Abstract
The discovery of the myofibroblast has opened new perspectives for the comprehension of the biological mechanisms involved in wound healing and fibrotic diseases. In recent years, many advances have been made in understanding important aspects of myofibroblast basic biological characteristics. This review summarizes such advances in several fields, such as the following: i) force production by the myofibroblast and mechanisms of connective tissue remodeling; ii) factors controlling the expression of α-smooth muscle actin, the most used marker of myofibroblastic phenotype and, more important, involved in force generation by the myofibroblast; and iii) factors affecting genesis of the myofibroblast and its differentiation from precursor cells, in particular epigenetic factors, such as DNA methylation, microRNAs, and histone modification. We also review the origin and the specific features of the myofibroblast in diverse fibrotic lesions, such as systemic sclerosis; kidney, liver, and lung fibrosis; and the stromal reaction to certain epithelial tumors. Finally, we summarize the emerging strategies for influencing myofibroblast behavior in vitro and in vivo, with the ultimate goal of an effective therapeutic approach for myofibroblast-dependent diseases.
Myofibroblasts regulate connective tissue remodeling by combining the extracellular matrix (ECM)–synthesizing features of fibroblasts with cytoskeletal characteristics of contractile smooth muscle cells. Since their first description in granulation tissue 40 years ago, remarkable progress has been made in understanding myofibroblast biological characteristics and their participation in physiological and pathological situations.1 It is well established that myofibroblasts have multiple origins, contribute importantly to connective tissue remodeling by exerting traction forces and synthesizing ECM components, regress and disappear by apoptosis on wound epithelialization, and may persist in fibrotic situations and cause organ dysfunction.1 Since our last review on the subject in this journal,1 many new findings have emerged to advance our understanding of myofibroblast biological features, but many questions remain unanswered. Unresolved questions include the following: i) what is the progenitor or precursor cell for the myofibroblast, ii) is there a specific myofibroblast marker, iii) what regulates myofibroblast contractile activity, and iv) what is the basis for myofibroblast persistence in chronic or progressive fibrosis? Recent rapid progress in microRNA (miRNA) and epigenetics research2 has furnished new tools and concepts for assessing molecular regulation of myofibroblast differentiation and perpetuation of the myofibroblast phenotype, as potentially mediated by epigenetic mechanisms. The ultimate aim is to suggest therapeutic strategies for influencing the widespread pathological situations that depend on this enigmatic cell.
Myofibroblast Basics
During normal tissue repair, such as skin wound healing, controlled and transient activation of myofibroblasts contributes to restoration of tissue integrity by forming a mechanically sound scar.1 For example, scars stabilize the heart muscle after myocardial infarction and tendon, bone, and cartilage after fracture or rupture.3 However, when myofibroblast activities become excessive and persist, beneficial tissue repair turns into the detrimental tissue deformities characteristic of organ fibrosis. In this review, we will discuss in more detail fibrosis of the skin, lungs, liver, and kidney. In addition to these organs, myofibroblasts play a substantial role promoting heart fibrosis and vascular remodeling, but because of space limitation, these topics are not covered herein, and we refer the reader to recent reviews of this rapidly growing body of literature.4,5 Another fibrotic condition is the desmoplastic or stromal reaction to epithelial tumors, during which myofibroblasts contribute to the mediator and mechanical environment that promotes tumor progression.6 We will discuss the similarities of the tumor-associated fibroblasts and myofibroblasts with those present in other fibrotic lesions.
Specific Aspects of Myofibroblast Contraction
High contractile activity of myofibroblasts is necessary for generating tissue contractures. In addition to the expression of α-smooth muscle actin (α-SMA; gene ACTA) in stress fibers, which promotes stronger force generation compared with other actin isoforms in fibroblastic cells, myofibroblasts appear to use specific modes of contraction.3,7 In contrast to the reversible and comparably short-lived contraction of striated and smooth muscles, myofibroblast contractile activity, together with ECM synthesis and degradation, leads to connective tissue remodeling, followed by irreversible and long contractures in a process that can span weeks, months, or even years.7 It is still unknown how myofibroblasts stabilize contractions that occur at the cellular or subcellular level to counteract the stress present in a tissue undergoing remodeling. Recent in vitro studies8 indicate that myofibroblasts use a lockstep or ratchet mechanism of cyclic and incremental contractile events. This mechanism consists of strong (micronewtons) and far-ranging (tens of micrometers) contractions mediated by RhoA/Rho-associated kinase and weak (approximately 100 pN) and short-ranging (approximately 0.4 μm) cyclic contractions promoted by changes in intracellular calcium concentrations.8 The model proposes that strong isometric contraction generates slack in the myofibroblast-associated fibrous and stressed collagen. Such tension-released fibrils are then straightened by the weak, but repeated, subcellular contractile events. By local ECM remodeling and/or deposition of new ECM, the shortened and repeatedly stressed collagen fibrils stabilize the status quo of the ECM, and a new myofibroblast contraction cycle can begin. It is intriguing that the level of stress (ie, the resistance of collagen fibers to pulling) may determine which mechanism of contraction will be engaged.
Characteristic Features of the Myofibroblast
Although there is considerable evidence to indicate the fibroblastic origin of myofibroblasts, other cell types, mostly from mesenchymal lineages, have been suggested as alternative or additional precursors.1 Because different subsets of myofibroblast precursors are recruited in different organs, we will further address the question of the myofibroblast origin later. To target the fibrotic activity of myofibroblasts, irrespective of their provenance, it is important to define common denominators, a need that has stimulated the search for specific molecular markers. The most widely used molecular marker of the differentiated myofibroblast in research and clinical diagnostics is the de novo expression of α- SMA.1 The convenience of a unique molecular marker has fostered the misconception that a myofibroblast must express α-SMA to be a myofibroblast. However, the most important defining feature of myofibroblasts is the de novo development of in vivo stress fibers and contractile force.3,7 Several novel markers and modulators of the myofibroblast phenotype have been suggested. These include endosialin in tumor-associated fibroblasts,9 P311 in hypertrophic scar myofibroblasts,10 integrin α11β1 in fibroblasts from various sources,11 osteopontin in cardiac or dermal fibroblasts,12 and periostin13,14 (Table 115–77). It remains to be confirmed whether these markers are tissue and/or condition specific or whether they are useful in identifying myofibroblasts in more general terms. To our knowledge, none of the other markers described over the years is unique to the myofibroblast.
Table 1.
Myofibroblast-Modulating Factors
| Myofibroblast-inducing factors | Myofibroblast-suppressing factors |
|---|---|
| Transcription-Regulating Factors | |
| Smad3 and Smad215 | Smad7 |
| MeCP216 | NF-κB30 |
| KLF517 | KLF415,22,31 |
| HMGA218 | PPARγ30,32 |
| SRF19,20 | Nkx2.533 |
| RTEF-121 | YB-134 |
| Sp1 and Sp317,22 | |
| C/EBPβ23 | |
| CSL24 | |
| c-Myb25 | |
| MRTF-A/MRTF-B20,26–28 | |
| Fli-129 | |
| Epigenetic Regulators and miRNAs | |
| DNMT135 | Other DNMTs16,42 |
| HDAC436,37 | miR-2943–48 |
| (HDAC6 and HDAC8)36 | |
| miR-19238 | Let718 |
| miR-13230 | miR-200a49 |
| miR-2139 | |
| miR-12940 | |
| miR-200b/c38 | |
| miR-216a41 | |
| Growth Factors, Cytokines, and Others | |
| TGFβ150–52 | Interferon-γ60 |
| P31110 | CXCL1061 |
| Wnt51,53 | |
| Jagged151,54 | |
| FAK52,55 | |
| NOX456–59 | |
| ECM, ECM-Modulating Proteins, and Physical Factors | |
| Stiff ECM3,62–65 | Soft ECM3,62,63 |
| ROS66,67 | |
| LOX68 | |
| LOXL269,70 | |
| Lysyl hydroxylase and PLOD271 | |
| Osteopontin72 | |
| Periostin13,14 | |
| CCN2 (CTGF)51,73,74 | |
| ED-A fibronectin1,75,76 | |
| Membrane-Bound and Surface-Expressed Proteins | |
| Endosialin (Tem1)9 | |
| Integrin | |
| α11β111 | |
| α3β111,53 | |
| αvβ311,50 | |
| αvβ550 | |
| Notch124,51,54,77 | |
The table summarizes recently identified proteins and factors that have regulated myofibroblast differentiation either directly or indirectly. For well-established myofibroblast modulators, such as TGFβ1, review articles are referenced.
C/EBP, CCAAT enhancer binding protein; CTGF, connective tissue growth factor; ED-A, extradomain A; FAK, focal adhesion kinase; Fli-1, friend leukemia integration; KLF, Kruppellike factor; PLOD, procollagen-lysine, 2-oxoglutarate 5-dioxygenase; ROS, reactive oxygen species; RTEF, R-transcription enhancer factor; Sp, specificity protein; SRF, serum response factor; YB-1, Y-box binding protein-1.
Myofibroblast-Inducing Factors and Conditions
Mechanical resistance of the ECM, in conjunction with the action of profibrotic transforming growth factor β1 (TGFβ1), is an amply documented primary stimulus for myofibroblast differentiation and persistence.1 Myofibroblasts develop in vitro and in vivo their highly contractile cytoskeletal apparatus only above a certain ECM stiffness threshold.3,62 Various fibrotic organs and tissues have recently exceeded this threshold at the micromechanical level.78–80 Tissue stiffness increases as a consequence of ECM-remodeling activities of fibroblasts and myofibroblasts.63 Contracting cells then generate the conditions that make them even more contractile in a detrimental feed-forward loop. The chicken-and-egg question of how fibroblastic cells are initiated to become contractile in the soft ECM present after the injury may be answered by recent biomechanical studies. In an animal model of liver fibrosis, increased tissue stiffness precedes the activation of fibroblastic cells and the accumulation of collagen, thus suggesting that such early mechanical changes may be sufficient to trigger the contraction cascade.68 Collagen cross-linking catalyzed by lysyl oxidase (LOX) enzymes is one possible factor responsible for early structural changes and tissue stiffening in these conditions.68 Fibrosis-specific and stable collagen cross-links are further formed by lysyl hydroxylase and pro-collagen-lysine, 2-oxoglutarate 5-dioxygenase, which are responsible for pyridinoline and aldehyde-derived collagen cross-links in the fibrotic skin.71 Furthermore, antibody-mediated inhibition of the enzyme LOX-like 2 (LOXL2) suppresses fibrosis and its progression in a variety of organ systems, including tumor desmoplasia.69 However, it remains unclear whether this effect is due to reduced ECM stiffness. The potential role of initial tissue stiffening in the onset of fibrosis is particularly interesting given that cultured myofibroblasts can activate latent TGFβ1 from a sufficiently stiffened ECM by integrin-mediated contraction.64,65 A purely mechanically driven mechanism of profibrotic growth factor activation has been experimentally proved in a cell-free system81 and is supported by the recently revealed structure of latent TGFβ1.82 These findings establish a direct link between the mechanical and chemical factors regulating myofibroblast differentiation and ultimately causing fibrosis.50,83 Table 1 summarizes myofibroblast-inducing and myofibroblast-inhibiting factors.
Myofibroblast Differentiation and Regulation of ACTA Gene Expression
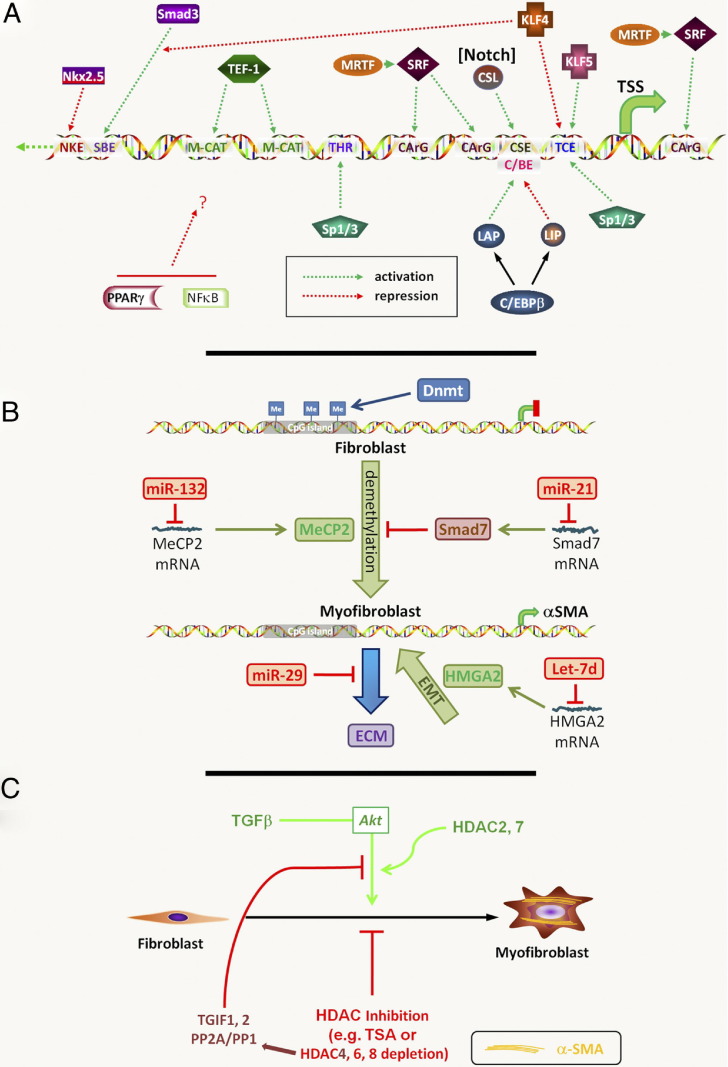
Because α-SMA expression is a common key element for detection of myofibroblast differentiation and a major player in contractile force production, most of the available data focus on the regulatory mechanisms underlying expression of this gene. Abundant information is available on transcriptional regulation of the ACTA gene, which indicates complex combinatorial mechanisms involving both stimulatory and inhibitory factors (Figure 1A and Table 1).
Figure 1.
Myofibroblast epigenetics. A: Transcriptional regulation of the ACTA gene in myofibroblast differentiation. Activating or repressing transcription factors are shown directed at the relative locations of their cognate binding elements in the ACTA gene promoter. The interaction of Kruppellike factor (KLF) 4 with Smad3 results in decreased Smad3 binding to the Smad-binding element (SBE). In contrast, MRTF enhances serum response factor binding to its cognate element to activate differentiation. PPARγ and NF-κB are also shown, but the location/nature of their direct interaction with the promoter is uncertain (indicated with a question mark). The activation of Notch signaling activates its downstream factor CSL. CArG, CC(A/T)6GG; C/EBP, CCAAT enhancer binding protein; CSL, from CBF1/RBP-J in mammals, suppressor of hairless [Su(H)] in drosophila and xenopus, and Lag-1 in Caenorhabditis elegans; IM-CAT, binding element with sequence CATCCT; LAP, liver activator protein; LIP, liver inhibitory protein; NKE, Nkx2.5 binding element; SRF, serum response factor; TEF, transcription enhancer factor; THR, TGF-β1 hypersensitivity region; TSS, transcription start site. B: DNA methylation and miRNAs in myofibroblast differentiation. Methylated CpG islands are shown in the ACTA gene promoter in the fibroblast, which are maintained by DNMT. The differentiated myofibroblast shows reduced methylation with activation of ACTA gene expression. The methylated DNA-binding protein MeCP2 promotes myofibroblast differentiation; however, multiple target genes are regulated by this protein. The indicated miRNA species regulate their target mRNAs with downstream consequences on myofibroblast differentiation, as indicated. Although ACTA is used as a hallmark gene of the myofibroblast, other fibrosis-relevant genes have been regulated by hypermethylation as well (see Myofibroblast DNA Methylation). C: Histone deacetylation and myofibroblast differentiation. The indicated HDAC isoforms modulate differentiation via multiple mechanisms. In addition, inhibition/depletion of HDAC4 suppresses TGFβ activation of Akt via 5′-TG-3′-interacting factor 1,2 (TGIF1,2) and protein phosphatase 2A/1 (PP2A/PP1).
Activators of the α-SMA Promoter
Among the activators are serum response factor and the transcription enhancer factor-1 family member R-transcription enhancer factor, which bind to CC(A/T)6GG (CArG) and CATTCCT (MCAT) elements, respectively, in the upstream regulatory sequence of the α-SMA promoter.19,21 In addition to the importance of Smad3 and its binding element,15 another element found to be important in TGFβ1-induced differentiation is a proximal TGFβ control element, to which several factors [eg, Krüppel-like factor 5, specificity protein (Sp) 1, and Sp3] can bind to activate transcription.17,21,22 Other upstream elements include the TGFβ hypersensitivity region and an Smad-binding element, which are activated by binding to Sp1/Sp3 and Smad3, respectively.15,22 Studies in several different cell types have implicated additional transcription factors, such as CCAAT enhancer–binding protein β, CSL (from CBF1/RBP-J in mammals, Suppressor of Hairless [Su(H)] in Drosophila and Xenopus, and Lag-1 in Caenorhabditis elegans) (a down3stream target of Notch signaling), and c-Myb, in regulation of ACTA gene expression (Table 1).23–25
An important factor linking ACTA gene transcription to the level of mechanical stress and the state of the contractile cytoskeleton is myocardin-related transcription factor A (MRTF-A).20,26,27 In a mechanism that involves the F-actin–organizing factor mDia1, MRTF-A docks to CArG elements and enhances the transcriptional activity of serum response factor.20 MRTF-A and MRTF-B also mediate TGFβ1-induced myofibroblast differentiation and transcription of smooth muscle genes in fibroblasts.28 Other mechanically regulated transcription of fibrosis-related genes have been recently reviewed.20
Repressors of the α-SMA Promoter
Several factors have down-regulated α-SMA expression and, thus, may be responsible for active suppression of myofibroblast differentiation (Table 1). Among these factors is Kruppellike factor 4, which can compete for binding to the TGFβ control element by its activators and interact with the Mad homology 2 (MH2) domain of Smad3 to suppress its binding to the ACTA promoter.31 Additional repressors are Nkx2.5, peroxisome proliferator–activated receptor (PPAR)-γ, and Y-box binding protein-1.32–34 In the case of Nkx2.5, reduction in its expression correlates with myofibroblast differentiation in lung fibroblasts. Thus, differentiation may be mediated by a derepression mechanism dependent on decreased expression of one or more of these repressors.
Smad-Independent Regulation of the α-SMA Promoter
In addition to Smad signaling, the multiple, complex signaling mechanisms implicated in regulation of myofibroblast differentiation may also involve mitogen-activated protein (MAP) kinases.15 Depending, in part, on the precursor cell type, Wnt signaling pathways may also be involved.53 Notch signaling is important in endothelial-to-mesenchymal transition and myofibroblast differentiation from lung fibroblasts.24,54 This is likely mediated by CSL interacting with its binding element identified in the ACTA promoter. Induction of Jagged1, a ligand for Notch1, results in activation of this signaling pathway, with subsequent activation of ACTA gene transcription. Impaired Notch signaling in vivo results in reduced myofibroblast differentiation in an animal model.54 Another important pathway in myofibroblast differentiation associated with the desmoplastic response in tumors is Hedgehog signaling, although the downstream target gene(s) have not been identified.84 All these differing signaling pathways are likely to work in concert at one level or another in more complex scenarios to ultimately affect ACTA gene expression, both directly and indirectly, via other regulatory genes.
Myofibroblast Epigenetics: A Fibrotic Memory?
Various approaches have demonstrated differences in the protein, gene, and transcriptional profiles between myofibroblast precursors from different origins and their fibrotic counterparts.85,86 Microarray studies have shown up-regulation of many genes, consistent with activation by TGFβ1, Wnt, and connective tissue growth factor (CCN2) signaling pathways, which are abnormally expressed in patients with fibrotic disorders.85,87 These fibrotic signatures tend to disappear in explanted fibroblasts compared with whole tissue from the same patients.88 A common observation in profiling studies is that specific signatures of myofibroblasts are retained over several passages in vitro, suggesting a myofibroblast memory that may be mediated by epigenetic modifications, with consequent preservation of the myofibroblast phenotype and its persistence. Epigenetic regulation of gene expression in a variety of fibrotic conditions has involved DNA methylation, modification of histones, and regulation of miRNAs targeting select genes (Figure 1, B and C).
Myofibroblast DNA Methylation
In the case of DNA methylation, modification at CpG islands represents an important mechanism for gene silencing, and this appears to be the case for the ACTA gene16,89 (Figure 1B). Lung alveolar epithelial type II cells, which do not express α-SMA, exhibit high levels of methylation in the three CpG islands identified in the regulatory regions of this gene and in intronic regions. In contrast, lung fibroblasts exhibit significantly lower levels of DNA methylation in this gene, and its inhibition using 5-aza-2′-deoxycytidine activates gene transcription. DNA methylation is not limited to the ACTA gene in fibrotic conditions. In lung fibroblasts, hypermethylation of the promoter region of the TYHY1 gene has been implicated in its silencing, correlating with fibrosis in the lung.89,90 In scleroderma fibroblasts, ECM-inhibitory genes, such as Smad7 or Fli-1 are also hypermethylated and consequently down-regulated, suggesting that DNA methylation could cause derepression of ECM genes involved in fibrosis.29
Similarly induced specific deficiency of DNA methyl transferases (DNMTs), using small-interfering RNAs, causes activation of ACTA gene transcription in these fibroblasts42 (Figure 1B). In the fibrotic kidney, DNMT1 is associated with persistent fibroblast activation and fibrogenesis via promotion of hypermethylation of RASAL1, a gene that encodes an inhibitor of the Ras oncogene.35 DNMT1+/− heterozygous mice exhibit reduced renal fibrogenesis and kidney fibrosis. Conversely, induced DNMT overexpression in lung fibroblasts, using expression plasmids, results in repression of ACTA gene expression and, thus, of myofibroblast differentiation.35 The use of these approaches to alter DNMT expression and/or inhibition of DNA methylation is likely to affect other genes as well, which may indirectly affect ACTA gene expression. This is the case in a study30 of hepatic stellate cells (HSCs), in which inhibition of DNA methylation results in activation of PPARγ and NF-κB, which then repress ACTA gene expression. Direct and indirect effects of the methylated DNA-binding protein MeCP2 have also been observed. MeCP2 binds directly to the ACTA gene promoter and is essential for ACTA gene expression.16 Moreover, MeCP2 deficiency significantly reduces pulmonary fibrosis and myofibroblast differentiation in an animal model. The relative contribution of these direct versus indirect mechanisms may be decisive in determining the ultimate effect on myofibroblast differentiation.
Myofibroblasts, Fibrosis, and miRNAs
In addition to epigenetic modulation through DNA methylation, different miRNAs appear to contribute to the myofibroblast memory43 (Figure 1B and Table 1). miR-29 expression is markedly reduced in skin biopsy specimens and explanted skin fibroblasts from patients with scleroderma, suggesting a post-translational epigenetic mechanism for increased ECM gene expression.44 In fibrotic lung, low levels of miR-29 correlate with high expression levels of profibrotic genes, including previously unrecognized ECM and ECM remodeling genes. Knockdown or TGFβ1-mediated down-regulation of miR-29 in cultured lung fibroblast derepressed fibrosis-associated genes.45 Down-regulation of miR-29 in cardiac fibroblasts in vitro and in vivo induces fibrosis-associated genes, whereas its overexpression has a fibrosis-suppressing effect.46 A comparable fibrosis-suppressing role has been demonstrated for miR-200a in the kidney49 and liver.47
Conversely, in an animal model of kidney fibrosis, up-regulation of miR-192 is reported on TGFβ1 signaling, and overexpression of an miR-192 analogue induces ECM production.38 Similarly, miR-129,40 miR-192, miR-200b/c,38 and miR-216a41 promote expression of fibrotic genes in mouse mesangial cells. Another miRNA involved in fibrosis is miR-132, which mediates inhibition of MeCP2 expression, reduces PPARγ expression, and consequently enhances ACTA gene expression in lung fibroblast.30 Such an indirect mechanism has also been reported for additional miRNA species. Among these, Let7 and miR-21 affect lung myofibroblast differentiation via effects on HMGA2 and Smad7, respectively, although additional effects on other target genes have not been excluded.18,39 Future miRNA profiling studies, such as the one recently started for bleomycin-induced lung fibrosis,91 will hopefully provide a more complete picture of the miRNA species involved in the different aspects of fibrosis.
Myofibroblast Histone Modifications
Histone modification is involved in the perpetuation of fibrosis. The importance of histone modification in regulation of ACTA gene expression is generally indirect.30 The histone deacetylases (HDACs) 4, 6, and 8 have been identified as the key HDACs involved in this mechanism36 (Figure 1C and Table 1). Although HDAC4 possibly affects the activation of Akt in TGFβ1-induced differentiation,37 the direct target genes of these modified histones have not been identified. Histone acetylation is usually associated with activation of target gene expression; thus, the mechanism of this inhibition on ACTA gene expression is likely due to activation of other genes that repress its expression. In addition to histone acetylation, methylation of histones is involved in myofibroblast development and fibrosis.30 Considering the rapid progress made in understanding epigenetic processes, it will be years until the first potential targets are identified and tested for their anti-fibrotic potential.
Organ Fibrosis
Systemic Sclerosis
The Role of Fibrosis in Systemic Sclerosis
Systemic sclerosis or scleroderma is a chronic autoimmune and fibrotic disease of unknown etiology with no effective disease-modifying therapies and significant mortality. The disease is highly heterogeneous in its clinical manifestations. Widespread fibrosis affecting virtually every organ distinguishes scleroderma from organ-based fibrotic processes, such as pulmonary fibrosis, keloids, glomerulosclerosis, and hepatic fibrosis. The dermis, lung parenchyma, heart, gastrointestinal tract, tendons, and ligaments are prominently affected, with endocrine organs, such as the thyroid gland, occasionally affected.51 Interestingly, neither the liver nor the central nervous system show significant fibrosis in scleroderma. Activated fibroblasts and myofibroblasts are the primary effector cells of fibrosis in scleroderma.51
The skin in scleroderma is characterized by replacement of the normal dermal architecture with collagen-rich connective tissue. Progressive dermal thickening and sclerosis obliterate eccrine and sebaceous glands, hair follicles, and small blood vessels. Analysis demonstrates excessive deposition of the main fibrillar collagens (types I and III) and types V and VII collagens, normally restricted to the dermal-epidermal junction.92 The extradomain A fibronectin splice variant, which is critical for myofibroblast differentiation, shows increased deposition.93 Other structural ECM molecules that are aberrantly expressed in scleroderma include fibronectin, proteoglycans, fibrillin, and elastin fibrils.94 Fibrillin accumulation is of particular interest, because fibrillin-1 regulates the activation of ECM-bound latent TGFβ1, and mice with a fibrillin-1 mutation (tight skin mice) develop a sclerodermalike phenotype.95 Microarray analysis of scleroderma skin reveals up-regulation of genes normally associated with bone and cartilage, including type XI collagen and cartilage oligomeric matrix protein (COMP). Integrins involved in cell-cell and cell-ECM adhesion and latent growth factor activation are also overexpressed in the lesional tissue.86,87
The Origin of Myofibroblasts in Scleroderma
Fibroblasts and myofibroblasts are the principal stromal cells responsible for the excessive ECM deposition and remodeling in scleroderma. Although in situ hybridization studies have identified an increased proportion of biosynthetically active fibroblasts in scleroderma dermis, in vitro clonal analysis of explanted skin fibroblasts shows substantial heterogeneity with respect to collagen synthesis, suggesting the existence of distinct subpopulations of dermal mesenchymal cells with different origins. Cells positive for α-SMA are present in the skin with lesional scleroderma but are not detected in healthy skin.93,96 Accumulation is prominent in areas with significant collagen deposition. Myofibroblastic cells are also observed in the esophagus and lung, even in the liver, despite the absence of fibrosis. Culture of bronchoalveolar lavage fluids from patients with scleroderma shows spontaneous outgrowth of α-SMA–positive cells with high production of collagen and fibronectin.51 In contrast, myofibroblasts are not detected in bronchoalveolar lavage fluids from healthy individuals. The number of myofibroblasts in the lesional skin in scleroderma correlates with both the extent of local collagen deposition and the clinical assessment of skin stiffness.97 Moreover, the myofibroblast score in the skin shows a decrease over 6 to 12 months in patients treated with cyclophosphamide, suggesting that myofibroblast levels in the skin may be used as biomarkers of changing skin involvement.
The origin of myofibroblasts in the fibrotic lesions has not been conclusively established. They may arise from the in situ activation of normally quiescent resident fibroblasts in response to extracellular triggers, such as TGFβ1, Wnt, Jagged/Notch, CCN2, endothelin-1, lysophophatidic acid, and other signaling molecules, as well as hypoxia and mechanical stress due to increased ECM stiffness.51 Each of these stimuli, which induce α-SMA expression and stress fiber formation in explanted healthy skin fibroblasts, has been aberrantly expressed or regulated in scleroderma. Alternatively, myofibroblasts in scleroderma lesional tissue may arise from other cell types, including vascular smooth muscle cells, pericytes,93 and endothelial cells under the influence of TGFβ198 or epithelial cells in the skin or lungs.99,100 Because both myofibroblasts and pericytes are associated with vascular remodeling and injury, which are prominent in patients with scleroderma, vascular smooth muscle cells and pericytes might provide the pathogenetic link between vascular injury and fibrosis characteristic of scleroderma. Although the significance of mesenchymal differentiation pathways in the context of scleroderma has not been conclusively demonstrated, and the mechanisms underlying such cellular plasticity in vivo remain mostly unknown, it is tempting to consider scleroderma therapies that are based on targeting these events.
Persistence of Scleroderma Fibroblasts
Historically, the study of scleroderma fibroblasts focuses primarily on the skin, because it is readily accessible for biopsy. Pioneering studies by Leroy101 30 years ago are the first to demonstrate the feasibility of studying explanted skin fibroblasts in scleroderma. These observations indicate that scleroderma-derived skin fibroblasts display a biosynthetically activated phenotype in vitro that is independent of extracellular signals. A cell-autonomous activated phenotype is maintained for multiple in vitro passages.101 These studies have been subsequently reproduced and extended by other investigators.102 In some studies, most explanted scleroderma fibroblasts are positive for α-SMA in vitro, with increased production of collagen and tissue inhibitor of metalloproteinase.103 Moreover, myofibroblasts explanted from scleroderma skin are resistant to Fas-induced apoptosis.96 Apoptosis resistance may be due to activation of the Akt prosurvival pathway and may account for the persistence of myofibroblasts in scleroderma skin.104 Another potential mechanistic explanation accounting for the persistent α-SMA expression in scleroderma is provided by the demonstration that pharmacological inhibition of focal adhesion kinase phosphorylation significantly attenuates the myofibroblast phenotype of these cells.55 Scleroderma fibroblasts show an elevated level of focal adhesion kinase phosphorylation, presumably reflecting their stimulation by autocrine TGFβ1. Consistent with the autocrine TGFβ1 hypothesis, explanted scleroderma fibroblasts show constitutive nuclear localization of phosphorylated Smad2/3, even in the absence of added TGFβ1.105 These studies suggest that an autocrine TGFβ1-stimulatory loop induces focal adhesion kinase phosphorylation and apoptosis resistance, with consequent persistence of α-SMA–expressing myofibroblasts.52
In addition to enhanced ECM production, scleroderma fibroblasts in culture produce chemokines and growth factors and spontaneously generate reactive oxygen radicals, such as H2O2, through the NADPH oxidase (NOX) complex pathway, which is up-regulated in scleroderma skin.66 In turn, intracellular production of reactive oxygen species is at least partially responsible for the constitutive up-regulation of collagen synthesis in these cells. The expression of surface receptors for relevant growth factors and chemokines, including TGFβ1, platelet-derived growth factor, and CCR2, are also elevated on scleroderma fibroblasts, suggesting an additional feed-forward amplification mechanism that might underlie the persistently activated phenotype.106
Renal Fibrosis
Renal interstitial fibrosis is a common pathological feature of chronic kidney disease (CKD), irrespective of etiology. The myofibroblast is a major player in the onset and evolution of renal fibrosis and has been implicated in pathogenesis.107
The Origin of Renal Myofibroblasts
Despite their pivotal role in disease progression, the source of renal myofibroblasts is still a matter of debate. Several progenitors have been proposed in addition to local fibroblasts, including circulating fibrocytes, local pericytes,108,109 and resident epithelial cells, through epithelial-to-mesenchymal transition (EMT).110 The possible contribution of EMT to fibrosis in vivo was recently challenged by a study108 using a mouse model of renal interstitial fibrosis and genetic cell lineage tracing. However, under the same conditions of kidney obstruction, but using a different approach, EMT was observed by another group.111 These conflicting data have been addressed elsewhere in several excellent critical articles.112,113
Tubuloepithelial-to-Mesenchymal Cross Talk Contributes to Kidney Myofibroblast Generation
It appears increasingly likely that loss of the normal, homeostatic microenvironment, due to alterations in the tubulointerstitium per se, triggers myofibroblast activation and causes progression of fibrosis.114 This established concept is based on the histological evidence that proximal tubule cell (PTC) injury precedes interstitial fibrosis and that regions of active interstitial fibrosis predominantly exhibit a peritubular, rather than a perivascular, distribution. This notion is supported by in vitro studies115 showing that cortical fibroblast proliferation is stimulated by paracrine signals generated by PTC in co-culture.
Moreover, in proteinuric nephropathies with progressive injury of the glomerular filtering barrier, abnormal uptake of ultrafiltered proteins by PTCs induces release of TGFβ1, which, in turn, promotes interstitial fibrogenesis.116 Re-establishment in vitro of epithelial cell polarization and differentiation inhibits proliferation of mesenchymal stem cells (MSCs) from co-cultured adipose tissue fragments,117 confirming that uninjured differentiated renal tubular cells in their normal configuration contribute to the maintenance of the homeostatic state of MSCs. A seminal work73 evaluating the effects in vitro of aristolochic acid on several PTC lines reveals that damaged epithelial cells markedly up-regulate expression of profibrotic TGFβ1 and CCN2. This up-regulation is associated with a marked increase in the percentage of cells in the G2/M phase of the cell cycle, which is confirmed in vivo using different acute kidney disease mouse models.73 The contribution of PTCs is further supported in a tetracycline-inducible transgenic mouse model, in which conditional overexpression of TGFβ1, limited to renal tubules, induces widespread peritubular fibrosis and focal nephron degeneration.114 During the course of this process, the remnants of one cell were removed by phagocytosis by neighboring cells with a mechanism akin to that seen in tubular atrophy.118 Outside these degenerating tubules, a marked proliferation of resident fibroblasts, without contribution of EMT and only sparse macrophages, is associated with progressive deposition of ECM.
Notch signaling is involved in orchestrating kidney development in tubular epithelial cells and plays a role in tubulointerstitial development.77 Interestingly, specific expression of cleaved Notch1 in tubular epithelial cells causes fibrosis in the surrounding interstitium, with a histological appearance that resembles the lesions seen in human CKD. Conversely, tubular-specific deletion of Notch signaling protects against kidney fibrosis.77 The importance of an altered tubule-interstitial microenvironment to fibrosis is also supported by the clinical observation that the severity of acute kidney injury (AKI) with massive tubular epithelial cell death is a robust predictor of progression to CKD.119 Even if mechanisms for AKI to CKD progression are unknown, it can be hypothesized that AKI progression toward CKD arises from incomplete repair of regenerating tubules. Another explanation is that persistently high profibrotic signaling activity in regenerating tubule epithelium produces paracrine activity that drives fibroblast proliferation and inflammation. Thus, in severe AKI and CKD, epithelial-mesenchymal cross talk alterations possibly lead to tubulointerstitial fibrosis and kidney failure.
It appears that an altered renal microenvironment is the main cause of myofibroblast differentiation in renal fibrosis, and cross talk between epithelial cells and fibroblasts is pivotal for maintenance of the local homeostatic microenvironment, a concept previously suggested for idiopathic pulmonary fibrosis120,121 and for stromal reaction to carcinoma.122 Therefore, interfering with tubular-mesenchymal cross talk emerges as a promising strategy for the development of new anti-fibrotic drugs, which are not solely directed to block myofibroblast activation.
Pulmonary Fibrosis
Structural Aspects of the Lung Relevant for Fibrosis
The adult human lung is a structurally complex organ system composed of >40 cell types. The upper conducting airway, composed of the trachea and a series of branching bronchi and bronchioles, terminates in the gas-exchanging alveoli of the lower respiratory tract. At each level, the epithelium-lined airways and endothelium-lined vasculature are integrated, both structurally and functionally, by an interconnected reticulum of mesenchymal cells and ECM extending from the upper airway down to the alveoli.123 The lung is susceptible to various forms of short-/long-term injuries, both airborne and blood borne, that may culminate in fibrosis. In addition, fibrosis may involve the small conducting airways (eg, asthma and chronic obstructive pulmonary disease), vasculature (eg, pulmonary hypertension), or pleura (eg, pleural fibrosis). Some forms of fibrosis, such as acute lung injury or cryptogenic organizing pneumonia, are at least partially reversible, whereas others, in particular idiopathic pulmonary fibrosis, are progressive and usually fatal.120,121 A central role for the myofibroblast in tissue remodeling and fibrosis involving these different tissue compartments and varied disease processes has been demonstrated.124
Origin of Lung Myofibroblasts
The concept that lung myofibroblasts may derive from multiple cellular sources, including bone marrow progenitors and the lung epithelium, has been previously reviewed.1 Depending on the experimental model and tools used, conclusions on participation of myofibroblast progenitors to lung fibrosis sometimes appear contradictory. For example, recent studies using a surfactant protein C-CreER(T2) knock-in allele to follow the fate of type II alveolar cells in vivo indicated no contribution to myofibroblasts in the fibrotic reaction to an acute lung injury involving intratracheal instillation of bleomycin.125 The resolving nature of bleomycin-induced lung fibrosis in mice contrasts with the progressive nature of the most recalcitrant forms of lung fibrosis in humans, in particular idiopathic pulmonary fibrosis. Whether chronicity of the injury may account for processes, such as EMT, remains to be determined. Chronicity and irreversibility of the fibrotic process may also be linked to the epigenetic (dys) regulation2,16,42,89 that controls the differentiation and fate of endogenous reparative cells.
Specific Features of Myofibroblasts in the Lung
The fate of myofibroblasts, regardless of their origin(s), in injured tissues may ultimately determine whether normal healing occurs or whether progression to end-stage fibrosis ensues.121,126 There is likely to be significant heterogeneity in lung fibroblasts, including anti-fibrotic subpopulations, such as Thy-1–expressing fibroblasts and lipofibroblasts.65,89,90 The mechanisms that produce an apoptosis-resistant myofibroblast phenotype have not been fully elucidated, although some of the same factors that mediate myofibroblast differentiation appear to promote myofibroblast survival.90,127,128 A member of the NOX family of enzymes, NOX4 has been required for TGFβ1-induced myofibroblast differentiation, ECM production, and contractility of lung myofibroblasts.56,57 NOX4 is up-regulated in lungs of human subjects with idiopathic pulmonary fibrosis, and genetic or pharmacological targeting of NOX4 attenuated lung fibrogenesis in two different murine models of lung injury.56 It is unknown if the profibrotic effects of NOX4 are related solely to activation of myofibroblasts or if its expression in alveolar epithelial cells may also contribute to fibrogenesis.58,59 It is also yet to be determined if targeting NOX4 modulates the apoptosis-resistant phenotype of myofibroblasts and whether this strategy may be effective in established fibrosis. However, the proof of concept for therapeutic targeting of prosurvival signaling with a protein kinase inhibitor in animal models of pulmonary fibrosis has been demonstrated.129
Another potential therapeutic strategy is to induce dedifferentiation of myofibroblasts as a mechanism for myofibroblast deactivation in progressive fibrotic disorders.130 Cellular plasticity, specifically the ability of differentiated cells to dedifferentiate into more primitive multipotent or pluripotent progenitor cells, appears to be important in the remarkable regenerative potential observed in amphibians. The extent to which such mechanisms (or the lack thereof) explain the more limited regenerative capacity in mammals is unknown. It has also been proposed that lung fibrosis may represent a process of disordered redevelopment, in which there exists an aberrant recapitulation of developmental pathways.131 Further studies on myofibroblast plasticity and its capacity for dedifferentiation may provide additional insights into tissue repair and fibrosis.
Liver Fibrosis
Characteristics of Liver Fibrosis
In experimental and clinical liver fibrosis, α-SMA–expressing myofibroblasts are the major source of fibrillar collagen and other ECM proteins. Although the impact of excessive and abnormal ECM deposition is well evaluated in the liver, little is known about the role of myofibroblast contraction. The contractive nature of liver fibrosis is suggested by the smaller size of the fibrotic liver (relative to the healthy liver) and its irregular or nodular surface due to underlying scar tissue. Collapse of the liver parenchyma results in a reduction of the distance between portal zones, which, together with ECM synthesis, is part of the fibrotic process. Distortion of liver architecture characterizing advanced fibrosis and shunting of the portal blood along porto-portal and porto-central fibrotic septa, away from hepatocytes, results in hepatic failure and portal hypertension. It is conceivable that these changes are mainly due to tissue remodeling and depend on contractile forces exerted by myofibroblasts in addition to ECM deposition.
Origins of Liver Myofibroblasts
Myofibroblasts are absent from normal liver. They are derived primarily from activated hepatic perisinusoidal cells (HSCs), at least in experimental liver injury.132 However, other potential precursor cells are embedded in the portal tract stroma around portal structures, including vessels and biliary structures. For example, portal fibroblasts (PFs) are able to acquire a myofibroblastic phenotype,133 and their contribution may be more important than generally assumed.134 The contribution of liver MSCs to the myofibroblast population is as yet unclear. These mesenchymal progenitor cells are usually identified by the combined expression of different specific surface marker proteins and their capacity to differentiate into different mesenchymal cell lineages. The potential of MSCs to differentiate into myofibroblasts has gained increasing attention; however, it is unclear whether this fate is beneficial or detrimental for normal tissue repair and the envisaged medical use of MSCs for tissue regeneration purposes.135 The association of MSCs with the well-known epithelial stem cells located in Hering's canals may constitute a niche, a concept that is being explored in the liver.136 Several studies have demonstrated that, after injury, many myofibroblasts may originate from bone marrow,137 but the contribution of these cells to collagen production is not known.138 Lineage-tracing studies fail to demonstrate the generation of myofibroblasts from hepatic epithelial cells (hepatocytes and cholangiocytes) by EMT.139 However, the controversy about the ability of hepatic epithelial cells to become myofibroblasts remains unsettled.113 Thus, the possibility that MSCs, bone marrow–derived cells, and EMT may represent alternative sources of myofibroblasts cannot be excluded.
Evidence for the Reversibility of Myofibroblast Differentiation in Specific Liver Subpopulations
It is increasingly accepted that, depending on the underlying causative factor (eg, alcohol abuse, viral hepatitis, or biliary cholestasis), liver fibrosis may involve different fibrogenic cell subpopulations. To our knowledge, no reliable markers have been identified that allow unambiguous discrimination between HSC- and PF-derived myofibroblasts. However, differences have been reported between these two fibrogenic cell populations, concerning the mechanisms underlying myofibroblastic differentiation, activation, and deactivation.140 After isolation from healthy rat liver and culturing under the same conditions, both HSCs and PFs acquire a myofibroblast phenotype. HSC-derived myofibroblasts display rounded and spread morphological characteristics with an enlarged cytoplasm and, more important, a poor survival after two to three passages. Conversely, PF-derived myofibroblasts have more elongated morphological characteristics and proliferate over multiple passages. In vivo, during liver repair, HSC- and PF-derived myofibroblasts display different functions because of their specific locations and microenvironment. By using a model of cultured precision-cut liver slices, the behavior of the myofibroblast subpopulations during remodeling differs depending on the experimental model,141 the pathological situation, and the disease cause.142 HSC-derived myofibroblasts can lose α-SMA expression without undergoing cell death, whereas in similar conditions, PF-derived myofibroblasts die by apoptosis. HSC-derived myofibroblasts are involved in blood flow regulation and hepatocellular healing. The appearance of the myofibroblast phenotype in HSCs may lead to disordered vascular remodeling after liver injury, inducing ischemia and then fibrosis. PF-derived myofibroblasts are involved in scarring.143
Remodeling of the Fibrotic Injury and Potential Therapies
The traditional view of liver fibrosis as an irreversible process is obsolete, and the development of liver fibrosis is thought to be a dynamic and potentially bidirectional process. Because of the liver's extraordinary regeneration potential, myofibroblast and fibrosis reversion strategies may be more applicable than in other organs, such as the lung.130 Spontaneous resolution of scarring is seen in animal models of liver fibrosis and in human trials in which the stimuli responsible for long-term or repeated hepatic damage are successfully removed. Key players in the process are myofibroblasts, matrix metalloproteinases, and matrix metalloproteinase inhibitors. However, it is still unclear whether advanced fibrotic or cirrhotic liver can revert back to normal architecture after the removal of profibrotic factors. It is conceivable that there is a point of no return, even for this highly regenerative organ. Important quantities of elastin and extensively cross-linked ECM components seem to be features of irreversibility.144 Numerous preclinical studies145 aimed at inhibiting myofibroblast activity and fibrosis progression in liver have been developed, but their translation into the clinic remains limited.
Stromal Reaction to Epithelial Tumors
Cancer cells do not exist independently but interact dynamically with local and distant host cells, best conceptualized as a gradually evolving microenvironment or even ecosystem.6 Nonredundant changes in a single element may inevitably alter the organization of the whole system and may drive tumor initiation, invasion, and metastasis. Morphological evidence of host participation in the primary tumor, the premetastatic niche, and metastatic sites includes the following: i) desmoplasia consisting of carcinoma-associated fibroblasts (CAFs) and ECM; ii) inflammation and immune response represented by lymphocytes, macrophages, and dendritic cells; and iii) angiogenesis evidenced by newly formed blood vessels and lymph vessels. Herein, we focus on the prominent role of CAFs as host cells in the tumor microenvironment. Phenotypically, CAFs closely resemble myofibroblasts exhibiting different levels of specific marker expression. This raises the possibility that tumor-associated myofibroblasts fall into a diversity of functional subtypes, similar to what has been shown for inflammatory cells and macrophages.122
Origins of Myofibroblasts and CAF in Tumor Stroma
Current understanding of the origin and molecular events surrounding the genesis of myofibroblasts and CAFs in tumor stroma is still a matter of debate. CAFs are thought to arise from several mobilized cell types, such as tissue-resident MSCs or fibroblasts, as well as recruited bone marrow–derived MSCs and fibrocytes.122 Alternatively, or in addition, CAFs may originate from multiple resident precursors, such as endothelial cells, myoepithelial cells, epithelial (cancer) cells, smooth muscle cells, adipocytes, and stellate cells. These findings suggest a high degree of plasticity and/or a diversity of origins for tumor myofibroblasts. This diversity may also explain the heterogeneous phenotype of myofibroblast populations observed within tumors.122
Specific Roles and Features of Myofibroblasts in the Tumor Environment
Traditionally, biomarker research has focused on molecular characteristics of the cancer cells, but increasing evidence suggests that myofibroblasts may refine the prognostic assessment of tumors and may provide predictive information about response to chemotherapy. Gene expression profiles of laser-capture microdissected tumor-associated host cells from primary breast tumors yield prognostic signatures strongly associated with a poor disease-specific survival. A wound-response signature, containing genes implicated in tissue remodeling, migration, angiogenesis, and inflammation, is established from 50 fibroblast cultures whose expression changed after exposure to 10% serum. Patients with breast cancer whose tumors express this wound-response signature have a markedly reduced overall survival and distant metastasis-free survival compared with those with tumors that do not express this signature.80 Immunohistochemical analysis of breast tumors also reveals that many of the proteins encoded by genes identified in the previously mentioned signatures are expressed by myofibroblasts, indicating their striking prognostic value. Increased expression of platelet-derived growth factor receptor β, LOXL2, caveolin-1, and CD10 in CAFs has been correlated with poor clinical outcome in multiple cancer types, such as breast, colon, pancreas, and liver.69,146–148 Increased co-expression of LOXL2 and α-SMA in colon and breast cancer suggests that myofibroblasts are the main producers of LOXL2. The ECM, including cross-linked collagen type I, has an influence on therapeutic response. Moreover, a tumor-associated host cell gene expression signature present in estrogen receptor–negative breast tumors predicts resistance to neo-adjuvant chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide.149
In the primary tumor microenvironment, resident human mammary and prostate fibroblasts exhibit autocrine signaling loops mediated by TGFβ1 and chemokines and differentiated into myofibroblasts.150 Epigenetic regulation by miRNAs and oxidative stress caused by reactive oxygen species in the primary tumor promote myofibroblast differentiation67 and secretion of senescence-associated pro-inflammatory cytokines, such as IL-6 and IL-8.151 When considering the tumor environment as an ecosystem, however, it is important to note that a tumor is not composed solely of cancer cells and myofibroblasts. The secretion of pro-inflammatory cytokines by myofibroblasts may influence the recruitment of inflammatory cells, which, in addition to their immunomodulatory activity, may also stimulate invasion of cancer cells. CAFs isolated from the initial hyperplastic stage in multistep skin tumorigenesis exhibit activated expression of the pro-inflammatory cytokine IL-6.152 More important, chemical and physical insults encountered during therapeutic interventions may also cause stress-induced cellular senescence and reciprocal activation of pro-inflammatory, prosurvival, and pro-invasive pathways in the local ecosystem and at distant anatomical locations.153
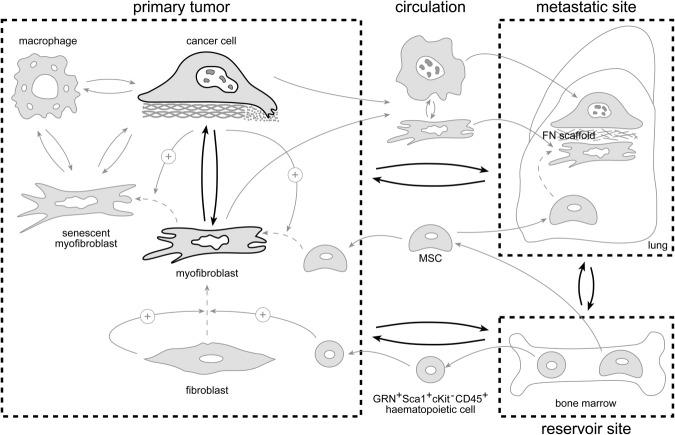
Primary tumor–induced remodeling of distant ecosystems, which initiates metastatic niche formation and host cell recruitment, suggests a crucial role for secretory products released into the circulation (Figure 2). The primary tumor signals to the bone marrow and stimulates the release of MSCs into the circulation, with recruitment by stromal cell–derived factor-1α chemokine gradients to the tumor site, where they are converted into myofibroblasts by TGFβ1.154 Moreover, osteopontin released by an aggressive xenograft mobilizes granulin-secreting ScaI+cKit−CD45+ bone marrow–derived hematopoietic cells into the circulation.72 These granulin-secreting cells home to a second otherwise quiescent or indolent tumor in the same animal. Granulin accumulation converts resident fibroblasts into myofibroblasts and stimulates progression of an otherwise indolent tumor.72 Secretory products from the primary tumor ecosystem recruit MSCs or hematopoietic cells from the bone marrow but also may promote myofibroblast differentiation in distant loci. Thus, TGFβ1, packaged in secreted nanovesicles termed exosomes, converts fibroblasts into myofibroblasts at distant organs and may prepare metastatic niches by focalized production of a fibronectin scaffold.155,156 Alternatively, metastatic cancer cells can bring their own soil, including myofibroblasts, from the primary ecosystem to the distant ecosystem, as shown for the lung. Comigrating myofibroblasts provide prosurvival signals in the circulation and an early growth advantage in the ectopic lung site.157
Figure 2.
Tumor myofibroblasts. A schematic view highlights the cross signaling (double arrows) within and between local and distant tumor ecosystems. Arrows indicate displacement of cells between ecosystems; dashed arrows, conversion from one cell type into another; positive arrows, stimulation; and bold arrows, importance of the signaling.
Thus far, we have emphasized the invasion- and metastasis-promoting effects of myofibroblasts. However, transgenic mouse experiments suggest that suppressive signals derived from resident tissue fibroblasts can also control tumor initiation and progression.80 For example, disruption of the TGFβ receptor type II (TGFBR2) gene in fibroblasts results in invasive squamous cell carcinoma of the forestomach.158 In a model of prostate cancer, loss of p53 function in fibroblasts precedes inactivation of p53 in the epithelium.159 Targeted genetic inactivation of the phosphatase and tensin homologue (PTEN), a tumor suppressor with lipid and protein phosphatase activity, in fibroblasts of mouse mammary glands accelerates the initiation and progression of ErbB2-driven mammary epithelial tumors characterized by massive accumulation of collagen type I and infiltration of F4/80-positive macrophages.160 In conclusion, the complex molecular mechanisms that govern cancer cell–myofibroblast interactions in the primary tumor and in distant ecosystems justify the need for systems-level approaches to advance the field.
Conclusion and Perspectives
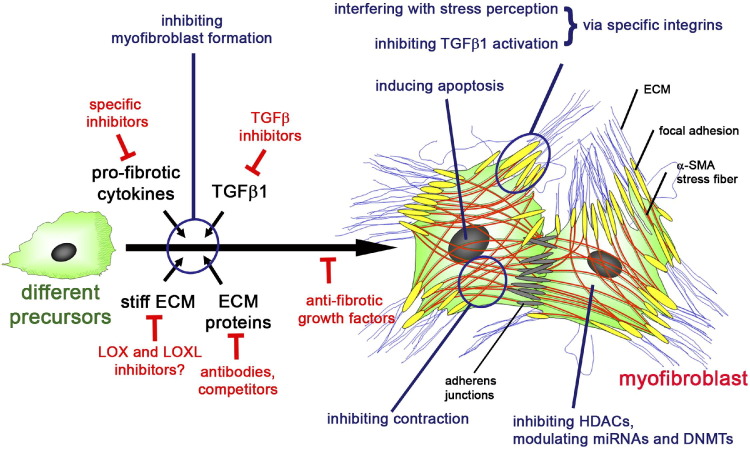
Based on the biological observations pinpointing the mechanisms of differentiation and activity of the myofibroblast, many attempts have been made to target them in anti-fibrotic therapies (Figure 3). However, no clinically effective compound has yet been identified, although several possibilities continue to be under investigation. Emerging and promising, but not yet clinically tested, strategies to counteract fibrosis include the use of miRNAs as potential therapeutic targets.43 Another strategy is to interfere with collagen cross-linking and ECM stiffening by controlling the activities of LOX,68 LOXL2,70 and specific lysyl hydroxylases.162
Figure 3.
The myofibroblast in the center of attention. Anti-fibrotic therapies can be designed to interfere with the extracellular chemical and mechanical factors that lead to myofibroblast formation from a variety of different precursors. Alternatively, one might interfere with intracellular signaling pathways, transcription regulators, and epigenetic mechanisms that specifically modulate myofibroblast differentiation. Other potential anti-fibrotic targets are specific features of the differentiated myofibroblast, such as α-SMA in the contractile apparatus, specific integrins, and ECM proteins. Other strategies aim to induce myofibroblast regression and/or apoptosis
(modified and reproduced with permission from the study by Hinz and Gabbiani161).
The capacity of cytokines to inhibit α-SMA expression by fibroblastic cells suggests a different strategy. In the case of interferon-γ, locally applied cytokine is found to be effective for treatment of Dupuytren's nodules in one study,60 but this result needs confirmation. Another more recent example for anti-fibrotic cytokines is CXCL10, which effectively treated lung fibrosis in an animal model.61 The complementary approach is to block profibrotic cytokines, among which TGFβ1 is the most attractive target. However, administration of TGFβ1-inhibiting drugs and antibodies has shown little success in animal and human studies, indicating that targeting a single cytokine is unlikely to be successful.52 More promising strategies appear to target TGFβ1 profibrotic cofactors, such as CCN274 or the ECM protein extradomain A fibronectin.75,76 Alternatively, TGFβ1 can be counteracted at the level of its activation from latent stores; possible targets in the activation process are fibroblast/myofibroblast-associated integrins αvβ3, αvβ5, and α8β1 integrin50 and the epithelial integrin αvβ6.163 It is conceivable that inhibiting these integrins has alternative suppressing effects on myofibroblast development. Other integrins that have contributed to fibrosis and myofibroblast differentiation through different pathways include α3β1, α11β1, and αvβ3, and all represent possible therapeutic targets.11
Other interesting approaches are based on the possibility of terminating myofibroblast persistence by inducing their apoptosis164 or their reversion to nonfibrogenic cell phenotypes.108 In the case of the former, the ability of nitric oxide to induce myofibroblast apoptosis165 suggests a possible therapeutic approach, such as by the use of phosphodiesterase-5 inhibitors.166 Finally, the experimental observation that the acetylated N-terminal peptide of α-SMA, Ac-EEED, inhibits both α-SMA and collagen type I expression, on intracellular delivery into myofibroblasts, makes this peptide a potential suppressor of fibrotic changes. This possibility has been successfully tested on experimental wound healing.167 We believe that these different approaches will eventually open new therapeutic avenues.
Footnotes
Supported by grants from the Canadian Institutes of Health Research (210820 and 219974 to B.H.), a grant from the Collaborative Health Research Program Natural Sciences and Engineering Research Council of Canada/Canadian Institutes of Health Research (1004005), a grant from the Heart and Stroke Foundation Ontario (NA7086 to B.H.), a grant from the Swiss National Science Foundation (3200-067254 to B.H.), grants from the NIH (HL-028737, HL-031963, HL-052285, and HL-091775 to S.H.P.; HL-067967 and HL-094230 to V.J.T.), grants from the University of Limoges and from the French Ministry of Research (A.D.), AR42309 (J.V.), grants from the Flemish League Against Cancer, Foundation Against Cancer, and the Fund for Scientific Research-Flanders (O.D.W. and M.M.).
References
- 1.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luco R.F., Allo M., Schor I.E., Kornblihtt A.R., Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144:16–26. doi: 10.1016/j.cell.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 4.van den Borne S.W., Diez J., Blankesteijn W.M., Verjans J., Hofstra L., Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 5.Coen M., Gabbiani G., Bochaton-Piallat M.L. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31:2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 6.Mareel M., Oliveira M.J., Madani I. Cancer invasion and metastasis: interacting ecosystems. Virchows Arch. 2009;454:599–622. doi: 10.1007/s00428-009-0784-0. [DOI] [PubMed] [Google Scholar]
- 7.Follonier Castella L., Gabbiani G., McCulloch C.A., Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010;316:2390–2401. doi: 10.1016/j.yexcr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Follonier Castella L., Buscemi L., Godbout C., Meister J.J., Hinz B. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J Cell Sci. 2010;123:1751–1760. doi: 10.1242/jcs.066795. [DOI] [PubMed] [Google Scholar]
- 9.Christian S., Winkler R., Helfrich I., Boos A.M., Besemfelder E., Schadendorf D., Augustin H.G. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. 2008;172:486–494. doi: 10.2353/ajpath.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan J., Peng X., Luo G., Ma B., Cao C., He W., Yuan S., Li S., Wilkins J.A., Wu J. Investigating the role of P311 in the hypertrophic scar. PLoS One. 2010;5:e9995. doi: 10.1371/journal.pone.0009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carracedo S., Lu N., Popova S.N., Jonsson R., Eckes B., Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285:10434–10445. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenga Y., Koh A., Perera A.S., McCulloch C.A., Sodek J., Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res. 2008;102:319–327. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 13.Vi L., Feng L., Zhu R.D., Wu Y., Satish L., Gan B.S., O'Gorman D.B. Periostin differentially induces proliferation, contraction and apoptosis of primary Dupuytren's disease and adjacent palmar fascia cells. Exp Cell Res. 2009;315:3574–3586. doi: 10.1016/j.yexcr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darby I.A., Vuillier-Devillers K., Pinault E., Sarrazy V., Lepreux S., Balabaud C., Bioulac-Sage P., Desmouliere A. Proteomic analysis of differentially expressed proteins in peripheral cholangiocarcinoma. Cancer Microenviron. 2010;4:73–91. doi: 10.1007/s12307-010-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu B., Wu Z., Phan S.H. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29:397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 16.Hu B., Gharaee-Kermani M., Wu Z., Phan S.H. Essential role of MeCP2 in the regulation of myofibroblast differentiation during pulmonary fibrosis. Am J Pathol. 2011;178:1500–1508. doi: 10.1016/j.ajpath.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan S.H. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandit K.V., Corcoran D., Yousef H., Yarlagadda M., Tzouvelekis A., Gibson K.F., Konishi K., Yousem S.A., Singh M., Handley D., Richards T., Selman M., Watkins S.C., Pardo A., Ben-Yehudah A., Bouros D., Eickelberg O., Ray P., Benos P.V., Kaminski N. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandbo N., Kregel S., Taurin S., Bhorade S., Dulin N.O. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-beta. Am J Respir Cell Mol Biol. 2009;41:332–338. doi: 10.1165/rcmb.2008-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan M.W., Hinz B., McCulloch C.A. Mechanical induction of gene expression in connective tissue cells. Methods Cell Biol. 2010;98:178–205. doi: 10.1016/S0091-679X(10)98008-4. [DOI] [PubMed] [Google Scholar]
- 21.Gan Q., Yoshida T., Li J., Owens G.K. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression. Circ Res. 2007;101:883–892. doi: 10.1161/CIRCRESAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 22.Cogan J.G., Subramanian S.V., Polikandriotis J.A., Kelm R.J., Jr, Strauch A.R. Vascular smooth muscle alpha-actin gene transcription during myofibroblast differentiation requires Sp1/3 protein binding proximal to the MCAT enhancer. J Biol Chem. 2002;277:36433–36442. doi: 10.1074/jbc.M203232200. [DOI] [PubMed] [Google Scholar]
- 23.Hu B., Ullenbruch M.R., Jin H., Gharaee-Kermani M., Phan S.H. An essential role for CCAAT/enhancer binding protein beta in bleomycin-induced pulmonary fibrosis. J Pathol. 2007;211:455–462. doi: 10.1002/path.2119. [DOI] [PubMed] [Google Scholar]
- 24.Noseda M., Fu Y., Niessen K., Wong F., Chang L., McLean G., Karsan A. Smooth muscle alpha-actin is a direct target of Notch/CSL. Circ Res. 2006;98:1468–1470. doi: 10.1161/01.RES.0000229683.81357.26. [DOI] [PubMed] [Google Scholar]
- 25.Buck M., Kim D.J., Houglum K., Hassanein T., Chojkier M. c-Myb modulates transcription of the alpha-smooth muscle actin gene in activated hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G321–G328. doi: 10.1152/ajpgi.2000.278.2.G321. [DOI] [PubMed] [Google Scholar]
- 26.Small E.M., Thatcher J.E., Sutherland L.B., Kinoshita H., Gerard R.D., Richardson J.A., Dimaio J.M., Sadek H., Kuwahara K., Olson E.N. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010;107:294–304. doi: 10.1161/CIRCRESAHA.110.223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masszi A., Speight P., Charbonney E., Lodyga M., Nakano H., Szaszi K., Kapus A. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol. 2010;188:383–399. doi: 10.1083/jcb.200906155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crider B.J., Risinger G.M., Jr, Haaksma C.J., Howard E.W., Tomasek J.J. Myocardin-related transcription factors A and B are key regulators of TGF-β1-induced fibroblast to myofibroblast differentiation. J Invest Dermatol. 2011;131:2378–2385. doi: 10.1038/jid.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Fan P.S., Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–2279. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]
- 30.Mann J., Chu D.C., Maxwell A., Oakley F., Zhu N.L., Tsukamoto H., Mann D.A. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705–714. doi: 10.1053/j.gastro.2009.10.002. 714.e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B., Wu Z., Liu T., Ullenbruch M.R., Jin H., Phan S.H. Gut-enriched Kruppel-like factor interaction with Smad3 inhibits myofibroblast differentiation. Am J Respir Cell Mol Biol. 2007;36:78–84. doi: 10.1165/rcmb.2006-0043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess H.A., Daugherty L.E., Thatcher T.H., Lakatos H.F., Ray D.M., Redonnet M., Phipps R.P., Sime P.J. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 33.Hu B., Wu Y.M., Wu Z., Phan S.H. Nkx2.5/Csx represses myofibroblast differentiation. Am J Respir Cell Mol Biol. 2010;42:218–226. doi: 10.1165/rcmb.2008-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang A., Liu X., Cogan J.G., Fuerst M.D., Polikandriotis J.A., Kelm R.J., Jr, Strauch A.R. YB-1 coordinates vascular smooth muscle alpha-actin gene activation by transforming growth factor beta1 and thrombin during differentiation of human pulmonary myofibroblasts. Mol Biol Cell. 2005;16:4931–4940. doi: 10.1091/mbc.E05-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bechtel W., McGoohan S., Zeisberg E.M., Muller G.A., Kalbacher H., Salant D.J., Muller C.A., Kalluri R., Zeisberg M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glenisson W., Castronovo V., Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta. 2007;1773:1572–1582. doi: 10.1016/j.bbamcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Guo W., Shan B., Klingsberg R.C., Qin X., Lasky J.A. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–L870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato M., Arce L., Wang M., Putta S., Lanting L., Natarajan R. A microRNA circuit mediates transforming growth factor-beta1 autoregulation in renal glomerular mesangial cells. Kidney Int. 2011;80:358–368. doi: 10.1038/ki.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V.J., Kaminski N., Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato M., Putta S., Wang M., Yuan H., Lanting L., Nair I., Gunn A., Nakagawa Y., Shimano H., Todorov I., Rossi J.J., Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato M., Wang L., Putta S., Wang M., Yuan H., Sun G., Lanting L., Todorov I., Rossi J.J., Natarajan R. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells. J Biol Chem. 2010;285:34004–34015. doi: 10.1074/jbc.M110.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu B., Gharaee-Kermani M., Wu Z., Phan S.H. Epigenetic regulation of myofibroblast differentiation by DNA methylation. Am J Pathol. 2010;177:21–28. doi: 10.2353/ajpath.2010.090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chau B.N., Brenner D.A. What goes up must come down: the emerging role of microRNA in fibrosis. Hepatology. 2011;53:4–6. doi: 10.1002/hep.24071. [DOI] [PubMed] [Google Scholar]
- 44.Maurer B., Stanczyk J., Jungel A., Akhmetshina A., Trenkmann M., Brock M., Kowal-Bielecka O., Gay R.E., Michel B.A., Distler J.H., Gay S., Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 45.Cushing L., Kuang P.P., Qian J., Shao F., Wu J., Little F., Thannickal V.J., Cardoso W.V., Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rooij E., Sutherland L.B., Thatcher J.E., DiMaio J.M., Naseem R.H., Marshall W.S., Hill J.A., Olson E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roderburg C., Urban G.W., Bettermann K., Vucur M., Zimmermann H., Schmidt S., Janssen J., Koppe C., Knolle P., Castoldi M., Tacke F., Trautwein C., Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 48.Wang B., Komers R., Carew R., Winbanks C.E., Xu B., Herman-Edelstein M., Koh P., Thomas M., Jandeleit-Dahm K., Gregorevic P., Cooper M.E., Kantharidis P. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol. 2012;23:252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang B., Koh P., Winbanks C., Coughlan M.T., McClelland A., Watson A., Jandeleit-Dahm K., Burns W.C., Thomas M.C., Cooper M.E., Kantharidis P. miR-200a prevents renal fibrogenesis through repression of TGF-beta2 expression. Diabetes. 2010;60:280–287. doi: 10.2337/db10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wipff P.J., Hinz B. Integrins and the activation of latent transforming growth factor beta1: an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Varga J., Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varga J., Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim K.K., Wei Y., Szekeres C., Kugler M.C., Wolters P.J., Hill M.L., Frank J.A., Brumwell A.N., Wheeler S.E., Kreidberg J.A., Chapman H.A. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu T., Hu B., Choi Y.Y., Chung M., Ullenbruch M., Yu H., Lowe J.B., Phan S.H. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174:1745–1755. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mimura Y., Ihn H., Jinnin M., Asano Y., Yamane K., Tamaki K. Constitutive phosphorylation of focal adhesion kinase is involved in the myofibroblast differentiation of scleroderma fibroblasts. J Invest Dermatol. 2005;124:886–892. doi: 10.1111/j.0022-202X.2005.23701.x. [DOI] [PubMed] [Google Scholar]
- 56.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amara N., Goven D., Prost F., Muloway R., Crestani B., Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carnesecchi S., Deffert C., Donati Y., Basset O., Hinz B., Preynat-Seauve O., Guichard C., Arbiser J.L., Banfi B., Pache J.C., Barazzone-Argiroffo C., Krause K.H. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waghray M., Cui Z., Horowitz J.C., Subramanian I.M., Martinez F.J., Toews G.B., Thannickal V.J. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 60.Pittet B., Rubbia-Brandt L., Desmoulière A., Sappino A.P., Roggero P., Guerret S., Grimaud J.A., Lacher R., Montandon D., Gabbiani G. Effect of gamma-interferon on the clinical and biologic evolution of hypertrophic scars and Dupuytren's disease: an open pilot study. Plast Reconstr Surg. 1994;93:1224–1235. doi: 10.1097/00006534-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Jiang D., Liang J., Campanella G.S., Guo R., Yu S., Xie T., Liu N., Jung Y., Homer R., Meltzer E.B., Li Y., Tager A.M., Goetinck P.F., Luster A.D., Noble P.W. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wells R.G. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 63.Grinnell F., Petroll W.M. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–361. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 64.Wipff P.J., Rifkin D.B., Meister J.J., Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y., Hagood J.S., Lu B., Merryman W.D., Murphy-Ullrich J.E. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–22393. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sambo P., Baroni S.S., Luchetti M., Paroncini P., Dusi S., Orlandini G., Gabrielli A. Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001;44:2653–2664. doi: 10.1002/1529-0131(200111)44:11<2653::aid-art445>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 67.Toullec A., Gerald D., Despouy G., Bourachot B., Cardon M., Lefort S., Richardson M., Rigaill G., Parrini M.C., Lucchesi C., Bellanger D., Stern M.H., Dubois T., Sastre-Garau X., Delattre O., Vincent-Salomon A., Mechta-Grigoriou F. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Georges P.C., Hui J.J., Gombos Z., McCormick M.E., Wang A.Y., Uemura M., Mick R., Janmey P.A., Furth E.E., Wells R.G. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 69.Barry-Hamilton V., Spangler R., Marshall D., McCauley S., Rodriguez H.M., Oyasu M., Mikels A., Vaysberg M., Ghermazien H., Wai C., Garcia C.A., Velayo A.C., Jorgensen B., Biermann D., Tsai D., Green J., Zaffryar-Eilot S., Holzer A., Ogg S., Thai D., Neufeld G., Van Vlasselaer P., Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 70.Barker H.E., Chang J., Cox T.R., Lang G., Bird D., Nicolau M., Evans H.R., Gartland A., Erler J.T. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Slot A.J., Zuurmond A.M., Bardoel A.F., Wijmenga C., Pruijs H.E., Sillence D.O., Brinckmann J., Abraham D.J., Black C.M., Verzijl N., DeGroot J., Hanemaaijer R., TeKoppele J.M., Huizinga T.W., Bank R.A. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- 72.Elkabets M., Gifford A.M., Scheel C., Nilsson B., Reinhardt F., Bray M.A., Carpenter A.E., Jirstrom K., Magnusson K., Ebert B.L., Ponten F., Weinberg R.A., McAllister S.S. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest. 2011;121:784–799. doi: 10.1172/JCI43757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 75.Kohan M., Muro A.F., White E.S., Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010;24:4503–4512. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muro A.F., Moretti F.A., Moore B.B., Yan M., Atrasz R.G., Wilke C.A., Flaherty K.R., Martinez F.J., Tsui J.L., Sheppard D., Baralle F.E., Toews G.B., White E.S. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bielesz B., Sirin Y., Si H., Niranjan T., Gruenwald A., Ahn S., Kato H., Pullman J., Gessler M., Haase V.H., Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu F., Mih J.D., Shea B.S., Kho A.T., Sharif A.S., Tager A.M., Tschumperlin D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olsen A.L., Bloomer S.A., Chan E.P., Gaca M.D., Georges P.C., Sackey B., Uemura M., Janmey P.A., Wells R.G. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bissell M.J., Hines W.C. Why don't we get more cancer?: a proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buscemi L., Ramonet D., Klingberg F., Formey A., Smith-Clerc J., Meister J.J., Hinz B. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 82.Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T., Springer T.A. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramirez F., Rifkin D.B. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bailey J.M., Swanson B.J., Hamada T., Eggers J.P., Singh P.K., Caffery T., Ouellette M.M., Hollingsworth M.A. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larsson O., Diebold D., Fan D., Peterson M., Nho R.S., Bitterman P.B., Henke C.A. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS One. 2008;3:e3220. doi: 10.1371/journal.pone.0003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sargent J.L., Whitfield M.L. Capturing the heterogeneity in systemic sclerosis with genome-wide expression profiling. Expert Rev Clin Immunol. 2011;7:463–473. doi: 10.1586/eci.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei J., Bhattacharyya S., Tourtellotte W.G., Varga J. Fibrosis in systemic sclerosis: emerging concepts and implications for targeted therapy. Autoimmun Rev. 2010;10:267–275. doi: 10.1016/j.autrev.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gardner H., Shearstone J.R., Bandaru R., Crowell T., Lynes M., Trojanowska M., Pannu J., Smith E., Jablonska S., Blaszczyk M., Tan F.K., Mayes M.D. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 2006;54:1961–1973. doi: 10.1002/art.21894. [DOI] [PubMed] [Google Scholar]
- 89.Sanders Y.Y., Tollefsbol T.O., Varisco B.M., Hagood J.S. Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. Am J Respir Cell Mol Biol. 2011;45:16–23. doi: 10.1165/rcmb.2010-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kis K., Liu X., Hagood J.S. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie T., Liang J., Guo R., Liu N., Noble P.W., Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics. 2011;43:479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rudnicka L., Varga J., Christiano A.M., Iozzo R.V., Jimenez S.A., Uitto J. Elevated expression of type VII collagen in the skin of patients with systemic sclerosis: regulation by transforming growth factor-beta. J Clin Invest. 1994;93:1709–1715. doi: 10.1172/JCI117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajkumar V.S., Howell K., Csiszar K., Denton C.P., Black C.M., Abraham D.J. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–R1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bayle J., Fitch J., Jacobsen K., Kumar R., Lafyatis R., Lemaire R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128:871–881. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- 95.Siracusa L.D., McGrath R., Ma Q., Moskow J.J., Manne J., Christner P.J., Buchberg A.M., Jimenez S.A. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skin mutation. Genome Res. 1996;6:300–313. doi: 10.1101/gr.6.4.300. [DOI] [PubMed] [Google Scholar]
- 96.Jelaska A., Korn J.H. Role of apoptosis and transforming growth factor beta1 in fibroblast selection and activation in systemic sclerosis. Arthritis Rheum. 2000;43:2230–2239. doi: 10.1002/1529-0131(200010)43:10<2230::AID-ANR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 97.Kissin E.Y., Merkel P.A., Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54:3655–3660. doi: 10.1002/art.22186. [DOI] [PubMed] [Google Scholar]
- 98.Li Z., Jimenez S.A. Protein kinase Cδ and the c-Abl kinase are required for transforming growth factor β induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum. 2011;63:2473–2483. doi: 10.1002/art.30317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aden N., Nuttall A., Shiwen X., de Winter P., Leask A., Black C.M., Denton C.P., Abraham D.J., Stratton R.J. Epithelial cells promote fibroblast activation via IL-1alpha in systemic sclerosis. J Invest Dermatol. 2010;130:2191–2200. doi: 10.1038/jid.2010.120. [DOI] [PubMed] [Google Scholar]
- 100.Zhou G., Dada L.A., Wu M., Kelly A., Trejo H., Zhou Q., Varga J., Sznajder J.I. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1120–L1130. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leroy E.C. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med. 1972;135:1351–1362. doi: 10.1084/jem.135.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trojanowska M. What did we learn by studying scleroderma fibroblasts? Clin Exp Rheumatol. 2004;22:S59–S63. [PubMed] [Google Scholar]
- 103.Kirk T.Z., Mark M.E., Chua C.C., Chua B.H., Mayes M.D. Myofibroblasts from scleroderma skin synthesize elevated levels of collagen and tissue inhibitor of metalloproteinase (TIMP-1) with two forms of TIMP-1. J Biol Chem. 1995;270:3423–3428. doi: 10.1074/jbc.270.7.3423. [DOI] [PubMed] [Google Scholar]
- 104.Jun J.B., Kuechle M., Min J., Shim S.C., Kim G., Montenegro V., Korn J.H., Elkon K.B. Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J Invest Dermatol. 2005;124:298–303. doi: 10.1111/j.0022-202X.2004.23559.x. [DOI] [PubMed] [Google Scholar]
- 105.Mori Y., Chen S.J., Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964–1978. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 106.Pannu J., Gardner H., Shearstone J.R., Smith E., Trojanowska M. Increased levels of transforming growth factor beta receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum. 2006;54:3011–3021. doi: 10.1002/art.22063. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V., Valerius M.T., McMahon A.P., Duffield J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin S.L., Kisseleva T., Brenner D.A., Duffield J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeisberg M., Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med. 2004;82:175–181. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 111.Inoue T., Okada H., Takenaka T., Watanabe Y., Suzuki H. A case report suggesting the occurrence of epithelial-mesenchymal transition in obstructive nephropathy. Clin Exp Nephrol. 2009;13:385–388. doi: 10.1007/s10157-009-0168-4. [DOI] [PubMed] [Google Scholar]
- 112.Zeisberg M., Duffield J.S. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 113.Popov Y., Schuppan D. Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive? Gastroenterology. 2010;139:722–725. doi: 10.1053/j.gastro.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 114.Koesters R., Kaissling B., Lehir M., Picard N., Theilig F., Gebhardt R., Glick A.B., Hahnel B., Hosser H., Grone H.J., Kriz W. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson D.W., Saunders H.J., Baxter R.C., Field M.J., Pollock C.A. Paracrine stimulation of human renal fibroblasts by proximal tubule cells. Kidney Int. 1998;54:747–757. doi: 10.1046/j.1523-1755.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- 116.Abbate M., Zoja C., Rottoli D., Corna D., Tomasoni S., Remuzzi G. Proximal tubular cells promote fibrogenesis by TGF-beta1-mediated induction of peritubular myofibroblasts. Kidney Int. 2002;61:2066–2077. doi: 10.1046/j.1523-1755.2002.00380.x. [DOI] [PubMed] [Google Scholar]
- 117.Udo K., Aoki S., Uchihashi K., Kawasaki M., Matsunobu A., Tokuda Y., Ootani A., Toda S., Uozumi J. Adipose tissue explants and MDCK cells reciprocally regulate their morphogenesis in coculture. Kidney Int. 2010;78:60–68. doi: 10.1038/ki.2010.68. [DOI] [PubMed] [Google Scholar]
- 118.Schelling J.R., Cleveland R.P. Involvement of Fas-dependent apoptosis in renal tubular epithelial cell deletion in chronic renal failure. Kidney Int. 1999;56:1313–1316. doi: 10.1046/j.1523-1755.1999.00684.x. [DOI] [PubMed] [Google Scholar]
- 119.Chawla L.S., Amdur R.L., Amodeo S., Kimmel P.L., Palant C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Selman M., Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 121.Thannickal V.J., Toews G.B., White E.S., Lynch J.P., 3rd, Martinez F.J. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 122.De Wever O., Demetter P., Mareel M., Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 123.Evans M.J., Van Winkle L.S., Fanucchi M.V., Plopper C.G. The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am J Respir Cell Mol Biol. 1999;21:655–657. doi: 10.1165/ajrcmb.21.6.3807. [DOI] [PubMed] [Google Scholar]
- 124.Araya J., Nishimura S.L. Fibrogenic reactions in lung disease. Annu Rev Pathol. 2010;5:77–98. doi: 10.1146/annurev.pathol.4.110807.092217. [DOI] [PubMed] [Google Scholar]
- 125.Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., Hogan B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thannickal V.J., Horowitz J.C. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Horowitz J.C., Lee D.Y., Waghray M., Keshamouni V.G., Thomas P.E., Zhang H., Cui Z., Thannickal V.J. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Horowitz J.C., Rogers D.S., Sharma V., Vittal R., White E.S., Cui Z., Thannickal V.J. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vittal R., Horowitz J.C., Moore B.B., Zhang H., Martinez F.J., Toews G.B., Standiford T.J., Thannickal V.J. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hecker L., Jagirdar R., Jin T., Thannickal V.J. Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res. 2011;317:1914–1921. doi: 10.1016/j.yexcr.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hardie W.D., Glasser S.W., Hagood J.S. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Friedman S.L. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 133.Desmoulière A. Hepatic stellate cells: the only cells involved in liver fibrogenesis?: a dogma challenged. Gastroenterology. 2007;132:2059–2062. doi: 10.1053/j.gastro.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 134.Dranoff J.A., Wells R.G. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hinz B. The Myofibroblast in Connective Tissue Repair and Regeneration. In: Ralphs C.A.J., editor. Woodhead Publishing Ltd; Cambridge: 2010. pp. 39–82. [Google Scholar]
- 136.Greenbaum L.E., Wells R.G. The role of stem cells in liver repair and fibrosis. Int J Biochem Cell Biol. 2011;43:222–229. doi: 10.1016/j.biocel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fujimiya T., Liu J., Kojima H., Shirafuji S., Kimura H., Fujimiya M. Pathological roles of bone marrow-derived stellate cells in a mouse model of alcohol-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2009;297:G451–G460. doi: 10.1152/ajpgi.00055.2009. [DOI] [PubMed] [Google Scholar]
- 138.Higashiyama R., Moro T., Nakao S., Mikami K., Fukumitsu H., Ueda Y., Ikeda K., Adachi E., Bou-Gharios G., Okazaki I., Inagaki Y. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology. 2009;137:1459–1466. doi: 10.1053/j.gastro.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 139.Chu A.S., Diaz R., Hui J.J., Yanger K., Zong Y., Alpini G., Stanger B.Z., Wells R.G. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guyot C., Lepreux S., Combe C., Doudnikoff E., Bioulac-Sage P., Balabaud C., Desmoulière A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 141.Guyot C., Combe C., Balabaud C., Bioulac-Sage P., Desmoulière A. Fibrogenic cell fate during fibrotic tissue remodelling observed in rat and human cultured liver slices. J Hepatol. 2007;46:142–150. doi: 10.1016/j.jhep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 142.Guyot C., Lepreux S., Combe C., Sarrazy V., Billet F., Balabaud C., Bioulac-Sage P., Desmouliere A. Fibrogenic cell phenotype modifications during remodelling of normal and pathological human liver in cultured slices. Liver Int. 2010;30:1529–1540. doi: 10.1111/j.1478-3231.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 143.Bosselut N., Housset C., Marcelo P., Rey C., Burmester T., Vinh J., Vaubourdolle M., Cadoret A., Baudin B. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics. 2010;10:1017–1028. doi: 10.1002/pmic.200900257. [DOI] [PubMed] [Google Scholar]
- 144.Ramachandran P., Iredale J.P. Reversibility of liver fibrosis. Ann Hepatol. 2009;8:283–291. [PubMed] [Google Scholar]
- 145.Popov Y., Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 146.Paulsson J., Sjoblom T., Micke P., Ponten F., Landberg G., Heldin C.H., Bergh J., Brennan D.J., Jirstrom K., Ostman A. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am J Pathol. 2009;175:334–341. doi: 10.2353/ajpath.2009.081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ikenaga N., Ohuchida K., Mizumoto K., Cui L., Kayashima T., Morimatsu K., Moriyama T., Nakata K., Fujita H., Tanaka M. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041–1051. doi: 10.1053/j.gastro.2010.05.084. 1051.e1-8. [DOI] [PubMed] [Google Scholar]
- 148.Sloan E.K., Ciocca D.R., Pouliot N., Natoli A., Restall C., Henderson M.A., Fanelli M.A., Cuello-Carrion F.D., Gago F.E., Anderson R.L. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Farmer P., Bonnefoi H., Anderle P., Cameron D., Wirapati P., Becette V., Andre S., Piccart M., Campone M., Brain E., Macgrogan G., Petit T., Jassem J., Bibeau F., Blot E., Bogaerts J., Aguet M., Bergh J., Iggo R., Delorenzi M. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 150.Kojima Y., Acar A., Eaton E.N., Mellody K.T., Scheel C., Ben-Porath I., Onder T.T., Wang Z.C., Richardson A.L., Weinberg R.A., Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodier F., Coppe J.P., Patil C.K., Hoeijmakers W.A., Munoz D.P., Raza S.R., Freund A., Campeau E., Davalos A.R., Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Erez N., Truitt M., Olson P., Arron S.T., Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 153.Gilbert L.A., Hemann M.T. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quante M., Tu S.P., Tomita H., Gonda T., Wang S.S., Takashi S., Baik G.H., Shibata W., Diprete B., Betz K.S., Friedman R., Varro A., Tycko B., Wang T.C. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hendrix A., Westbroek W., Bracke M., De Wever O. An ex(o) citing machinery for invasive tumor growth. Cancer Res. 2010;70:9533–9537. doi: 10.1158/0008-5472.CAN-10-3248. [DOI] [PubMed] [Google Scholar]
- 156.Webber J., Steadman R., Mason M.D., Tabi Z., Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 157.Duda D.G., Duyverman A.M., Kohno M., Snuderl M., Steller E.J., Fukumura D., Jain R.K. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhowmick N.A., Chytil A., Plieth D., Gorska A.E., Dumont N., Shappell S., Washington M.K., Neilson E.G., Moses H.L. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 159.Hill R., Song Y., Cardiff R.D., Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 160.Trimboli A.J., Cantemir-Stone C.Z., Li F., Wallace J.A., Merchant A., Creasap N., Thompson J.C., Caserta E., Wang H., Chong J.L., Naidu S., Wei G., Sharma S.M., Stephens J.A., Fernandez S.A., Gurcan M.N., Weinstein M.B., Barsky S.H., Yee L., Rosol T.J., Stromberg P.C., Robinson M.L., Pepin F., Hallett M., Park M., Ostrowski M.C., Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hinz B., Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78–82. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van der Slot A.J., Zuurmond A.M., van den Bogaerdt A.J., Ulrich M.M., Middelkoop E., Boers W., Karel Ronday H., DeGroot J., Huizinga T.W., Bank R.A. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004;23:251–257. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 163.Horan G.S., Wood S., Ona V., Li D.J., Lukashev M.E., Weinreb P.H., Simon K.J., Hahm K., Allaire N.E., Rinaldi N.J., Goyal J., Feghali-Bostwick C.A., Matteson E.L., O'Hara C., Lafyatis R., Davis G.S., Huang X., Sheppard D., Violette S.M. Partial inhibition of integrin alpha(v) beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 164.de Andrade J.A., Thannickal V.J. Innovative approaches to the therapy of fibrosis. Curr Opin Rheumatol. 2009;1:649–655. doi: 10.1097/BOR.0b013e328330da9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang H.Y., Phan S.H. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 166.Gonzalez-Cadavid N.F., Rajfer J. Treatment of Peyronie's disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol. 2010;7:215–221. doi: 10.1038/nrurol.2010.24. [DOI] [PubMed] [Google Scholar]
- 167.Hinz B., Gabbiani G., Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157:657–663. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]