Abstract
The abundance of a cytoplasmic mRNA in eukaryotes often determines the level of the encoded protein product. The rates at which an mRNA is synthesized, exported, and degraded collectively contribute to its abundance in all cell types. Numerous mRNAs, particularly those encoding structural proteins, are very stable, with half-lives in the order of many hours. In contrast, mRNAs encoding regulatory proteins, including oncoproteins, cytokines, and signaling proteins, are relatively unstable with half-lives of an hour or less. As a result, modest changes in their decay rates affect their levels over a relatively short time period. This is particularly important to ensure rapid responses to extracellular signaling events. Messenger RNAs often harbor sequence elements that dictate their degradation rates. Adenylate uridylate (A+U)-rich elements (AREs), first identified in 1986, are perhaps the best characterized sequences that promote rapid mRNA degradation. These elements, localized within 3′-untranslated regions, sometimes contain AUUUA pentamers within an overall U-rich sequence, but this does not always define a bona fide ARE. Thus, experimental validation is essential before bestowing upon a suspected A+U-rich sequence the title of “ARE.” This chapter describes a reporter gene system that permits quantitative assessment of the effects of candidate A+U-rich sequences on mRNA half-life. This system employs tetracycline-controlled transcriptional silencing of the reporter gene, isolation of total-cell RNA at selected time points, quantitative reverse transcriptase polymerase chain reaction analysis of reporter mRNA levels, and nonlinear regression analysis of mRNA level as a function of time to quantitatively define parameters describing mRNA decay kinetics. Finally, this chapter describes more specialized assays to characterize ARE-mediated mRNA decay pathways, including deadenylation, and discusses decapping.
1. Introduction
mRNA decay plays a major role in regulating the precise timing and expression of many gene products in eukaryotes. It is, therefore, important to consider how the process of mRNA decay affects gene expression. The rate at which an mRNA decays determines not only its steady-state levels, but also its rate of disappearance in response to transcriptional silencing of its cognate gene. The decay rate of an mRNA also determines the length of time necessary for it to reach its steady-state level upon transcriptional induction of its gene: the shorter the mRNA half-life, the sooner it reaches steady state (Ross, 1995). Messenger RNA decay rates are often not fixed, but can change in response to a variety of stimuli. This dynamic control of mRNA decay rates contributes significantly to rapid changes in cellular gene expression programs in response to hormonal, environmental, and/or developmental cues.
Within the structure of an mRNA are elements that determine its decay rate. These are found in the 5′-untranslated region (UTR), the protein coding region, and/or the 3′-UTR. The most widespread, and best characterized, family of mRNA degradation elements consists of adenylate uridylate (A+U)-rich elements (AREs), located within 3′-UTRs. ARE-bearing mRNAs are present in species ranging from humans to the yeast Saccharomyces cerevisiae (Duttagupta et al., 2003). In mammals, AREs direct rapid degradation of many mRNAs encoding oncoproteins, cytokines, and G-protein-coupled receptors, to name just a few (Wilson et al., 1999). AREs have been categorized into three broad classes based on sequence characteristics: class I—one or more AUUUA pentamers within a U-rich sequence; class II—tandem repeats of AUUUA; and class III—U-rich sequences lacking AUUUA motifs (Chen et al., 1995; Wilusz et al., 2001). Bioinformatic analyses predict that as many as 7% (≈2000) of the mRNAs in the human transcriptome harbor AREs (Bakheet et al., 2001). However, very few of these AREs have actually been validated experimentally.
Diverse RNA–protein and protein–protein interactions serve as the effectors of ARE-mediated mRNA decay (AMD; Knapinska et al., 2005; Wilusz et al., 2001). Numerous A+U-binding proteins (AUBPs) have been cloned over the years. As their name implies, these proteins bind to AREs to promote AMD; the exception is the Hu family of AUBPs, which serve to block AMD, thereby inducing, rather than silencing, the expression of ARE-mRNAs. In some cases, micro-RNAs (miRNAs) are also required for optimal AMD (Jing et al., 2005). It is likely that AUBPs and miRNAs act to recruit the decay machinery to an ARE-mRNA whereby deadenylation, decapping, and 3–5′ and/or 5′–3′ degradation deconstruct the transcript. In a limited number of cases, an ARE promotes endonucleolytic cleavage of the mRNA rather than deadenylation as the rate-limiting step in degradation (Stoeckle, 1992; Zhao et al., 2000).
Over the years, various methods have been developed to determine mRNA half-life, including kinetic labeling techniques, drug-dependent transcriptional inhibition, or use of promoters regulated by transient induction or repression (Harrold et al., 1991; Speth and Oberbaumer, 1993). Kinetic labeling techniques are limited in that they require analysis of relatively abundant and stable mRNAs (Loflin et al., 1999). It is thus not suited for analyses of low-copy mRNAs or those suspected of having short half-lives. A second approach to measuring mRNA decay kinetics is to chemically inhibit transcription and follow changes in abundance of an mRNA of interest as a function of time. While actinomycin D is typically utilized, there are examples in which this drug treatment has stabilized an mRNA that is otherwise known to be degraded rapidly (Shyu et al., 1989; Speth and Oberbaumer, 1993). Furthermore, because actinomycin D and other RNA polymerase inhibitors arrest all mRNA synthesis, these approaches generally measure the decay of specific transcripts within dying cell populations. Finally, a transient-induction assay can be performed to monitor mRNA decay after growth factor stimulation (Shyu et al., 1989). Also known as transcriptional pulsing, its underlying principle is to provide a stimulus, such as a growth factor for the FOS promoter, that will lead to a short burst of mRNA synthesis, after which decay of the mRNA can be followed. While this approach can be successful, the inducible promoter must be under very precise on–off regulation. However, there are few eukaryotic promoters that meet this stringent qualification (Loflin et al., 1999). A further caveat of this approach is that the transcriptional stimuli must not include components capable of modulating mRNA decay kinetics.
To circumvent these problems, we and others have utilized the tetracycline-responsive transcription system described by Gossen and Bujard (1992). A gene of interest is cloned downstream of a minimal cytomegalovirus (CMV) promoter linked to a tetracycline-responsive element immediately upstream. Cells are cotransfected with this plasmid and a plasmid expressing a tetracycline-controlled transactivator protein (tTA). In the absence of tetracycline, tTA binds the promoter and activates transcription of the transgene. Upon addition of tetracycline, tTA dissociates from the promoter, and transcription of the transgene ceases. Decay of the transgene-encoded mRNA is then followed to establish mRNA decay kinetics. Specific mechanisms contributing to mRNA catabolism, such as deadenylation and decapping, can also be monitored.
2. Reporter Gene System
The experiments of Shaw and Kamen (1986) first demonstrated that insertion of the ARE from the granulocyte–macrophage colony-stimulating factor (GM-CSF) gene into the 3′-UTR of the rabbit β-globin gene reduced product mRNA half-life to less than 30 mins; β-globin mRNAs have a normal half-life of 20–30 h (Ross and Sullivan, 1985). Since that time, the rabbit β-globin (Rβ) gene has proven to be an effective reporter gene for analyses of AMD. However, other reporter genes have been utilized with success, including green fluorescent protein (GFP), β-galactosidase, and firefly luciferase.
We utilize the Rβ gene, as it encodes a very stable mRNA. Thus, the effect of an ARE on mRNA can be quite pronounced. For tetracycline-responsive transcription, we cloned the Rβ gene into the plasmid pTRE (BD Biosciences) to generate the plasmid pTRE-Rβ (Fig. 3.1). The SV40 polyadenylation signal from pTRE was excised so that transcription termination and polyadenylation signals from the Rβ gene are utilized. The Rβ gene contains a unique BglII restriction enzyme site that lies in the 3′-UTR adjacent to the stop codon. This site provides a convenient insertion point for any ARE, or suspected ARE, for analyses of mRNA decay. Even though the BglII site contains the last two bases of the stop codon, the site can be filled in with Klenow or T4 DNA polymerase for blunt-end cloning without disrupting the stop codon. For short AREs (<60 bp), two complementary deoxyribonucleotides harboring 5′-GATC can be commercially synthesized, annealed, kinased, and used for ligation to BglII-digested pTRE-Rβ. The 5′-GATC overhangs on the annealed deoxyribonucleotides providing the sticky ends for annealing and ligation to BglII-digested pTRE-Rβ. Longer AREs can be obtained by polymerase chain reaction (PCR) using gene-specific primers incorporating BamHI and/or BglII restriction sites; both enzymes produce 5′-GATC overhangs. If it is not possible to engineer either of these restriction enzyme sites, the blunt-end PCR product can be cloned into plasmid pTRE-Rβ after digesting the plasmid with BglII and performing a fill reaction using Klenow and all four deoxyribonucleotides.
Fig 3.1.
Tetracycline-responsive reporter plasmid for mRNA decay analyses. The 1.7-kb Rβ gene was cloned into the XbaI-HindIII site of plasmid pTRE (BD Biosciences). (Upon cloning, the HindIII site was destroyed as indicated by the slash.) This deleted the SV40 polyadenylation sequence in pTRE such that the Rβ polyadenylation sequence in exon 3 is utilized. The minimal CMV promoter, which lacks the enhancer, is labeled Pmin CMV; it is linked to seven copies of the tetracycline operator sequence, labeled tetO.The unique BglII site located just downstream of the stop codon is used for insertion of candidate AREs. Restriction sites between Pmin CMV and the Rβ gene are listed as well; these are not unique in the plasmid. For the Rβ gene, filled boxes are exons; open boxes are introns.
3. Construction of the Reporter Gene-ARE Plasmid
The following procedure describes the cloning of a PCR product into the BglII site of plasmid pTRE-Rβ. The PCR product is designed to incorporate a BamHI site into the 5′ end and a BglII site into the 3′ end; this facilitates screening of colonies for insertion of the PCR product in the proper orientation.
Procedure
-
PCR amplify the desired ARE-mRNA fragment using gene specific primers flanking a suspected ARE. The forward primer should incorporate a BamHI site, GGATCC. The reverse primer should incorporate a BglII site, AGATCT. We usually add a “GC clamp,” 5′-GCAC-3′, at the 5′ ends of primers, followed by nucleotides for the restriction site. Primer domains complementary to template cDNA should have a Tm of 58–60 °C.
(Note: To reiterate, the Tm does not include the restriction site or the GC clamp.)
-
If the template is a plasmid, 1–10 ng is sufficient. Other suitable templates include 0.5 ng of commercially available cDNA (BD Biosciences). If total-cell RNA or poly(A)+ mRNA is available, cDNA can be synthesized in a reverse transcriptase reaction prior to PCR. We have used the Access RT-PCR kit from Promega with success. It is also useful to use Pfu-based polymerases, as they possess proofreading activity, which minimizes misincorporation errors. We suggest basing amplification cycles as follows:
96 ° C, 2 min
10× [96 °C, 40 s; Tm (from step 1) −6 °C, 1 min; 72 ° C, 3 min]
15× (96 °C, 40 s; 62 °C, 1 min; 72 °C, 3 min)
-
72 °C, 10 min
(Note: the 96 °C melting temperature assumes that a Pfu-based polymerase is used. Additionally, these amplification cycles are a starting point and may need adjustment depending on the characteristics of the sequence to be amplified.)
Verify correct product size by fractionating 5 μl of the PCR reaction in a 1% agarose gel. Fractionate the remainder of the PCR reaction in a preparative 1% agarose gel and excise the band using a clean spatula. Purify the DNA in the gel slice using a gel extraction kit (Qiagen) and elute the DNA into 50 μl of deionized H2O. This step removes the template DNA, which is particularly important for a plasmid template.
Digest the DNA fragment overnight with restriction endonucleases BamHI and BglII at 37°C. Purify the digested fragment using the High PCR purification kit (Roche) and elute the DNA in 50 μl deionized H2O.
The plasmid pTRE-Rβ can be digested with BglII at the same time as the PCR product from step 4.
Self-ligation of the plasmid during subsequent ligation procedures can be minimized by removal of the 5′ phosphates generated during linearization. Add 1 μl of calf intestinal alkaline phosphatase (Promega) to the BglII digest of plasmid pTRE-Rβ and continue incubation for 1 h at 37 °C. Perform phenol–chloroform extraction once to remove enzymes. Precipitate the DNA with ethanol and resuspend it at a concentration of 100 ng/μl in deionized H2O.
Use 1 μl of linearized pTRE-Rβ fragment and 3 μl of purified, digested PCR product in a ligation reaction with 1 μl T4 DNA ligase (Promega) either at room temperature for at least 1 h or overnight at 15 °C.
Use 1 μl of ligation product to transform Escherichia coli DH5α, or comparable, electrocompetent cells. Use a voltage setting of 2.5 kV to electroporate bacterial cells. Add 1 ml Luria broth (LB) and incubate at 37 °C for 1 h with shaking at 250 rpm. Spread 50 μl of the culture on an LB agar plate containing 50 μg/ml ampicillin and culture for 14 to 16 h at 37 °C.
Select 5 to 10 colonies for the screening process and culture in 4 ml LB + ampicillin with shaking at 250 rpm in a 37 °C incubator for 14 to 16 h. Harvest DNA from cultures utilizing the Qiagen miniprep kit. Digest purified DNA using restriction endonucleases EcoRI and BglII for 2 h at 37 °C. Run samples in a 1% agarose gel. Digestion of clones will produce three fragments. For a negative clone, one of these will be ≈80 bp, too small to see on a 1% agarose gel. A positive clone (i.e., with the insert in the proper orientation) will produce a fragment of 80 bp plus the size of the ARE or 3′-UTR sequence inserted; this should be readily detectable for AREs larger than 60 to 70 bp.
An alternative to restriction enzyme screening is to utilize colony PCR (Dallas-Yang et al., 1998; Ward, 1992). The forward primer is the following Rβ sequence: 5′-GAT ATA CAC TGT TTG AGA TGA GG-3′. The reverse primer is the same reverse primer used to generate the ARE-specific PCR product from step 1 given earlier. A PCR product is generated only if the insert is in the proper orientation.
At completion of the cloning process, the reporter plasmid, pTRE-Rβ-ARE, can be purified in larger quantities using any number of methods. We typically use midiprep kits from Qiagen with excellent results.
4. Cell Culture and Transfection
The reporter gene is under the control of a tetracycline-regulated promoter, allowing transcription of the reporter cassette in cells expressing the tetracycline-controlled transactivation protein, tTA, when tetracycline is absent (Gossen and Bujard, 1992). Addition of tetracycline or its derivatives rapidly silences reporter transcription to initiate the time course for mRNA decay. A significant advantage to using a Tet-responsive system versus actinomycin D, for example, is that addition of tetracycline arrests transcription of the reporter mRNA only and does not otherwise adversely influence cell physiology. It is important to perform parallel transfections of plasmid pTRE-Rβ and pTRE-Rβ-ARE to allow comparison of mRNA decay kinetics and thus assessment of the effects of a candidate ARE upon mRNA decay. In the experiments described here, we utilized HeLa Tet-Off cells (BD Biosciences), which constitutively express tTA. (The HeLa cell line is a human cervical carcinoma.) A spectrum of cell lines is available from BD Biosciences. However, we have found that it works just as well to cotransfect reporter plasmids and plasmid pTet-Off (BD Biosciences), which expresses tTA. This permits analysis of AMD in specialized cell lines that are not available commercially.
There are two additional important points to note. First, together with the plasmids noted earlier, we cotransfect plasmid pEGFP-C2 (BD Biosciences), which encodes green fluorescent protein (EGFP); reporter mRNA levels can thus be normalized to levels of EGFP mRNA to control for possible variations in transfection efficiency or total-cell RNA recovery. Second, it is essential to limit the amount of reporter plasmid transfected, as it is relatively easy to saturate the AMD machinery (Laroia et al., 2002; Loflin et al., 1999; Xu et al., 1998). High-level expression of ARE-mRNAs leads to their stabilization and misinterpretation of the experimental results. To circumvent this problem, we transfect no more than 50 ng of reporter plasmid per 1.5 × 105 cells (Lasa et al., 2000). This allows sufficient reporter mRNA expression for reliable detection by quantitative RT-PCR.
Procedure
HeLa Tet-Off cells are cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated, fetal bovine serum (FBS), L-glutamine, and penicillin/streptomycin (P/S). Cells are maintained at 37 °C in 5% CO2 and should be monitored daily and split every 3–5 days depending on their degree of confluency.
On day 1, seed approximately 1.5 × 105 cells per well in two 6-well plates, as transfections are performed in duplicate. Cells are cultured in 2 ml DMEM, 10% FBS, L-glutamine and P/S (per well), and incubated at 37 °C overnight in a CO2 incubator.
-
On day 2, transfection mixes are prepared in a 15-ml Falcon tube for each reporter plasmid to be transfected. For each well, pipette the following: 1.5 μg of Bluescript plasmid pBSKII (Invitrogen) or an equivalent carrier DNA, 200 ng of plasmid pEGFP-C2, 50 ng of pTRE-Rβ reporter plasmid (±ARE), and 150 μl of OPTI-MEM (serum-free medium; Invitrogen) in a total volume of 160 μl.
(Note: If using cells that do not express the tTA transcription factor, add 500 ng of plasmid pTet-Off (BD Biosciences) to the transfection mix.)
Mix and then add 20 μl Superfect transfection reagent (Qiagen). Vortex the tube for 5 s and incubate it for 10 min at room temperature to allow DNA: Superfect complexes to form. Add 1 ml of DMEM, 10% FBS, and L-glutamine, mix well by pipetting up and down 5–6 times, and incubate for 10 min.
(Note: For multiple reactions, amounts are scaled up. For example, for two 6-well plates of cells transfected with the same reporter plasmid, multiply the aforementioned amounts by 12.)
Wash cells once with 2 ml PBS while DNA: Superfect complexes are forming. Add 1 ml of the DNA: Superfect transfection mixture to each well of a 6-well plate. Rock the plate back and forth several times to ensure that the cells are bathed in transfection mixture. Culture the cells for 4 h at 37 °C in a CO2 incubator.
-
Remove transfection mixture from the cells and replace with fresh DMEM, 10% FBS, L-glutamine, and P/S. Culture the cells for 2 days at 37 °C in a CO2 incubator.
Note: Transfection conditions and methods will undoubtedly vary among different cell lines. Regardless of the method of transfection employed; however, it is important to keep in mind that the amounts of reporter plasmids must be limited to prevent saturation of the cellular AMD machinery. Thus, in our experience for cell lines other than HeLa Tet-Off, we have tried to maintain 50 ng of reporter plasmid per 1.5 × 105 cells. Nonetheless, it may be important to examine various amounts of reporter plasmids with specialized cell lines.
5. Time Course and RNA Isolation
The mRNA decay time course is initiated by addition of the tetracycline derivative doxycycline to the culture medium. The advantages of doxycycline are that it is more stable and more soluble than tetracycline (http://www.bdbiosciences.com). Doxycycline addition quickly silences transcription of the reporter gene. RNA is then isolated at selected time points after transcriptional silencing. Because of the sensitivity of quantitative RT-PCR, isolation of DNA-free RNA is essential. This is particularly important with plasmid transfections, as reporter genes will be present in many, many copies per cell compared to endogenous genes.
Procedure
On day 4, 2 days following transfection, remove growth medium and initiate time course by the addition of 2 ml DMEM, 10% FBS, L-glutamine, and P/S containing 2 μg/ml doxycycline (Sigma). A stock solution of 1 mg/ml doxycycline can be prepared in sterile deionized H2O and stored at −20°C. Doxycycline is added to a concentration of 2 μg/ml in fresh growth medium and then added to the cells. It is important to apply doxycycline to cells premixed in fresh medium; in our experience, simply adding aliquots of the doxycycline stock solution to the existing culture medium significantly retards the arrest of reporter transcription.
For RNA isolation we use the RNeasy kit (Qiagen). Unless otherwise noted, all buffers are included in the kit. Harvest cells from each plate after 0, 0.5, 1, 2, 4, and 6 h by aspirating the medium and adding 350 μl of RLT lysis buffer containing β-mercaptoethanol. [Prepare a 10-ml stock solution of RLT buffer + 100 μl of β-mercaptoethanol (BioRad), which is stable at room temperature for 1 month.] Scrape the cell lysis mixture using a polyethylene scraper (Corning). Remove the lysate using a 1-ml Pipetman; it may be necessary to cut off the end of the pipette tip due to lysate viscosity. Use a clean razor blade to cut ≈2 mm from the end of the pipette tip. Pipette the viscous lysate into a QIAshredder cartridge (Qiagen) and spin in a microcentrifuge at room temperature for 2 min at maximum speed. This procedure fragments chromatin and improves RNA yields greatly. Place the collection tube on dry ice until all time point samples are collected.
Thaw frozen samples on ice. Add 350 μl of 70% ethanol to the sample and mix well. Pipette the mixture (≈700 μl) through an RNeasy mini-column and spin it in a microcentrifuge at room temperature for 15 s at 10,000 rpm.
Add 350 μl of buffer RW1 into the column and spin in a microcentrifuge at room temperature for 15 s at 10,000 rpm. Discard the flow through. Add 10 μl of DNase I stock solution to 70 μl of buffer RDD and add this mixture to the column. Incubate the column at room temperature for 15 min.
Add 350 μl of buffer RW1 into the column, spin in a microcentrifuge at room temperature for 15 s at 10,000 rpm, and discard the flow through. Transfer the column to a new collection tube, add 500 μl of buffer RPE into the column, and spin in a microcentrifuge at room temperature for 15 s at 10,000 rpm. Discard the flow through. Repeat the wash with 500 μl of buffer RPE, spin as before, and discard the flow through. Spin the column an additional 2 min at full speed to remove any remaining liquid from the column.
Transfer the RNeasy column to a fresh tube. To elute RNA, add 30 μl of deionized H2O directly onto the column membrane and spin it in a microcentrifuge at room temperature for 1 min at 10,000 rpm. Store the collected RNA at −80 °C.
Ensure RNA quality by fractionating 3 μl of RNA sample in a 1% agarose minigel to observe intact 28S and 18S ribosomal RNAs. The gel need not contain denaturing agents. Quantify RNA spectrophometrically by measuring absorbance at 260 nm.
6. Quantitation of mRNA Levels
While Northern blot analysis and RNase protection assays can detect changes in mRNA abundance, we find that quantitative, real-time RT-PCR (qPCR) is a relatively quick, easy, and sensitive means to measure mRNA levels for determinations of decay kinetics. Isolated RNA is first reverse transcribed to cDNA and subsequently amplified by PCR in the same reaction tube. We use fluorescent probes to measure the amount of DNA amplified (Bustin, 2000; Nolan et al., 2006). The gene-specific probe contains a fluorescent molecule at its 5′ end and a fluorescence quencher molecule at its 3′ end. During each annealing step, primers specific for Rβ reporter mRNAs and for the EGFP internal control mRNA hybridize to their respective complementary sequences. As Taq DNA polymerase extends DNA from the primers, it encounters the fluorescent probe, causing cleavage of the fluorescent molecule, releasing it from the quencher molecule, thus enhancing fluorescence emission. The first PCR cycle number in which fluorescence reaches its detection threshold is referred to as CT. Low CT values indicate large amounts of an mRNA, whereas high CT values indicate low amounts of an mRNA. In the following protocol, Rβ-specific primers amplify a portion of the mRNA coding region; thus, the same primer/probe set can be used regardless of the sequence cloned into the 3′-UTR for decay analyses. Additionally, the Rβ-specific primers span two different exons in the mRNA; this minimizes amplification of potentially contaminating plasmid DNA (which would include the intervening intron). Finally, because the probe for the internal control EGFP mRNA has a different fluorophore from the Rβ-specific probe, PCR reactions can be multiplexed. That is, both EGFP and Rβ mRNAs can be assayed at the same time in the same tube. This makes the internal control normalizations more accurate and reduces sample numbers, which saves PCR reagents.
Procedure
-
We perform one-step qPCR using the SuperScript III platinum one-step qRT-PCR kit (Invitrogen) with a MX3005P multiplex quantitative PCR system (Stratagene). Prepare sufficient reaction mix for all samples and a no template control (NTC). Assemble the PCR master mix in sterile optical tubes, 8× strips with optical caps (Stratagene). For each sample, mix 0.5 μl each of Rβ forward and reverse primers (stock concentrations 15 pmol/μl), 0.5 μl each of EGFP forward and reverse primers (stock concentrations 15 pmol/μl), 0.5 μl of Rβ probe (stock concentration 5 pmol/μl), 0.5 μl of EGFP probe (stock concentration 5 pmol/μl), and 1 μl Super Script III RT platinum with 25 μl of the 2 × reaction mix, included in the kit, in a total volume of 49.5 μl per sample. The following sequences are used for the Rβ primers: forward 5′-GTGAACTGCACTGTGACAAGC-3′ and reverse 5′-ATGAGTAGACAGCACAATAACCAG-3′. The following sequences are used for the EGFP primers: forward 5′-GCGA-CACCCTGGTGAACC-3′ and reverse 5′-GATGTTGTGGCGGATC TTGAAG-3′. The sequence for the Rβ probe is as follows: 5′-/56-FAM/CGTTGCCCAGGAGCCTGAAGTTCTCA/3BHQ_1/-3′ where FAM and BHQ_1 are fluorescein amidite and Black Hole Quencher-1, respectively. The following is the sequence for the EGFP probe 5′-Cal610/CACCTTGATGCCGTTCTT CTGCTTGTCG/3BHQ_2/-3′ where Cal 610 is CAL Fluor Red 610. We have also successfully used other long-wavelength probes (e.g., Texas red) in combination with Black Hole Quencher-2 (BHQ_2) for this application. Fluorescent probes and primers are from Integrated DNA Technologies, Inc. (http://www.idtdna.com).
(Note: It is very important to minimize the exposure of fluorescent probes and PCR reactions containing them to room light. Store stock tubes in foil at −20 °C.)
Assemble one separate mix to be used for the no reverse transcriptase (RT) control in which Super Script III RT platinum is omitted.
Add 49.5 μl of the PCR master mix to each qPCR tube (exclude no RT control). Use the separate mix lacking the RT enzyme (from step 2) in place of the master mix for the no RT control. Add 300 ng of RNA from each time point (isolated as described earlier) for a final volume of 55 μl per qPCR sample tube within the 8× strip. Exclude RNA from the NTC tube. Add 0.5 μl of platinum Taq polymerase (Invitrogen) to each sample. Mix the samples by repeated pipetting 5–6 times, avoiding formation of bubbles. Incubate samples on ice until they are ready to load into the thermocycler.
Program the qPCR thermocycler as follows: 50°C, 15 min; 95°C, 2 min; 50× (95°C, 15 s; 60°C, 30 s).
At cycle completion, remove samples. These can be stored at −20 °C for later gel analysis of band sizes for quality control.
-
Verify PCR products on a 2% agarose gel to confirm correct amplification products. The expected product sizes are 160 bp for Rβ and 90 bp for EGFP. Data analysis is described in the next section.
Note: As with any PCR-based procedure, it is essential to be judicious about using clean gloves, pipettes, and work surfaces to prevent contamination with primers or plasmids that are not intended to enter the experiment.
7. Analysis of qPCR Data for mRNA Half-Life
To analyze qPCR data from the previous section it is necessary to derive relative RNA abundance parameters from resolved CT values. While many qPCR manuals indicate that fold differences in RNA abundance between samples may be extracted from the differences in resolved CT values using 2ΔCT, this algorithm assumes that probe fragment amplification efficiency is 100%. To avoid this assumption, we generate standard curves that relate CT as a function of RNA mass for each measured transcript (R/2 and EGFP) under multiplexed amplification conditions. These standard curves are generated by performing the qPCR assay using serial dilutions of RNA from cells transfected with plasmids pTRE-Rβ and pEGFP-C2 and then plotting CT values versus log10 of RNA mass. Ideally, this experiment should consist of at least six RNA dilutions encompassing a large range of masses, run at least in duplicate. Theoretically, the CT values of the dilution series should yield a linear relationship given by the equation:
| (3.1) |
where A is the slope, which is related to the amplification efficiency, and B is the y intercept. The line determined by least-squares fit should have an R2 value very close to 1 (≥0.985) (http://www.stratagene.com). Points in the nonlinear range usually are RNA dilutions at very low concentrations (i.e., below detection limits) or are at very high concentrations that saturate the assay. Thus, the line delimits the CT range over which the qPCR assay is linear.
Using procedures described previously, we transfected HeLa Tet-Off cells with the pTRE-Rβ and pEGFP-C2 plasmids and prepared total-cell RNA 2 days later. We performed qPCR for both mRNAs in the same tube using a dilution series of total-cell RNA from 0.1 to 2000 ng. The assay was linear over CT values ranging from 22 to 36 for Rβ and 21 to 28 for EGFP (Fig. 3.2). Least-squares fit of data yielded the equation CT = –4.715 * (log10 RNA mass) + 34.79 with R2 = 0.991 for Rβ, and CT = –2.506 * (log10 RNA mass) + 29.28 with R2 = 0.998 for EGFP. While this is a convenient entry point to begin experiments, we recommend individual laboratories creating standard curves using the thermocycler and RT/ qPCR reagent kit that will be utilized routinely.
Fig 3.2.
Standard curves for qPCR analyses of Rβ reporter and EGFP internal control mRNAs. HeLa Tet-Off cells (BD Biosciences) were transfected with plasmids pT R E - R β and pEGFP-C2 (BD Biosciences) as described in the text. After 2 days, total-cell RNA was isolated. A titration of RNA masses from 0.1 to 2000 ng was analyzed by qPCR using fluorescent probes specific for Rβ (A) and EGFP (B) mRNAs using a MX3005P quantitative PCR system (Strategene). The included software analyzed CT versus log10 RNA mass by least-squares fit to determine the slope and y intercept of the line and R2.The derived equations for each mRNA are listed in the text. Only the linear portions of the assays are shown in the graphs.
With standard curves and equations in hand, CT values from qPCR analyses of RNAs from transfections of reporter Rβ and EGFP plasmids permit determination of a relative mass of both Rβ and EGFP mRNAs from each time point RNA. Since knowing the CT value yields log10 RNA mass, performing the simple calculation 10log (RNA mass) permits determination of relative Rβ or EGFP mRNA mass for each time point. Since EGFP is used for normalization, the mass ratio Rβ/EGFP is calculated for each time point. The mass ratios for each time point are then divided by the mass ratio for time zero. Thus, the mass ratio for time zero is set to 100%. This analysis yields a series of percentages that illustrate the amount of Rβ reporter mRNA (either with or without an ARE) remaining at each time point. The percentages should decline progressively if the reporter mRNA decays significantly over the time course of the experiment. Because mRNA decay generally approximates first-order kinetics (Belasco and Brawerman, 1993), a semilogarithmic plot of “percent Rβ mRNA remaining” versus “time in doxycycline” is normally linear. These data may be resolved using the first-order decay function
| (3.2) |
where [RNA]t is the percent Rβ mRNA remaining at each time t, [RNA]0 is the percent Rβ mRNA at t = 0, and k is the first-order decay constant (Belasco and Brawerman, 1993; Ross 1995). Messenger RNA half-life is then calculated from
| (3.3) |
Procedure
Using thermocycler software, print amplification plots and individual text reports (indicating CT numbers) for FAM (Rβ) and Cal Fluor Red (EGFP).
Record CT values at each time point for Rβ reporter and EGFP mRNAs in an Excel spreadsheet (Microsoft Corp.; available upon request; Fig. 3.3). From the equation CT = A*(log10 RNA mass) + B, determined from its standard curve, calculate the relative mRNA mass of Rβ at each time point. Use the CT values at each time point for EGFP to calculate relative mass of EGFP mRNA at each time point using the equation for EGFP derived from its standard curve. There will be two mass values for each of Rβ and EGFP at each time point since qPCR reactions are run in the duplicate.
Calculate the mass ratio Rβ/EGFP for each time point and then calculate the mean of each mass ratio for each time point (again, since qPCR reactions are run in duplicate for each time point). The time zero, mean mass ratio is defined as 100% Rβ reporter mRNA remaining. Divide the mean mass ratio for each time point by the mean mass ratio for time zero and multiply by 100%. This series of percentages represents how much Rβ mRNA remains at each time point compared to time zero.
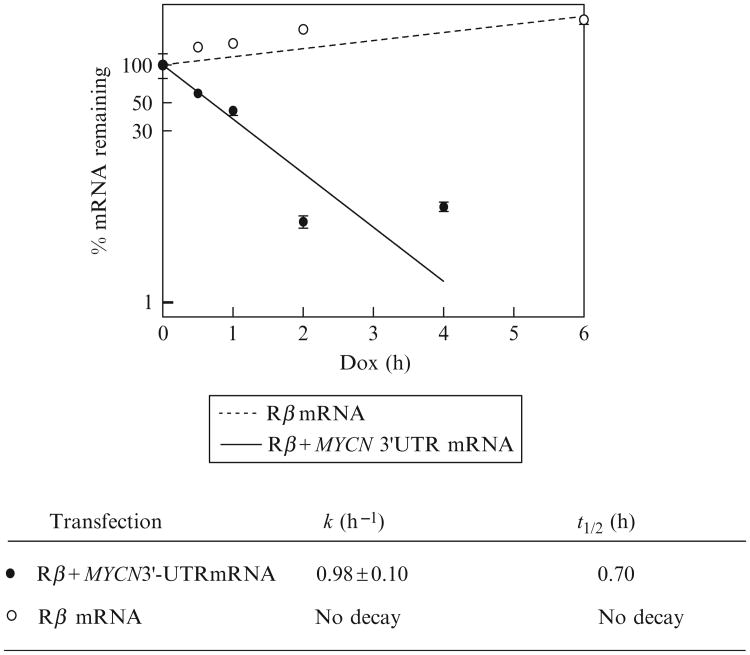
Enter the “percent Rβ mRNA remaining” (y axis) and “time in doxycycline” (x axis) values into a statistical software package such as Prism (GraphPad, San Diego) to prepare a semilogrithmic plot of “percent” versus “time.”; Solve first-order decay constants (k) by nonlinear regression analysis using Eq. (3.2). Figure 3.4 is an example analysis of mRNA decay kinetics of Rβ mRNA versus Rβ + MYCN3′-UTR mRNA; the C6 4 22.45 531.45 latter mRNA has the MYCN proto-oncogene 3′-UTR in place of the Rβ 3′-UTR. 5.
Calculate reporter mRNA half-lives (t1/2) using Eq. (3). For example, see Fig. 3.4.
Fig 3.3.
Excel spreadsheet of qRT-PCR analysis for mRNA half-life determinations. The human neuroblastoma cell line NBL-WS was cotransfected with plasmids pTet-Off and pEGFP-C2 (BD Biosciences) and either pTRE-Rβ or pTRE-Rβ/MYCN. For plasmid pTRE-Rβ/MYCN, the 3′-UTR of the human MFCV gene replaces the Rβ 3′-UTR.The MYCN3′-UTR contains two AREs that confer rapid degradation of MYCN mRNA (Manohar et al., 2002). Following a time course of doxycycline treatment to block transcription of the reporter genes, RNA was isolated and analyzed by qPCR. CT values for EGFP and Rβ-MYCN mRNAs were recorded for each time point. Utilizing standard curves, which provide the slopes (A) and y intercepts (B) for EGFP and Rβ-MYCN reporter mRNA, CT values were converted to relative RNA masses of Rβ-MYCN and EGFP mRNAs. The relative mass ratios of Rβ/EGFP were then calculated for each time point and averaged from duplicate samples for each time point. The Rβ/EGFP ratio for time zero was defined as 100 %. Mass ratios from subsequent time points were divided by the mass ratio for time zero and multiplied by 100%. Similar analyses were also performed for cells transfected with pTRE-Rβ, encoding wild-type β-globin (not shown). SD, standard deviation.
Fig 3.4.
Decay kinetics of Rβ and Rβ-MYCN mRNAs in NBL-WS neuroblastoma cells. The percentage of each reporter mRNA remaining at each doxycycline time point was plotted versus time in doxycycline. These data were then analyzed by nonlinear regression to Eq. (3.2) using Prism 3.0.3 (GraphPad) to calculate first-order decay constants, k. Messenger RNA half-lives were then calculated using t1/2 = ln 2/k. Data indicate that Rβ does not decay in NBL-WS cells across the timescale of this experiment, whereas the MYCN3′-UTR promotes rapid degradation of the reporter mRNA with a half-life of ≈45 min.
Notes: From multiple half-life determinations it is possible to perform statistical tests to ascertain differences in half-lives between two or more mRNAs (e.g., Student's t test). This could include comparisons of a wild-type ARE with various mutant forms or a reporter ARE-mRNA in the presence or absence of an extracellular stimulus. The possibilities are numerous.
Traditionally, mRNA decay is considered a first-order process because the decay kinetics of many mRNAs evaluated to date largely approximate this behavior, at least within the accuracy of available measurement strategies. However, the quantitative nature of qPCR-based methods, coupled with their higher throughput relative to more traditional approaches for tracking mRNA abundance (e.g., Northern blots or RNase protection assays), permits consideration of alternative models of cellular mRNA decay. This may be particularly relevant for partitioned systems, where subpopulations of specific mRNAs may be restricted to discrete cellular locations. For example, heat shock and other cellular stresses can localize mRNAs into stress granules or other subcellular structures (Hoyle et al., 2007; Kedersha and Anderson, 2002), whereas the cellular distribution of other mRNAs may include fractions associated with components of the cytoskeleton (Czaplinski and Singer, 2006). Also, the decay of some mRNAs may be mediated by multiple competing pathways, such as nonsense codon containing transcripts, that may be targeted by both nuclear and cytoplasmic turnover mechanisms (Maquat, 2004) and/or decay mechanisms utilizing distinct trans-acting complexes (Gehring et al., 2005). Provided (i) specific mRNAs decay at different rates among their various subcellular locations and (ii) these mRNA subpopulations exchange slowly on the timescale of their decay, more complex distributions of “percent mRNA remaining”; versus “time in doxycycline” would be anticipated. For example, a bimodal mRNA population where one subset does not decay over the time course of the experiment may be modeled by
| (3.4) |
where [RNAdecaying]0 is the initial RNA fraction subject to measureable decay following first-order kinetic constant k, whereas [RNAstable] is the RNA fraction excluded from the decaying population. In theory, [RNAdecaying]0 + [RNAstable] = [RNA]0 from Eq. (3.2), thus if [RNAstable] = 0, Eq. (3.4) simplifies to yield Eq. (3.2). Conversely, a bimodal mRNA population where both subsets decay with different kinetics may be modeled by
| (3.5) |
where [RNAfast]0 and [RNAslow]0 represent the initial RNA fractions in each of the fast- and slow-decaying populations, whose abundances as a function of time are governed by k1 and k2, respectively. Accordingly, the cellular half-lives of each mRNA subpopulation may be extracted from
| (3.6) |
| (3.7) |
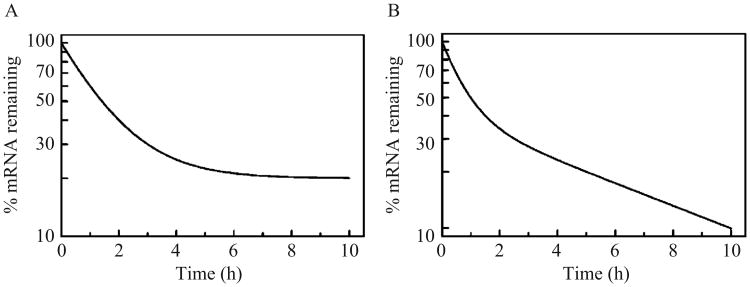
Representative regression solutions describing each of these models are shown in Fig. 3.5. We anticipate that consideration of both single exponential and more complex models of mRNA decay kinetics will yield novel insights into the mechanisms regulating mRNA localization and decay kinetics in cells.
Fig 3.5.
(A) Decay kinetics of an mRNA population where 80% of the total decays with a half-life of 1 h, while the remainder does not significantly decay over the time course of the experiment, modeled using Eq. (3.4). (B) Decay of a mixed mRNA population modeled using Eq. (3.5), where 60% of the initial mRNA pool decays with a half-life of 30 min, while the remainder decays with a 5-h half-life.
8. Messenger RNA Decay Pathways
The assays described so far quantitatively assess the effects of an ARE upon the normally stable reporter mRNA encoding rabbit β-globin. However, they do not address the mRNA decay pathway that a particular ARE might affect. This information can provide clues as to the trans-acting factors and enzymes involved. For most of the cases examined, an ARE promotes rapid deadenylation (Chen and Shyu, 1995; Knapinska et al., 2005; Wilusz et al., 2001). Decapping then ensues, followed by 3′–5′ and/or 5′–3′ degradation of the mRNA body. Moreover, an ARE stimulates the rate of each of these processes (Chen et al., 2001; Gao et al., 2001; Mukherjee et al., 2002). There are exceptions, however. For example, interleukin-1 induces degradation of the ARE-mRNA encoding the chemokine GROa via an endonucleolytic cleavage within the 3′-UTR (Stoeckle, 1992). We next describe methods to examine deadenylation specifically.
8.1. Deadenylation assay by Northern blotting
The Northern blot procedure provides a read out of mRNA size. As such, the time-dependent reduction in mRNA size during decay time course assays provides a visual means to quantify deadenylation rates (Ezzeddine et al., 2007; Shyu et al., 1991). [It can also demonstrate if rate-limiting endonucleolytic cleavage initiates mRNA decay (Herrick and Ross, 1994; Stoeckle, 1992).] Given the relatively small size of the Rβ reporter mRNA (≈700 nucleotides), deadenylation analyses are still readily possible even after insertion of a 200-nucleotide ARE into the mRNA (≈900 nucleotides total). However, for the analysis of a relatively large 3′-UTR (>1 kb) cloned into the Rβ reporter, the mRNA will be sufficiently large to preclude reliable measurements of poly(A) tail length. In this event, the large reporter mRNA can be cleaved into two portions using oligonucleotide-directed RNase H cleavage and Northern blotting, a method referred to as H mapping (Brewer and Ross, 1988, 1990). In this case, the oligodeoxyribonucleotide is chosen to anneal within 200–400 nucleotides of the 3′ end of the mRNA so that the RNase H cleavage within the mRNADNA duplex liberates a small 3′ portion of the mRNA; this 3′ portion (with its associated poly(A) tail) can be detected easily by high-resolution Northern blot analysis. An additional advantage of this method is that low abundance, 3′-decay intermediates can sometimes be observed (Brewer, 2000; Brewer and Ross, 1988). These intermediates can also provide clues regarding the mRNA decay enzymes involved.
Procedure
Prepare a stock solution of 4 M KCl and a stock solution of buffer TM [40 mM Tris–HCl (pH 7.5), 60 mM MgCl2] in RNase-free deionized H2O.
Precipitate purified RNAs from mRNA decay assays with ethanol, resuspend in 20 μl of 1 mM EDTA (pH 7.4), and heat at 70°C for 10 min for RNA denaturation.
Add the gene-specific oligodeoxyribonucleotide (0.5 μg; 14–20 nucleotides in length) to the mixture and incubate at 20 °C for 15 min.
Add 1 μl of 4 M KCl to the mixture and incubate at 20 °C for 15 min. Following incubation, add 20 μl of buffer TM and 1 μl RNase H (Phadia).
Incubate the reaction at 37 °C for 30 min to cleave mRNA:DNA duplexes.
Perform phenol–chloroform extraction and precipitate the RNA using ethanol. Fractionate RNAs by denaturing gel electrophoresis and detect the 3′ end of the mRNA by blot hybridization using the appropriate 32P-labeled or fluorescent probe. As a marker, one RNA sample can be processed as described earlier but incubated with 1 μg of oligo(dT12–18) (Phadia) at the same time as the gene-specific oligodeoxyribonucleotide. This will produce a band corresponding to mRNA without a poly(A) tail following the Northern blot procedure.
From the measured lengths of the poly(A) tail as a function of time, calculate the deadenylation rate as nucleotides per minute (Shyu et al., 1991).
8.2. Poly(A) tail assay by polymerase chain reaction
Northern blot procedures are somewhat time-consuming. An alternative is to utilize PCR-based approaches. There are a number of these, referred to as poly(A) tail assays, in the literature (Sallés and Strickland, 1999; Sallés et al., 1999). We created an anchored RT-PCR assay to measure poly(A) tail length on an mRNA (Rassa et al., 2000). In this assay, purified RNA is ligated at its 3′ end to an oligodeoxyribonucleotide (“anchor” primer) that contains a 3′-modified amino group. This modification prevents concatamer formation of the anchor primer during the ligation procedure. Ligated RNA is then reverse transcribed using a primer complementary to the anchor primer. The cDNA is amplified by PCR in the presence of [α-32P]dATP using a forward primer specific to an mRNA of interest. Products are fractionated by gel electrophoresis and visualized by autoradiography or by a phosphorimager.
Procedure
Isolate poly(A)+ RNA from ≈1.5 × 105 cells (each time point of an mRNA decay experiment). A number of commercially available kits are available, such as polyATract from Promega, which provide excellent results.
To the resulting RNA samples add 50 pmol of 3′ amino-modified oligodeoxyribonucleotide 5′-P-GGTCACCTTGATCTGAAGC-NH2-3′ and 1 μl T4 RNA ligase (New England Biolabs) in a total volume of 10 μl. Incubate the reaction at 37°C for 30 min.
Boil the reaction for 5 min and cool it to room temperature.
Add 50 pmol of primer 5′-GCTTCAGATCAAGGTGACCTTTTT-3′, which is complementary to the anchor primer and the five 3′-most residues of the ligated mRNA poly(A) tail. Perform a reverse transcription reaction in a final volume of 50 μl at 45 °C for 45 min using the Titan One Tube RT-PCR system (Roche Applied Science).
Add 1 μl DNase I-free RNase A (Promega) and incubate at 37°C for 15 min.
-
For PCR analysis, add 50 pmol of forward primer, which is gene specific, and 1 μCi [α-32P]dATP. The primer annealing site should be ≈50 nucleotides from the polyadenylation site.
(Note: For Rβ reporter mRNAs either with or without an ARE insertion, the forward primer can anneal 3′ of the BglII insertion site. Thus, the same primer set could be used to analyze a panel of ARE mutations, for example.)Perform amplification as follows: 25× (95°C, 30 s; 52°C, 60 s; 68°C, 60 s).
Fractionate one-fifth of the reaction and 32P-labeled DNA standards on a 6% polyacrylamide/7 M urea gel, dry it, and visualize bands by autoradiography or by a phosphorimager.
To calculate the longest poly(A) tail length, determine the size of the longest PCR product using standards and subtract the sizes of both primers and the distance of the 3′ end of the forward primer from the polyadenylation site. Repeat the calculations for each time point sample and determine deadenylation rates as described in the previous section. Alternatively, one-dimensional density measurements of individual lanes from autoradiographs or phosphorimager scans can permit distributions of poly(A) tail lengths to be described for individual mRNAs and tracked as a function of mRNA decay time.
8.3. Decapping
As noted earlier, the major decay mechanism of AMD is exonucleolytic removal of the poly(A) tail, followed by decapping and 3′–5′ and/or 5′–3′ degradation of the mRNA body. The rate of each step appears subject to influence by AREs. Both 3′–5′ and 5′–3′ pathways appear to involve hydrolysis of the 7mGpppG cap structure. In mammalian cells, the 5′–3′ pathway requires decapping enzymes that have been analyzed extensively (Wang et al., 2002). In the 3′–5′ decay pathway, exosome proteins and an exoribonuclease-dependent scavenger activity function together to hydrolyze the 5′ cap structure (Wang and Kiledjian, 2001). Thus, analyses of ARE-dependent decapping are of paramount importance to understanding the effects of individual AREs on AMD. See methods detailed by Kiledjian and colleagues in this volume for details.
9. Concluding Remarks
Since their discovery in 1986, AREs and AMD factors and enzymes have been subject to intense investigation by many laboratories. However, it is important to note that AREs can also control translation of an mRNA in a growing number of cases (Kawai et al., 2006; Liao et al., 2007; Mazan-Mamczarz et al., 2006; Paludan et al., 2001; Vasudevan and Steitz, 2007; Zhang et al., 2002). Moreover, in some cases, predicted “AREs” do not confer instability at all upon a heterologous mRNA. As such, for analysis of novel A+U-rich sequences, it is important for individual investigators to consider that a suspected ARE might not promote AMD but rather translational control. Alternatively, the sequences might confer AMD in one physiological state but translational control in another. The AMD field has provided many surprises and will probably continue to do so. Thus, investigators might consider translation assays, especially if a candidate ARE from a gene of interest does not promote mRNA degradation.
Acknowledgments
We thank Dr. Susan Cohn (University of Chicago) for providing NBL-WS neuroblastoma cells and a plasmid containing Rβ linked to the MYCN 3′-UTR and Dr. Kristina Sinsimer (Princeton University) for performing the Rβ and EGFP standard curves for qPCR.
References
- Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS. ARED: Human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–254. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J, Brawerman G. Editorial reviews. Elsevier Science and Technology Books; 1993. Control of messenger RNA stability; pp. 1–517. [Google Scholar]
- Brewer G. Regulation of c-myc mRNA decay in vitro by a phorbol ester-inducible, ribosome-associated component in differentiating megakaryoblasts. J Biol Chem. 2000;275:33336–33345. doi: 10.1074/jbc.M006145200. [DOI] [PubMed] [Google Scholar]
- Brewer G, Ross J. Poly(A) shortening and degradation of the 3′ A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988;8:1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G, Ross J. Messenger RNA turnover in cell-free extracts. Methods Enzymol. 1990;181:202–209. doi: 10.1016/0076-6879(90)81122-b. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;2:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- Chen CY, Shyu AB. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Czaplinski K, Singer RH. Pathways for mRNA localization in the cytoplasm. Trends Biochem Sci. 2006;31:687–693. doi: 10.1016/j.tibs.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Dallas-Yang Q, Jiang G, Sladek FM. Avoiding false positives in colony PCR. Biotech. 1998;4:580–582. doi: 10.2144/98244bm14. [DOI] [PubMed] [Google Scholar]
- Duttagupta R, Vasudevan S, Wilusz CJ, Peltz SW. A yeast homologue of Hsp70, Ssa1p, regulates turnover of the MFA2 transcript through its AU-rich 3′ untranslated region. Mol Cell Biol. 2003;23:2623–2632. doi: 10.1128/MCB.23.8.2623-2632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzeddine N, Chang TC, Zhu W, Yamashita A, Chen CY, Zhong Z, Yamashita Y, Zheng D, Shyu AB. Human TOB, an antiproliferative transcription factor, is a poly(A)-binding protein-dependent positive regulator of cytoplasmic mRNA deadenylation. Mol Cell Biol. 2007;27:7791–7801. doi: 10.1128/MCB.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Wilusz CJ, Peltz SW, Wilusz J. A novel mRNA-decapping activity in HeLa cytoplasmic extracts is regulated by AU-rich elements. EMBO J. 2001;20:1134–1143. doi: 10.1093/emboj/20.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring NH, Kunz JB, Neu-Yilik G, Breit S, Viegas MH, Hentze MW, Kulozik AE. Exon-junction complex components specify distinct routes of nonsense-mediated mRNA decay with different cofactor requirements. Mol Cell. 2005;20:65–75. doi: 10.1016/j.molcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrold S, Genovese C, Kobrin B, Morrison SL, Milcarek C. A comparison of apparent mRNA half-life using kinetic labeling techniques vs decay following administration of transcriptional inhibitors. Anal Biochem. 1991;198:19–29. doi: 10.1016/0003-2697(91)90500-s. [DOI] [PubMed] [Google Scholar]
- Herrick DJ, Ross J. The half-life of c-myc mRNA in growing and serum-stimulated cells: Influence of the coding and 3′ untranslated regions and role of ribosome translocation. Mol Cell Biol. 1994;14:2119–2128. doi: 10.1128/mcb.14.3.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle NP, Castelli LM, Campbell SG, Holmes LE, Ashe MP. Stress-dependent relocalization of translationally primed mRNPs to cytoplasmic granules that are kinetically and spatially distinct from P bodies. J Cell Biol. 2007;179:65–74. doi: 10.1083/jcb.200707010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Kawai T, Lal A, Yang X, Galban S, Mazan-Mamczarz K, Gorospe M. Translational control of cytochrome c by RNA-binding proteins TIA-1 and HuR. Mol Cell Biol. 2006;26:3295–3307. doi: 10.1128/MCB.26.8.3295-3307.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Anderson P. Stress granules: Sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- Knapinska AM, Irizarry-Barreto P, Adusumalli S, Androulakis I, Brewer G. Molecular mechanisms regulating mRNA stability: Physiological and pathological significance. Curr Genomics. 2005;6:471–486. [Google Scholar]
- Laroia G, Sarkar B, Schneider RJ. Ubiquitin-dependent mechanism regulated rapid turnover of AU-rich cytokine mRNAs. Proc Natl Acad Sci USA. 2002;99:1842–1846. doi: 10.1073/pnas.042575699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa M, Mahtani KR, Finch A, Brewer G, Saklatvala J, Clark AR. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol. 2000;20:4265–4274. doi: 10.1128/mcb.20.12.4265-4274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao B, Hu Y, Brewer G. Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nat Struct Mol Biol. 2007;14:511–518. doi: 10.1038/nsmb1249. [DOI] [PubMed] [Google Scholar]
- Loflin PT, Chen CY, Xu N, Shyu AB. Transcriptional pulsing approaches for analysis of mRNA turnover in mammalian cells. Methods. 1999;17:11–20. doi: 10.1006/meth.1998.0702. [DOI] [PubMed] [Google Scholar]
- Manohar CF, Short ML, Nguyen A, Nguyen NN, Chagnovich D, Yang Q, Cohn SL. HuD, a neuronal-specific RNA-binding protein, increases the in vivo stability of MYCN RNA. J Biol Chem. 2002;277:1967–1973. doi: 10.1074/jbc.M106966200. [DOI] [PubMed] [Google Scholar]
- Maquat LE. Nonsense-mediated mRNA decay: Splicing, translation, and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Lal A, Martindale JL, Kawai T, Gorospe M. Translational repression by RNA-binding protein TIAR. Mol Cell Biol. 2006;26:2716–2727. doi: 10.1128/MCB.26.7.2716-2727.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D, Gao M, O'Connor JP, Raijmakers R, Pruijn G, Lutz CS, Wilusz J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protocol. 2006;3:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Paludan SR, Ellermann-Eriksen S, Kruys V, Mogensen SC. Expression of TNF-alpha by herpes simplex virus-infected macrophages is regulated by a dual mechanism: Transcriptional regulation by NF-kappa B and activating transcription factor 2/Jun and translational regulation through the AU-rich region of the 3′ untranslated region. J Immunol. 2001;167:2202–2208. doi: 10.4049/jimmunol.167.4.2202. [DOI] [PubMed] [Google Scholar]
- Rassa JC, Wilson GM, Brewer GA, Parks GD. Spacing constraints on reinitiation of paramyxovirus transcription: The gene end U tract acts as a spacer to separate gene end from gene start sites. Viro. 2000;274:438–449. doi: 10.1006/viro.2000.0494. [DOI] [PubMed] [Google Scholar]
- Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Sullivan TD. Half-lives of beta and gamma globin messenger RNAs and of protein synthetic capacity in cultured human reticulocytes. Blood. 1985;66:1149–1154. [PubMed] [Google Scholar]
- Sallés FJ, Richards WG, Strickland S. Assaying the polyadenylation state of mRNAs. Methods. 1999;17:38–45. doi: 10.1006/meth.1998.0705. [DOI] [PubMed] [Google Scholar]
- Sallés FJ, Strickland S. Analysis of poly(A) tail lengths by PCR: The PAT assay. Methods Mol Biol. 1999;118:441–448. doi: 10.1385/1-59259-676-2:441. [DOI] [PubMed] [Google Scholar]
- Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Shyu AB, Greenberg ME, Belasco JG. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Speth C, Oberbaumer I. Expression of basement membrane proteins: Evidence for complex post-transcriptional control mechanisms. Exp Cell Res. 1993;204:302–310. doi: 10.1006/excr.1993.1037. [DOI] [PubMed] [Google Scholar]
- Stoeckle MY. Removal of a 3′ non-coding sequence is an initial step in degradation of gro alpha mRNA and is regulated by interleukin-1. Nucleic Acids Res. 1992;20:1123–1127. doi: 10.1093/nar/20.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128:1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiao X, Carr-Schmid A, Kiledjian M. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc Natl Acad Sci USA. 2002;99:12663–12668. doi: 10.1073/pnas.192445599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kiledjian M. Functional link between the mammalian exosome and mRNA decapping. Cell. 2001;107:751–762. doi: 10.1016/s0092-8674(01)00592-x. [DOI] [PubMed] [Google Scholar]
- Ward AC. Rapid analysis of yeast transformants using colony-PCR. Biotech. 1992;13:350. [PubMed] [Google Scholar]
- Wilson GM, Sun Y, Lu H, Brewer G. Assembly of AUF1 oligomers on U-rich RNA targets by sequential dimer association. J Biol Chem. 1999;274:33374–33381. doi: 10.1074/jbc.274.47.33374. [DOI] [PubMed] [Google Scholar]
- Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- Xu N, Loflin P, Chen CY, Shyu AB. A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse tragedy. Nucleic Acid Res. 1998;26:558–565. doi: 10.1093/nar/26.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Kruys V, Huez G, Gueydan C. AU-rich element-mediated translational control: Complexity and multiple activities of trans-activating factors. Biochem Soc Trans. 2002;30:952–958. doi: 10.1042/bst0300952. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Chang FC, Furneaux HM. The identification of an endonuclease that cleaves within an HuR binding site in mRNA. Nucleic Acids Res. 2000;28:2695–2701. doi: 10.1093/nar/28.14.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]