.
Mice and single germ cell lineage labeling
CAG-cre/Esr1 mice [B6.Cg-Tg(CAG-cre/Esr1*)5Amc/J] (Hayashi and McMahon, 2002) and R26R-EYFP mice [B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J] (Srinivas et al., 2001) were acquired from the Jackson Laboratory. All mice used in the present analysis are of mixed genetic background from the cross of these two strains. Mice were genotyped according to protocols from the JAX Mice database. To obtain fetuses for lineage marking, adult female R26R-EYFP mice were mated with male CAG-cre/Esr1 mice (Fig. 1), and midday on the day a vaginal plug appeared was designated as E0.5. Birth usually occurred between E19 and E20. The day of birth was designated postnatal day (P) 0. Tamoxifen was dissolved in corn oil (Sigma) and a single dose was injected intraperitoneally at 0.2 mg per 40 g body weight into pregnant female R26R-EYFP mice at E10.5 or E11.5. All procedures were approved by IACUC.
Immunostaining and microscopy
Fetal and neonatal gonads were dissected under a dissecting microscope in PBS and fixed immediately in cold 4% paraformaldehyde. Gonads were then incubated with primary antibodies overnight. After incubation on the next day with fluorescein-conjugated secondary antibodies (Dy Light 488 or 568; Jackson ImmunoResearch Laboratories), gonads were washed with PBST (PBS with 0.1% Tween 20) and stained with DAPI to visualize nuclei. Gonads were mounted on slides with mounting medium (Vector Labs), and analyzed using confocal microscopy (Leica SP5). Serial images of each germ cell clone were acquired. Three-dimensional models (supplementary material Movie 1) were generated by Imaris software (Bitplane). Germ cell numbers quantitated in each ovary were determined as described previously (Pepling and Spradling, 2001). To analyze the lineage-labeled oocytes in 4-week-old mice, fixed and frozen whole ovaries were serially sectioned. Following staining for YFP and VASA, the number of lineage-labeled germ cells of each ovary was determined by examining every section of the entire ovary. Primary antibodies used: VASA (1:400; Abcam), GFP (1:1000; Aves Labs), SCP3 (1:100; Abcam), phospho-histone H3 (1:100; Cell Signaling Technology) and cleaved PARP (1:200; Cell Signaling Technology).
Assessment of cyst structure
For each clone, a subgroup of adjacent YFP+ cells was scored as a separate cyst if its constituent cells were not connected to other YFP+ cells, even by a thin cytoplasmic bridge (e.g. Fig. 3I), as determined from three-dimensional reconstructions. For purposes of recording (supplementary material Tables S1-S13) single cells were listed as cysts of size n=1. Whether YFP will pass through cyst intercellular bridges cannot easily be predicted at any given time because movement through these structures may be blocked or regulated. We deduced that YFP did not move through female fetal cysts because the average clone size at E14.5 was 30 when labeling was carried out at E10.5 but only 17 when labeling was carried out at E11.5. By contrast, the corresponding values for E14.5 testis cysts were 40 and 38, suggesting that these cysts do allow YFP movement.
Fig. 3.
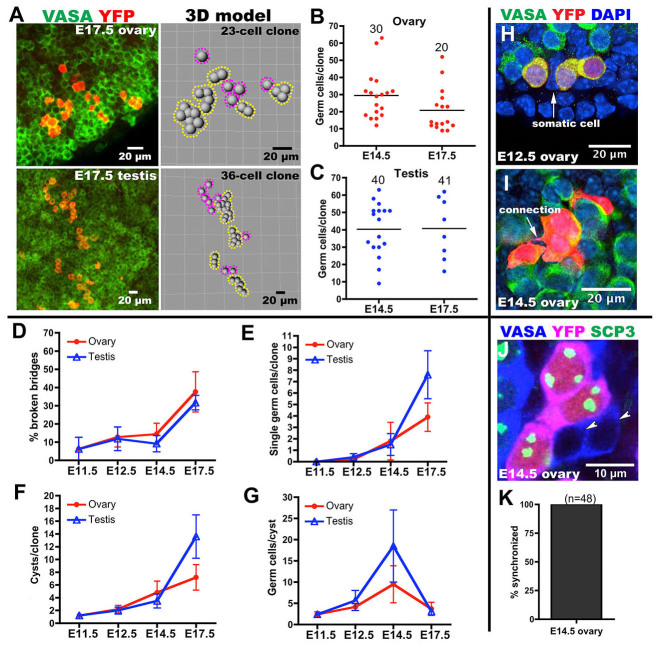
Germline cyst fragmentation. (A) Images (left) and models (right) of individual germ cell clones from lineage-labeled mouse E17.5 fetal ovaries (above) and testes (beneath). Yellow dashed lines surround cysts and purple dashed lines denote single cells. (B,C) Germ cell clone sizes analyzed in ovaries (B) or testes (C) from E14.5 or E17.5. The average number of germ cells per clone size is shown and is indicated by a bar. (D-G) Percentage of broken bridges per clone (D), single germ cells per clone (E), cysts per clone (F) and germ cells per cyst (G) as a function of time for ovary or testis. Error bars indicate s.d. (H) A somatic cell (arrow) separates two subsets of clonal germ cells. (I) A stretched cytoplasmic bridge (arrow) connects two subsets of clonal germ cells. (J) Meiotic synchrony of clonally related germ cells. A nest of E14.5 ovarian germ cells is shown containing three cells that are in meiosis, as shown by SCP3 staining (green), and that are clonally related (YFP, red); two clonally unrelated cells from the nest (arrowheads) have not entered meiosis. (K) Quantification of meiotic synchrony and lineage relationships in 48 germ cell nests analyzed as in J. Clonally related cells were always synchronized with respect to SCP3 expression.
Assessment of bridge breakage
The frequency of intercellular bridges that had broken in any germ cell clone of n cells (n>1) was determined by assuming that the cells originated as a single linear cyst. Then: fraction of broken bridges=(number of derived cysts-1)/(n-1).
Comparing the cortex and medullar regions
To compare germline cyst development in the cortex and medullar regions of the ovary, where germ cells have been reported to develop on different schedules (Byskov et al., 1997), we scored the regional location of all clones, as shown in supplementary material Fig. S2. For ovaries isolated between E11.5 and E14.5, the ovary was divided into two equal regions - a surface region and a mesonephros-proximal region - along the axis separating the surface and mesonephros. Similarly, for ovaries between E17.5 and P4, the cortical and medullar regions were defined as encompassing the distal or proximal zones between the ovarian surface and the hilum.
RESULTS
Analyzing PGC development by single germ cell lineage tagging
Deciphering the role that fetal germline cysts play in specifying the number of primordial follicles and in expanding male stem cell precursors requires the ability to mark germ cell lineages. We developed a particularly informative method, termed single germ cell lineage tagging, that allows the lineage of individual germ cells to be followed (see Materials and methods). The method utilizes low-level Tamoxifen (Tmx)-mediated induction of Cre recombinase activity to activate heritable R26R-YFP expression in just a few isolated, random cells within all tissues (Hayashi and McMahon, 2002) (Fig. 1B).
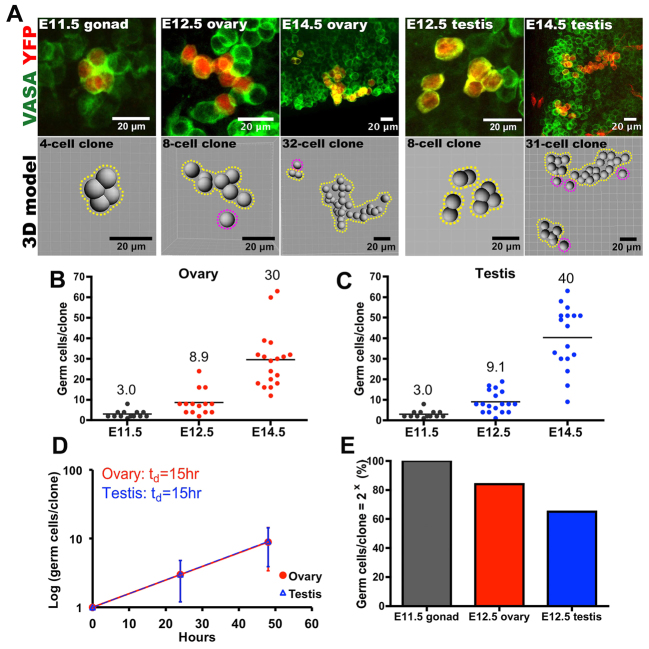
To test the system in fetal gonads, we injected pregnant mice of appropriate genotype at E10.5 with Tmx and determined by whole-mount confocal microscopy that only zero or one clone of labeled germ cells was present in most gonads 1 day later (Fig. 1C; supplementary material Fig. S1G). E10.5 is an ideal time for marking germ cells because 85% are single and only 13% are interconnected in groups of two at this stage (Pepling and Spradling, 1998). Somatic gonadal cells were also labeled at low frequency (Fig. 1C), but germ cells were readily distinguished based on their expression of the germ cell-specific mouse VASA homolog (also known as DDX4 or MVH). No YFP-labeled cells were evident in the absence of Tmx injection or the creER transgene (supplementary material Fig. S1A-C). The levels of Tmx used did not significantly perturb total germ cell number or impact sex determination (supplementary material Fig. S1D-F). The number of clones remained constant on subsequent days in the absence of further Tmx (supplementary material Fig. S1I), but the number of cells within each clone increased between E11.5 and E14.5 (Fig. 2A-D), but not thereafter, as expected. Clone size in both sexes increased exponentially, with a doubling time of 15 hours (Fig. 2B-D), in agreement with germ cell doubling times of 16 hours as measured from counts of all germ cells (Tam and Snow, 1981). Overall, we fully characterized the lineages of 104 female and 60 male embryonic germ cells (supplementary material Tables S1-S13), determined their regional location within the ovary, and reconstructed the three-dimensional structure and interconnections of the marked cells using Imaris software (Fig. 2A; supplementary material Movie 1).
Fig. 2.
Germline cyst formation documented by lineage tracing. (A) Images (above) and models (beneath) of individual germ cell clones at various stages (E11.5-E14.5) after labeling at E10.5. In the three-dimensional models, uniform spheres were used to position the germ cells. Cysts, i.e. groups of clonally labeled germ cells that remain in contact, are enclosed by yellow dashed lines. Single germ cells that have broken free are indicated by purple dashed lines. (B,C) The size of each germ cell clone in ovaries (B) or testes (C) during cyst formation (E11.5-E14.5). The average clone size is shown and is indicated by a bar. (D) The growth rate of labeled ovarian and testis germ cell clones, indicating a doubling time of 15 hours. Error bars indicate s.d. (E) The percentage of germ cell clones of a size that corresponds to a power of two in E11.5 and E12.5 fetal gonads.
All PGCs develop into cysts
Germline cyst formation can be detected in principle by the shape of the special endoplasmic reticulum-like organelle known as the fusome, by the formation of permanent intercellular bridges, or by mitotic synchrony. A stable fusome has not been detected in mouse germ cells (Pepling and Spradling, 1998). In Drosophila and mice, confocal microscopy of gonads stained to highlight bridges has proved insufficient to reliably decipher cyst structure (de Cuevas et al., 1997; Mork et al., 2012). However, individual E10.5 germ cells that develop into cysts will form clones of a size (number of cells) that is a power of two due to mitotic synchrony, whereas the clone size of individual germ cells undergoing complete divisions will not be so constrained. Following labeling at E10.5, 100% of germ cell clones contained one, two, four or eight labeled cells at E11.5 (supplementary material Table S1), whereas at E12.5 65-85% of clone sizes corresponded to powers of two (mostly four, eight and 16 cells), indicating ongoing cyst production (Fig. 2E; supplementary material Tables S2, S9). Injecting Tmx at E11.5 confirmed these findings (supplementary material Tables S8, S13). We concluded that all germ cells at E10.5 initiate development as synchronously dividing cysts, but that this synchrony soon starts to become disrupted.
Germline cysts partially fragment and separate
The structure of the labeled germ cell clones deduced from three-dimensional confocal analyses revealed a likely explanation for their progressive loss of synchrony (Fig. 2A). Initially, clones formed tightly organized clusters similar to the cysts described in other species (Fig. 2A) (Pepling et al., 2007). Surprisingly, however, between E11.5 and E14.5 cysts underwent partial fragmentation into smaller cysts, the component cells of which remained interconnected. Cells derived from a single progenitor were counted as separate cysts when the two groups became physically separated without any detectable connection (Fig. 2A; supplementary material Tables S2, S3, S9, S10).
The cycles of cells in different derivative cysts should drift apart, even though the cells within each cyst should remain synchronized. By E14.5, marked female E10.5 germ cells formed an average of 30±14 cells in 4.8±3.5 cysts, whereas male cells formed 40±15 cells in 3.6±2.6 cysts. In the ovary, germ cell clones in the surface/cortex region did not show significant regional differences in size or cyst number from clones in the mesonephros/medullar region (supplementary material Fig. S2). Our clone size measurements probably overestimate the variation in germ cell production from individual mature E10.5 PGCs. A fraction of the germ cells marked at E10.5 are still immature PGCs that will divide once more prior to forming cysts, thereby labeling two adjacent cysts (clones of 63 and 60 cells in supplementary material Table S3), whereas other marked germ cells are already part of two-cell cysts at E10.5 and will label only half a cyst (clones of 12-16 cells in supplementary material Table S3; see Materials and methods).
By E14.5 mitotic divisions have ceased and all female germ cells have entered meiosis within synchronous local groups (supplementary material Fig. S4). At this time, ∼12% of bridges have broken to release smaller cysts. By E17.5, however, the fraction of broken bridges has increased to 40% (Fig. 3D). Fragmentation might occur because cytokinesis is finally completed between two component cells, or as a result of external mechanical forces exerted by somatic cells that encircle many bridges (Pepling and Spradling, 2001). Fragmenting cysts sometimes showed evidence of mechanical stress (Fig. 3I) and two derivative cysts were often separated by a somatic cell (Fig. 3H). Germline cysts within adult testes undergoing fragmentation show similar structures (Nakagawa et al., 2007; Nakagawa et al., 2010).
Germ cells within derived cysts remain developmentally synchronized
Despite fragmentation, cyst cells that remained interconnected at E14.5 showed strong evidence of developmental synchrony as they entered meiosis. After staining mouse gonads containing YFP-labeled germ cell clones for the meiotic marker synaptonemal complex protein 3 (SCP3; or SYCP3 - Mouse Genome Informatics) we identified many germ cell nests whose constituent cells differed in meiotic status (Fig. 3J). In every case, all clonally related cells within the nest were in the same meiotic state (Fig. 3K). Even single germ cells in E17.5 ovaries were in the same meiotic state as other clonally related germ cells. The precise synchrony of sister cells within a cyst was not always perfect with respect to mitosis however (supplementary material Fig. S3), suggesting that some bridges functionally close before the cells separate.
Cyst fragmentation and germ cell apoptosis are independent
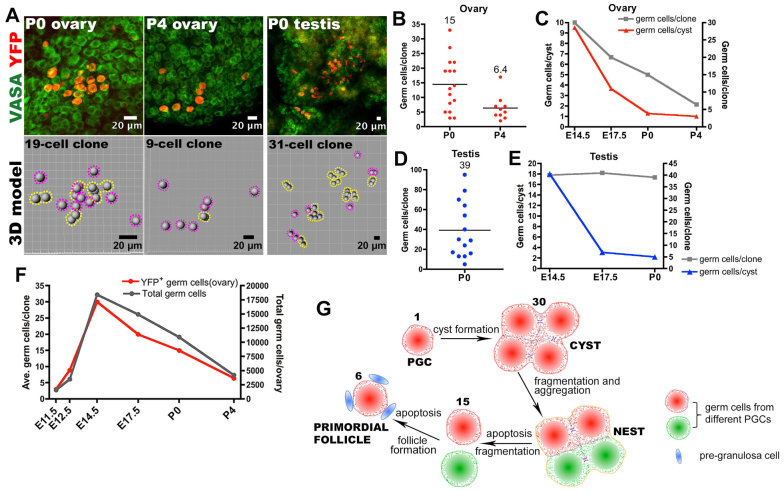
Previously, germ cell apoptosis (Coucouvanis et al., 1997) was thought to contribute heavily to the final breakdown of cysts during primordial follicle formation (Pepling and Spradling, 2001). Our results from analyzing cyst structure indicate that cyst breakdown and apoptosis do not precisely coincide. Male and female cysts both fragment extensively (Fig. 3D-G); however, male germ cells underwent very little apoptosis (Fig. 3B,C). In E12.5 mouse fetal ovaries, only 0.2% of germ cells expressed the apoptotic marker cleaved PARP, too low a value for apoptosis to account for the observed loss of 12.8% of the intercellular bridges. Female cysts broke down almost completely to single cells by P0, but female germ cells did not complete apoptosis until 4 days later (Fig. 4B,C). Thus, apoptosis is unlikely to be the major cause of cyst breakdown leading to primordial follicle formation.
Fig. 4.
Primordial follicle formation elucidated by lineage tracing. (A) Images (above) and models (beneath) of individual germ cell clones at P0 and in P4 ovaries near the end of primordial follicle formation. Yellow dashed lines encircle cysts and purple dashed lines denote single germ cells. (B) Female germ cell clone size at P0 and P4 plotted as in Fig. 3. (C) Comparing germ cells per cyst with germ cells per clone shows that cyst fragmentation precedes the completion of apoptosis. (D) Male germ cell clone at P0 plotted as in Fig. 3. (E) Stability of male germ cells (gray) but instability of cysts (blue) between E14.5 and P0. (F) The behavior of marked (YFP+) ovarian germ cell clones parallels changes in total germ cells. (G) Model of mouse PGC development. Each PGC (red) initially forms a cyst, which fragments into smaller cysts and aggregates with cysts from different PGCs (green) to form a nest. Further fragmentation (in females, as shown) and apoptosis leads to primordial follicle formation. The average number of germ cells derived from an initial PGC at each step of this process is indicated. Male fetal germ cell development (not shown) is similar, except that there is little apoptosis and cysts break down almost to single cells without entering meiosis.
Formation of oocytes or single germ cells from female or male cysts
Interestingly, in females an average of 6.4 primordial follicles, each containing a single oocyte, was generated from single E10.5 germ cells. This is similar to the average number of cysts, 4.8, that had arisen by E14.5 when germ cell division ceases. The mean efficiency of primordial follicle production can be calculated as the average yield of primordial follicles per clone divided by the average clone size at E14.5: 6.4/30=21%. In males, the initial cysts continued to break down and reached an average size of only 2.2 cells per cyst at P0 (Fig. 4D,E). Synchronous divisions had restarted by this time, so male germ cell cysts might break down nearly to single cells between E17.5 and P0.
We verified that lineage-tagged germ cells are representative of all germ cells. Counts of total germ cells followed a very similar profile to tallies of tagged germ cells (Fig. 4F). Thus, our results (Fig. 4G) can be safely extrapolated to germ cells within the entire fetal testis and ovary. This is not surprising because we sampled a very substantial fraction of the total germ cell population: 164 cells from an estimated total of ∼500 germ cells in the E10.5 gonad.
DISCUSSION
Mouse fetal germ cells invariably form interconnected cysts that partially fragment
Our studies demonstrate that germline cysts constitute a fundamental stage in the development of both male and female mouse gametes. All E10.5 germ cells behave as a uniform population and initiate cyst formation. However, unlike Drosophila cysts, mouse cyst cells do not remain interconnected and synchronous throughout the entire period of mitotic expansion. Instead, by E14.5 they fragment into multiple smaller cysts of variable size, the component germ cells of which, in females, enter meiosis synchronously.
Cyst formation and partial fragmentation can help explain previous observations regarding mouse female germline development. Synchronous germ cell mitoses occur between E10.5 and E14.5, but mitotic clusters corresponding to powers of two only predominate initially (Pepling and Spradling, 1998). Even though cell cycles remain synchronized within the cyst fragments, the random sites of bridge breakage will ensure that cyst number no longer corresponds to a power of two. Mixing and adherence of cysts derived from separate progenitors confirms a role for aggregation in premeiotic germ cell development (Mork et al., 2012), but our experiments rule out the possibility that nests form de novo by normal cell division and aggregation (Bendel-Stenzel et al., 2000; Gomperts et al., 1994; Mork et al., 2012).
Cyst fragmentation during premeiotic germline development, similar to that which we observed here, might occur in diverse species, as cysts whose component cells partially lose synchrony and diverge in number from a power of two have previously been described in multiple species (Büning, 1994). Cyst fragmentation and mixing might serve to diversify cysts of different sizes spatially throughout the gonad.
Fetal male cysts provide a model for studying stem cell production and regeneration
Our results provide new perspectives on the nature and origin of spermatogonial stem cells (SSCs) within adult testes. In adult male mice, cysts, referred to as undifferentiated spermatogonia, develop downstream from SSCs (Chiarini-Garcia and Russell, 2001) and their interconnecting bridges break at low frequency (Klein et al., 2010; Nakagawa et al., 2010). Under conditions of regeneration in flies and mice (Brawley and Matunis, 2004; Nakagawa et al., 2007; Sheng and Matunis, 2011) some male cyst cells break apart completely and replenish stem cell function. Our studies show that fetal male germ cells do not simply arrest and await hormonal signals for meiotic entry, but programmatically follow the cyst breakdown pathway to form the initial population of stem cells found shortly after birth (Yoshida et al., 2006). Thus, a regeneration process that is sporadic and hard to study in adult testes might be more easily analyzed during fetal stages.
Our results add a note of caution to the view that SSCs comprise a heterogeneous, stochastic population in a shifting equilibrium with respect to cyst structure (Nakagawa et al., 2010; Yoshida, 2012). Despite the fact that fragmentation sites appear to be stochastic, fetal germ cells as a whole develop in a highly regular manner from single progenitors to large cysts, cyst fragments, and back down nearly to individual cells under strict temporal control.
Cyst fragmentation may control oocyte formation
Our studies of female mouse germ cell development suggest that a fundamental relationship exists between cyst number at the time of meiotic entry and oocyte production. A large proportion of fetal germ cells in diverse organisms die by apoptosis prior to forming primordial follicles (Matova and Cooley, 2001), but the reasons for this have remained unclear. In the mouse, we accurately determined that 79% of all germ cells die before forming follicles. In Drosophila, all cysts contain 16 cells that form exactly one oocyte; hence, the remaining 15 cells (94%), which serve as nurse cells, eventually undergo apoptosis. It is unlikely that germ cells die primarily to maintain a balance of germline and somatic cells (Gondos, 1973). Such balancing would be likely to involve the apoptosis of entire cysts, as occurs in Drosophila if insufficient somatic cells are available locally to form a follicle (Drummond-Barbosa and Spradling, 2001). Instead, we found that at least some daughters of every E10.5 PGC gave rise to oocytes within primordial follicles (supplementary material Table S6).
The approximate correspondence between the number of cysts (4.8) at E14.5 and the number of primordial follicles (6.4) per progenitor, suggests that each cyst present at the time of meiotic entry generates a single oocyte, like a Drosophila cyst. Indeed, Drosophila cysts of abnormal sizes still form single oocytes if their interconnections remain functional (Yue and Spradling, 1992; Lilly et al., 2000). The reason this occurs is likely to be due to the asymmetric nature of the cyst-forming divisions. At least in Drosophila, a polarized microtubule cytoskeleton is produced from spindle remnants following each division, the minus ‘downstream’ ends of which point to the original cell. The oocyte normally forms from the most downstream cell, which is the initial cell in 16-cell Drosophila cysts. However, any fragment of a cyst formed in this manner will also have exactly one cell that is still downstream of the others. Thus, whatever the extent or type of cyst fragmentation that occurs prior to meiotic entry, each remaining cyst would contain one downstream cell that might develop into an oocyte and be preserved in a primordial follicle. Proteins that stabilize the meiotic cycle are proposed to be transported in a minus-end-directed fashion into the downstream cell to account for the production of a single final oocyte. The hypothesis that each mouse oocyte derives from a different E14.5 cyst must be tested in the future by even more detailed cell lineage tracking than was accomplished here.
Cysts have several postulated functions that might explain why it would be advantageous to derive oocytes in this manner. The most significant potential role would be to improve oocyte quality. Cyst cells destined to die (’nurse-like’ cells) might transport functional mitochondria and other materials through the bridges into the downstream cell, thereby generating oogonial Balbiani bodies (Cox and Spradling, 2003; Pepling et al., 2007). Simultaneously, oogonia would offload damaged components and parasites into the nurse cells, much as damaged molecules are transferred through the bud neck during sporulation in yeast (Ünal and Amon, 2011). Balbiani body formation is not essential for fertility (Cox and Spradling, 2006; Greenbaum et al., 2009) but whether it enhances fitness has not been tested. Indeed, one prediction of this model is that the quality of oocytes in primordial follicles may vary, depending on the size of the cyst from which they derive and hence their initial organelle content. Intercellular bridges and synchronous mitoses are observed in human germ cells at corresponding stages in their development (Gondos, 1973). Consequently, gaining a better understanding of how germline cysts contribute to specifying oocytes and to enhancing their quality should be an important focus of future research.
Supplementary Material
Acknowledgments
We thank Dr Eugenia Dikovskaia and the Carnegie mouse facility staff for invaluable advice and assistance; Dr Chen-Ming Fan and Dr Christoph Lepper for advice on mouse husbandry and lineage labeling techniques; and Dr Alex Bortvin, Dr Chen-Ming Fan, Dr Joseph Gall and members of the A.C.S. laboratory for comments on the manuscript.
Footnotes
Funding
This work was funded by the Howard Hughes Medical Institute. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.093864/-/DC1
References
- Bendel-Stenzel M. R., Gomperts M., Anderson R., Heasman J., Wylie C. (2000). The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech. Dev. 91, 143–152 [DOI] [PubMed] [Google Scholar]
- Bowles J., Knight D., Smith C., Wilhelm D., Richman J., Mamiya S., Yashiro K., Chawengsaksophak K., Wilson M. J., Rossant J., et al. (2006). Retinoid signaling determines germ cell fate in mice. Science 312, 596–600 [DOI] [PubMed] [Google Scholar]
- Brawley C., Matunis E. (2004). Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304, 1331–1334 [DOI] [PubMed] [Google Scholar]
- Büning J. (1994). The Insect Ovary. London: Chapman and Hall; [Google Scholar]
- Byskov A. G., Guoliang X., Andersen C. Y. (1997). The cortex-medulla oocyte growth pattern is organized during fetal life: an in-vitro study of the mouse ovary. Mol. Hum. Reprod. 3, 795–800 [DOI] [PubMed] [Google Scholar]
- Cheng J., Türkel N., Hemati N., Fuller M. T., Hunt A. J., Yamashita Y. M. (2008). Centrosome misorientation reduces stem cell division during ageing. Nature 456, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini-Garcia H., Russell L. D. (2001). High-resolution light microscopic characterization of mouse spermatogonia. Biol. Reprod. 65, 1170–1178 [DOI] [PubMed] [Google Scholar]
- Coucouvanis E. C., Sherwood S. W., Carswell-Crumpton C., Spack E. G., Jones P. P. (1997). Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp. Cell Res. 209, 238–247 [DOI] [PubMed] [Google Scholar]
- Cox R. T., Spradling A. C. (2003). A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development 130, 1579–1590 [DOI] [PubMed] [Google Scholar]
- Cox R. T., Spradling A. C. (2006). Milton controls the early acquisition of mitochondria by Drosophila oocytes. Development 133, 3371–3377 [DOI] [PubMed] [Google Scholar]
- de Cuevas M., Lilly M. A., Spradling A. C. (1997). Germline cyst formation in Drosophila. Annu. Rev. Genet. 31, 405–428 [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D., Spradling A. C. (2001). Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231, 265–278 [DOI] [PubMed] [Google Scholar]
- Gomperts M., Garcia-Castro M., Wylie C., Heasman J. (1994). Interactions between primordial germ cells play a role in their migration in mouse embryos. Development 120, 135–141 [DOI] [PubMed] [Google Scholar]
- Gondos B. (1973). Intercellular bridges and mammalian germ cell differentiation. Differentiation 1, 177–182 [Google Scholar]
- Greenbaum M. P., Yan W., Wu M. H., Lin Y. N., Agno J. E., Sharma M., Braun R. E., Rajkovic A., Matzuk M. M. (2006). TEX14 is essential for intercellular bridges and fertility in male mice. Proc. Natl. Acad. Sci. USA 103, 4982–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum M. P., Iwamori N., Agno J. E., Matzuk M. M. (2009). Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol. Reprod. 80, 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum M. P., Iwamori T., Buchold G. M., Matzuk M. M. (2011). Germ cell intercellular bridges. Cold Spring Harb. Perspect. Biol. 3, a005850 doi:10.1101/cshperspect.a005850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., McMahon A. P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 [DOI] [PubMed] [Google Scholar]
- Kai T., Spradling A. C. (2004). Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 428, 564–569 [DOI] [PubMed] [Google Scholar]
- Klein A. M., Nakagawa T., Ichikawa R., Yoshida S., Simons B. D. (2010). Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7, 214–224 [DOI] [PubMed] [Google Scholar]
- Koubova J., Menke D. B., Zhou Q., Capel B., Griswold M. D., Page D. C. (2006). Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 103, 2474–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly M. A., de Cuevas M., Spradling A. C. (2000). Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev. Biol. 218, 53–63 [DOI] [PubMed] [Google Scholar]
- Matova N., Cooley L. (2001). Comparative aspects of animal oogenesis. Dev. Biol. 231, 291–320 [DOI] [PubMed] [Google Scholar]
- McLaren A. (1984). Meiosis and differentiation of mouse germ cells. Symp. Soc. Exp. Biol. 38, 7–23 [PubMed] [Google Scholar]
- Mork L., Tang H., Batchvarov I., Capel B. (2012). Mouse germ cell clusters form by aggregation as well as clonal divisions. Mech. Dev. 128, 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Nabeshima Y., Yoshida S. (2007). Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell 12, 195–206 [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Sharma M., Nabeshima Y., Braun R. E., Yoshida S. (2010). Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328, 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling M. E., Spradling A. C. (1998). Female mouse germ cells form synchronously dividing cysts. Development 125, 3323–3328 [DOI] [PubMed] [Google Scholar]
- Pepling M. E., Spradling A. C. (2001). Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 [DOI] [PubMed] [Google Scholar]
- Pepling M. E., Wilhelm J. E., O’Hara A. L., Gephardt G. W., Spradling A. C. (2007). Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc. Natl. Acad. Sci. USA 104, 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X. R., Matunis E. (2011). Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development 138, 3367–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam P. P., Snow M. H. (1981). Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J. Embryol. Exp. Morphol. 64, 133–147 [PubMed] [Google Scholar]
- Ünal E., Amon A. (2011). Gamete formation resets the aging clock in yeast. Cold Spring Harb. Symp. Quant. Biol. 76, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. (2012). Elucidating the identity and behavior of spermatogenic stem cells in the mouse testis. Reproduction 144, 293–302 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Sukeno M., Nakagawa T., Ohbo K., Nagamatsu G., Suda T., Nabeshima Y. (2006). The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133, 1495–1505 [DOI] [PubMed] [Google Scholar]
- Yue L., Spradling A. C. (1992). hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 6, 2443–2454 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.