Abstract
Objective
The main histopathological focus of systemic sclerosis (SSc) has concentrated on fibrotic changes. We investigated the microvasculature alterations in the skin of patients with SSc at various stages of disease duration with whole-field digital microscopy.
Methods
Twenty consecutive patients with SSc, 1 with Raynaud's phenomenon (RP) without SSc, and 4 healthy controls underwent punch biopsy on the medial forearm. Eighteen patients were included in the primary analysis. Two with recent-onset diffuse cutaneous disease, 1 repeat SSc biopsy, and 1 patient with RP without SSc were also evaluated. All specimens were processed with histochemical stains and immunohistochemistry. We analyzed microvasculature abnormalities in an objective and systematic manner taking advantage of recent advances in whole-field digital microscopy. This analysis was coupled with ultrastructural evaluation performed with transmission electron microscopy (TEM).
Results
Whole-field digital microscopy and TEM of SSc skin biopsies revealed that endothelial abnormalities are a universal feature regardless of clinical features and/or duration of disease. These features were not seen in any healthy control specimens or in the single RP patient samples. Whole-field digital microscopy identified increased interstitial edema (31.0% ± 9.6% vs 17.6% ± 3.3% in controls; p = 0.009) and fibrosis (75.6% ± 5.7% vs 66.1% ± 9.8% in controls; p = 0.02) in all patients with SSc. Lower CD34 staining was seen in SSc compared to healthy controls (0.32% ± 0.22% vs 1.31% ± 0.34%; p < 0.0001) and within the SSc population with interstitial lung disease (0.55% ± 0.22% vs 0.15% ± 0.16%; p = 0.01). Perivascular and interstitial infiltrate of mast cells was present in all SSc specimens.
Conclusion
Whole-field digital microscopy offers a means of rapidly carrying out objective, fully quantitative, and reproducible measurements of microscopic features of SSc microvascular change. The universal morphologically abnormal endothelial cells and interstitial edema in all patients with SSc biopsied suggests that SSc may be intrinsically a disease of the endothelium characterized by vascular leak.
Keywords: SCLERODERMA, MICROSCOPY, SYSTEMIC SCLEROSIS, VASCULOPATHY, HISTOPATHOLOGY, MICROVASCULATURE
Systemic sclerosis (SSc; scleroderma) is considered a multi-organ system disease characterized by activation of immune cells and production of autoantibodies1. Histopathologically, the main focus has traditionally concentrated on fibrotic changes and collagen turnover2,3,4,5. However, clinical experience strongly suggests that there are 3 stages of SSc: edematous, fibrotic, and atrophic6. Therefore, characterization of microvasculature changes may provide insight into disease progression, allow for earlier diagnosis, and identify a critical opportunity for intervention7,8.
The earliest clinical symptom of SSc is most often related to disturbances in the peripheral vascular system, appreciated as Raynaud's phenomenon (RP; vasospasm) with capillary nailbed change (abnormal features of angiogenesis)9,10. Unfortunately, there is difficulty in the diagnosis of SSc at this early stage, before the development of obvious skin sclerosis11. LeRoy and Medsger proposed criteria for the early diagnosis and classification of SSc, which require RP with capillary nailbed changes and/or SSc-specific serologies12. Quantification of capillary nailbed change requires specialized equipment and training not uniformly available to all practitioners12,13,14. Additionally, autoantibody-negative disease is known to exist. Finally, SSc without skin involvement (sine scleroderma) is also well described, emphasizing that this disease can have a devastating outcome, independent of fibrosis15,16. It has been proposed that to overcome the high degree of variability in clinical features, diagnosis, and therapeutic approaches to SSc, the scientific community must find standardized and uniform procedures for early diagnosis and management of patients17.
Historically, skin biopsies have been used to characterize SSc. These biopsy specimens have adequately characterized the fibrotic aspect of the disease18,19,20,21. A correlate of this fibrosis is also well defined in the clinic by use of the modified Rodnan skin score (mRSS)22,23. SSc has been described as a prototypic fibrotic disorder21. However, it has also been proposed that the vessel wall itself is the origin of this disease and that the immune response is mediated by a process in which injury to the vessel wall cannot be contained or suppressed24. While the early phase of SSc has been qualified by endothelial cell apoptosis, perivascular edema, and inflammatory infiltrate in the perivascular space and clinical progression defined by increasing dermal fibrosis in skin biopsy specimens25,26,27, the microvascular changes on skin biopsy have not been described satisfactorily22. Edema is recognized as an important marker of disease severity in early disease28,29 and unfortunately can interfere with performance of the mRSS30. Thus, development of a way to score microvascular leak and edema in SSc has important clinical implications.
Recent advances in whole-field digital microscopy offer a means of rapidly carrying out quantitative, reproducible measurements of microanatomical features in high-resolution pathology images and in large image datasets with control for intraobserver and interobserver variability31,32,33,34. Its use for systematic examination of subtle differences exhibited by diseased tissue has potential to improve the characterization of the histologic stage, prognosis, and treatment responses in SSc.
We investigated alteration in skin microvasculature in SSc by the use of whole-field digital microscopy coupled with ultrastructural evaluation by transmission electron microscopy (TEM).
MATERIALS AND METHODS
Study population
The study was accepted by the Institutional Review Board (IRB) of the University of Utah (IRB number 00038705). Inclusion criteria included adult patients (age ≥ 18 years) with a diagnosis of SSc as accepted by the American College of Rheumatology (ACR)35, or early SSc as described by LeRoy and Medsger12. Twenty consecutive patients seen at the University of Utah SSc Center between February 2011 and July 2011 underwent a 6-mm punch biopsy on the left or right medial forearm. The skin biopsy specimen was divided, as follows: one-quarter of the specimen fixed in buffered formalin, one-quarter fixed in 4°C Karnovsky's fixative immediately after excision, and the remainder snap-frozen in liquid nitrogen for molecular studies. Fixed specimens were subsequently embedded in paraffin and Epon 812 for light and electron microscopy studies, respectively. Biopsy samples from 4 healthy controls that had nevus removal on the forearm were included for comparison. All controls were women of age range 19 to 64 years with absence of chronic medical conditions. An additional control had RP without capillary nailbed change and was receiving a calcium channel blocker (CCB).
Clinical data collection
Clinical features of disease were recorded for each patient as follows: disease duration from both first RP and non-RP symptoms; presence of capillary nailbed abnormality; presence of digital ulcerations; pulmonary arterial hypertension (PAH) determined by right heart catheterization; scleroderma renal crisis; mRSS; presence of nonpitting edema in the hands/fingers, graded 0 (absent), 1 (mild), 2 (moderate), 3 (severe); presence of interstitial lung disease (ILD) determined by high-resolution computed tomography of the chest; and symptoms of inflammation (tendon friction rubs, arthritis, pruritus). Immunosuppression regimens were recorded for each patient. All patients were taking CCB. The patients with PAH were receiving additional vasodilators. No patient was taking corticosteroids.
Histochemistry and whole-field digital microscopy
Sections from the paraffin block were stained for H&E and histochemical stains and immunohistochemistry: Masson's trichrome, elastic, and Prussian blue stains were also performed by standard protocols using the automatic Artisan Special Stains System (Dako, Carpinteria, CA, USA). The distribution of these findings in the dermis-adnexa, interstitium, or vessel wall was evaluated on light microscopy and the staining color threshold of the ImageScope 10.0 Color deconvolution analysis algorithm was used to accurately identify and quantify elastic content in the skin biopsies on the basis of its black color. Prussian blue stain was used to define the distribution of heme components, and to ensure vasculopathy was not related to hemosiderin deposition.
To define the distribution and extent of fibrosis, Masson's trichrome stain was used for evaluation of collagen content. Interstitial edema was defined by a digitized assessment of the strength of trichrome stain as areas with a decrease in trichrome stain strength where there was clearing and separation of extracellular matrix elements. Areas of fibrosis were determined by the strong blue staining of the trichrome stain. Quantification of trichrome was done with the Aperio ScanScope using the standardized color deconvolution algorithm.
Aldehyde Fuchsin-Alcian blue and Alcian blue (pH 2.5) stains were used to define areas of less intense staining with the trichrome stain to ensure that this was not due to accumulation of mucin or other glycosaminoglycans. Aldehyde Fuchsin-Alcian blue stain demonstrates sulfated mucosubstances and the nonsulfated mucosubstances do not react with the aldehyde fuchsin, but will color with the Alcian blue (pH 2.5) solution (second stain). In normal dermis there is scant amount of ground substance composed mainly of nonsulfated acid mucopolysaccharides that are identified with Alcian blue.
Immunohistochemical staining was performed on sections 4 μm thick of formalin-fixed, paraffin-embedded samples for collagen IV, laminin, CD34, and mast cell tryptase. Sections were air-dried at room temperature and then placed in a 60°C oven for 30 min to melt the paraffin. All staining steps were performed at 37°C on the automated immunostainer (BenchMark® XT, Ventana Medical Systems, Tucson, AZ, USA). Briefly, the slides were deparaffinized with EZ Prep solution (Ventana Medical Systems). Protease 2 was applied for 8 min (collagen IV) or 12 min (laminin). Pretreatment was performed with CC1 for 8 min (CD34). No pretreatment was needed (mast cell tryptase). Mouse monoclonal antibodies were applied. Sections were detected using the IView DAB detection kit (Ventana Medical Systems), which is a biotinylated goat anti-mouse/anti-rabbit secondary, streptavidinhorseradish peroxidase system, with DAB (3-3’ diaminobenzidine) as the chromogen. Sections were counterstained with hematoxylin (Ventana Medical Systems) for 8 min. Sections were dehydrated in graded alcohols (70%, 95% × 2, and 100% × 2), cleared in xylene, and then coverslipped. Specifications of antibodies were as follows: collagen IV (Dako; Clone CIV22, 1:200, 40 min incubation), laminin (Leica/Novocastra, Norwell, MA, USA; Clone LAM-89, 1:100, 40 min incubation), mast cell tryptase (Dako; Clone AA1, 1:400, 24 min incubation), and CD34 (Ventana Medical Systems; Clone QBEND40, pre-dilute, 32 min incubation).
To define the microvasculature, a combination of collagen type IV (a stain for basal lamina), laminin (a glycoprotein that is the major constituent of basement membrane), and CD34+ stains were applied to biopsy specimens. Aperio ScanScope and standardized color deconvolution and microvessel density algorithms were then used to quantify edema and microvascular density (MVD), while controlling for the total area of the biopsy; the quantification is not computer operator-dependent. Assessment of edema (vascular leak) is not subjective because computer image analysis quantifies the intensity of staining and assigns percentage as well as degree of staining (light to strong) in relation to the whole area analyzed. These areas are also identified in the H&E stain and match the light blue trichrome-stained areas.
Ultrastructural evaluation
Skin biopsy specimens for electron microscopy were processed following routine protocols; sections 1 μm thick were stained with toluidine blue and representative sections examined by TEM (Hitachi H-7100) in the Research Microscopy Laboratory, University of Utah. Tissue was examined according to an ultrastructural classification scheme that assesses endothelial cell swelling, endothelial nuclear enlargement, and basal lamina accumulation. These measures were graded 0 (none) to 3+ (extensive)32.
Statistical analysis
Statistics were analyzed using SAS version 9.2 (SAS, Cary, NC, USA). None of these variables violated the assumption of normality. Patient characteristics were summarized using means and frequency where appropriate. We used general linear models to compare age-adjusted characteristics. However, age was not a significant correlate of the characteristics. Therefore, we presented unadjusted means and used T tests for analysis of continuous variables and chi-square to assess associations between clinical and bivariate biopsy variables. T test was used to compare biopsy outcomes by the subgroups of patients defined by bivariate clinical characteristics. Spearman correlation was used to assess clinical and biopsy variables.
RESULTS
In our SSc study population in which diagnosis of SSc was accepted by the ACR criteria (Table 1)35, 16 subjects were women, 16 were white, and 2 were Hispanic. The age range was 34 to 79 years (mean age 56.8 yrs). The mRSS at the time of biopsy ranged from 4 to 19. Sixteen patients had skin thickening at the biopsy site. Sixteen patients had finger edema. The distribution of SSc subsets was 16 limited cutaneous SSc and 2 diffuse cutaneous SSc. Sixteen were antinuclear antibody-positive with a titer > 1:160, 3 subjects had anti-Scl-70 antibody, 7 had anticentromeric antibody, and 6 had RNA III polymerase antibodies. There was evidence of end-organ damage due to SSc (digital ulcerations, PAH, ILD, and/or scleroderma renal crisis) in 18 patients. No physical examination features were significantly associated with biopsy findings in this SSc population. However, the 2 patients with early SSc who met the LeRoy and Medsger criteria12 had significant hand and foot edema correlations with vascular leak on whole-field digital microscopy (p = 0.02). The one patient with RP without SSc taking a CCB did not have any appreciable hand or foot edema or vascular leak on microscopy.
Table 1.
Clinical characteristics of patients with systemic sclerosis (SSc).
| Characteristic | |
|---|---|
| Sex | |
| Female | 16** |
| Male | 2 |
| Age, yrs, mean ± SD | 56.8 ± 10.7 |
| Duration of RP yrs, mean ± SD | 11.7 ± 9.8 |
| Type of SSc | |
| Diffuse | 2** |
| Limited | 16 |
| Duration of SSc, yrs*, mean ± SD | 7.4 ± 8.2 |
| First SSc symptoms | |
| Skin thickening | 3** |
| Digital ulcer | 2 |
| Dyspnea | 2 |
| Polyarthritis | 3 |
| Reflux | 1 |
| Immunosuppression | |
| Azathioprine | 1** |
| Mycophenolate mofetil | 2 |
| Methotrexate | 2 |
| None | 13 |
From first non-Raynaud's symptom
Frequency. RP: Raynaud's phenomenon.
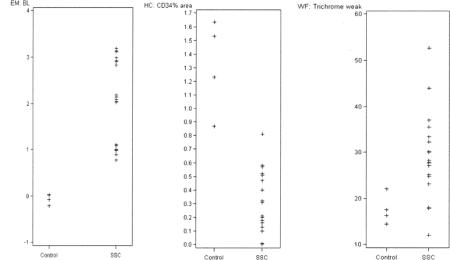
Five of the patients with SSc were on immunosuppression at the time of biopsy. One patient was biopsied 6 months after therapy for PAH and immunosuppression were initiated. In this patient, electron microscopy features did not change, but CD34 stain increased by 34%.
Whole-field digital microscopy
All patients with SSc had an increase in interstitial edema compared to normal control biopsy samples (Tables 2 and 3; Figure 1). Whole-field digital microscopy revealed significantly more interstitial edema among patients with SSc (31.0% ± 9.0%) compared to controls (17.6% ± 3.3%). Fibrosis was also significantly increased in patients (75.6 ± 5.7) compared to controls (66.1 ± 9.8). Additionally, the aldehyde fuchsin-Alcian blue and Alcian blue pH 2.5 stains showed only 2 cases with positive Alcian blue in the papillary and reticular dermis, but no significant stain was demonstrated with aldehyde fuchsin-Alcian blue. These findings support that the areas of clear appearance on trichrome stain represent areas of edema rather than mucin accumulation. None of the vessels stained for Prussian blue, suggesting that hemosiderin-related injury is not responsible for endothelial changes and vascular leak.
Table 2.
Whole-field digital microscopy findings. Data are mean ± SD.
| Findings | Control | SSc | p |
|---|---|---|---|
| Interstitial edema, % | 17.6 ± 3.3 | 31.0 ± 9.0 | 0.009* |
| Fibrosis, % | 66.1 ± 9.8 | 75.6 ± 5.7 | 0.02* |
| CD34+ microvessel density, % | 1.31 ± 0.34 | 0.32 ± 0.22 | < 0.0001* |
| Mean vessel area, % | 49.3 ± 11.8 | 43.8 ± 9.5 | 0.32 |
| No. vessels | 1515 ± 1087 | 1006 ± 1417 | 0.30 |
Significant.
Table 3.
Systemic sclerosis (SSc) clinical correlations with microscopy findings.
| Clinical Features | TEM: Basal Lamellation | Immunohistochemistry: CD34 Area | Whole-field Digital Microscopy: Trichrome Stain |
|---|---|---|---|
| SSc duration (1st non-RP symptom) | 0.05 (0.83)* | –0.08 (0.74)* | –0.50 (0.04)* |
| mRSS | 0.34 (0.16)* | –0.04 (0.87) | –0.09 (0.71) |
| ILD | — (0.84)** | –0.45 (0.06) | 0.19 (0.43) |
| Scl-70 antibody | — (0.38)** | –0.04 (0.88) | 0.06 (0.80) |
| RNA polymerase III | — (0.66)** | –0.13 (0.60) | –0.21 (0.39) |
| Centromeric antibody | — (1.00)** | 0.02 (0.93) | 0.07 (0.76) |
| Hand edema | — (0.80)** | 0.23 (0.35) | –0.07 (0.76) |
| Foot edema | — (0.07)** | 0.05 (0.85) | –0.02 (0.92) |
TEM: transmission electron microscopy; RP: Raynaud's phenomenon; mRSS: modified Rodnan skin score; ILD: interstitial lung disease.
Spearman's Rho (p value)
Fisher's exact p value.
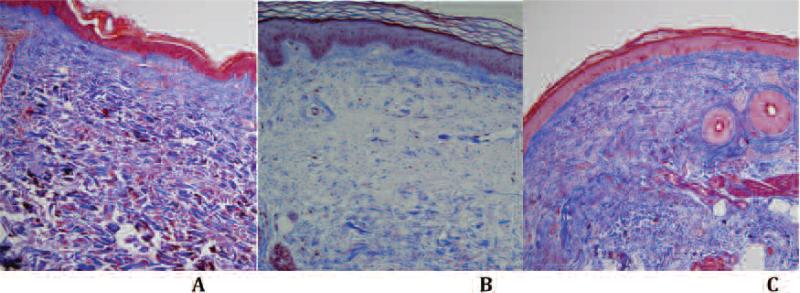
Figure 1.
Trichrome stain results. A. Control skin biopsy without abnormalities in the epidermis and dermis without inflammation, edema, or significant fibrosis. B. Edema is present in the superficial dermis and appears as clear blue staining. C. Diffuse dermal fibrosis is visible.
Immunohistochemistry
The extent of CD34 stain, which is related to microvascular repair, was lower among patients with SSc (1.31% ± 0.34%) than among controls (0.32% ± 0.22%; p < 0.001). However, when the percentage stain was quantified by area and microvessel density, this significance was lost (Tables 2 and 3; Appendix 1). Of interest, CD34 stain was lower among patients with ILD (mean 0.15 ± 0.16) than among patients without ILD (0.40% ± 0.22%; p = 0.01) even after controlling for vessel density (data not shown). This finding highlights one of the strengths of whole-field digital microscopy, which allows reproducible quantification of abnormalities. In our analysis, mast cell number per area was not significantly increased compared to the healthy controls. However, they were present in all patients with SSc in a perivascular and interstitial location, in contrast to the healthy controls.
Ultrastructural evaluation
On TEM, all SSc specimens had variable degrees of lamellation of basal lamina, with 1 to 3 layers seen (Table 3, Figure 2), and endothelial swelling was seen in all SSc specimens. These changes were not seen in the single patient with RP who was taking a CCB.
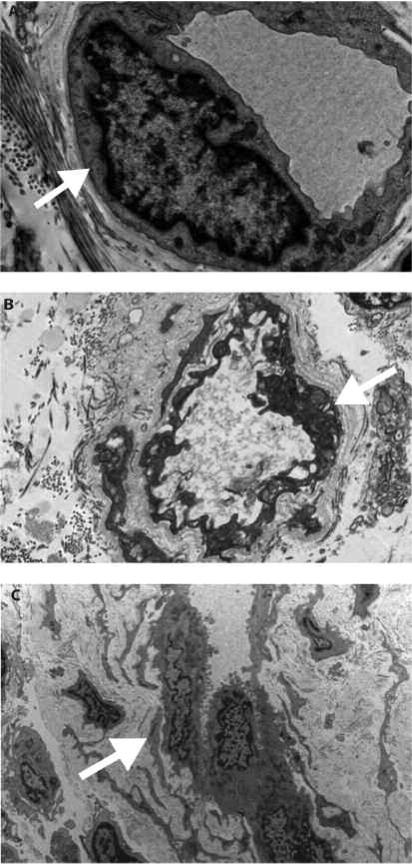
Figure 2.
Transmission electron microscopy. A. Control skin specimen shows a dermal capillary with thin basement membrane (arrow) and flattened endothelial cell. B. Capillary from a scleroderma case shows mild lamellation (arrow) of the basement membrane. C. A more extensive lamellation (arrow) of the basement membrane can be seen. The endothelial cells show abundant cytoplasm and enlarged nuclei.
DISCUSSION
Currently, the diagnosis of SSc relies heavily on clinical judgment. An improvement in diagnostic criteria through incorporation of objective measures is needed17,36. Our electron microscopy findings corroborate previous descriptions of microvascular change in SSc, including swollen endothelial cells with a duplicated, lamellated appearance of the basement membrane26,27. We found that regardless of SSc disease type, disease duration, or clinical features, all capillaries in SSc patients appeared to have these features of swollen endothelium and lamellation of basal lamina. These findings were also present in 2 patients with early SSc12 but not in a patient with RP without capillary nailbed changes. Thus, these features suggest oxidative injury and endothelial dysfunction, and establish SSc as a disease of the endothelium37. Under standing the link between endothelium dysfunction and interstitial edema has diagnostic and therapeutic implications. It is particularly important to study additional patients with early, diffuse SSc and RP without SSc to understand this mechanism.
Whole-field digital microscopy offers a means of rapidly carrying out quantitative, reproducible measurements of microanatomical features of microvascular change in SSc. Endothelium in patients with SSc is morphologically abnormal and this is one of the earliest changes found in this disease38. Previous histopathology descriptions of vasculopathy in SSc demonstrated that regardless of the extent of fibrosis, abnormalities of blood vessels are universal in patients with SSc26,27. The dissociation between vasculopathy and fibrosis as part of the natural history of the disease might explain the finding that some patients have signs of vascular leak without fibrosis, the entity of SSc sine scleroderma. Additionally, the mRSS decreases in a majority of patients over extended time, but the vascular complications of SSc seem to be progressive. Mast cells and CD34 cells have been proposed as important to the disease pathogenesis based on their presence in descriptive studies27,39,40. Our study suggests that whole-field digital microscopy of skin biopsy specimens, particularly in regard to quantification of vessel number, cell types, and vascular leak, may be useful both for research and to personalize clinical care.
We found that interstitial edema, a marker of vascular leak, was a central feature in all SSc specimens. This early stage of disease without fibrosis may be more responsive to disease-targeted therapies, because successful antifibrotic therapies do not exist17. It is possible that the interstitial edema is driven by another process, such as vascular injury, that does not improve over time. While total microvessel density (MVD) was not significantly different between patients with SSc and controls (likely because of differences in SSc disease duration), when CD34+ staining was quantified by MVD, SSc specimens had less CD34+ staining than controls. Further, there was significantly less CD34+ staining among SSc with ILD as compared to those without ILD. This finding is of interest because normal endothelial repair requires CD34 to be expressed on small-vessel endothelium41 to promote chemokine-mediated trafficking42. In addition to leukocyte adhesion or “homing” during the inflammatory process, CD34 expression plays a role in stem/progenitor cell localization/adhesion in the basement membrane. Our findings highlight that the endothelium remains a principal cell type of interest in SSc pathogenesis, since endothelial activation and abnormalities in the vessel walls are thought to be essential for vascular leak27,43,44. Although the initial trigger for endothelial injury in SSc remains unknown, it is thought that reperfusion injury, autoantibodies, infectious agents, and/or environmental factors conspire to perpetuate endothelial injury in genetically predisposed hosts45. The endothelium is a critical regulator of subsequent immune dysfunction46; thus understanding the process of vascular leak in SSc is of paramount importance.
Mast cells have been proposed as important in disease pathogenesis based on their presence in descriptive studies27,39,40,47. In our study, all patients with SSc had mast cells in a perivascular and interstitial location. The numbers of mast cells were not different statistically from control samples and appeared to be within normal limits, in contrast to previous descriptions32. This may be a sampling artifact or variation related to duration of SSc in our cases; nevertheless, the significance of mast cells as well as their function in SSc pathogenesis remains to be established.
Quantification of vasculopathy by whole-field digital microscopy may also be helpful for determining response to treatment. One patient who was rebiopsied 6 months after the initiation of immunosuppression and therapy for PAH showed no changes in clinical features, while the CD34 staining increased, suggesting that repeat biopsy may have a role in tracking therapeutic response.
Our study has some limitations. It was cross-sectional: a longitudinal study would be of greater value for determining the significance of our findings. While the pathologist was not blinded to the fact that the samples carried a diagnosis of SSc, she was blinded to the clinical severity, progression, and characteristics of the patients. In addition, we had only 2 participants with diffuse SSc; thus we were unable to do analysis by disease subtype. We have included these patients in the analysis because their findings are consistent with those of the limited subset, and suggest a common mechanism of disease. The importance of understanding the mechanism of vasculopathy in SSc is underscored by the fact that our findings did not display any correlation with a large number of other clinical variables. The population studied all had RP with capillary nailbed changes, but included only 1 patient with RP without SSc; thus we do not know if these findings are a consequence of RP, whether primary or secondary, or are related to SSc. Additionally, all patients with SSc were taking a CCB; thus the effects of this medication on the findings are also unknown. The value of repeat skin biopsies and the proper interval of time between biopsies remain to be determined. We restricted our controls to skin biopsy specimens from healthy participants requiring biopsy in the forearm location for nevus removal. We were able to find only 4 such patients during the study period. Given the small number of control specimens, our observations need to be confirmed with a larger number of samples. Nonetheless the study had adequate power to detect the differences in interstitial edema and MVD per post hoc analysis.
Our study demonstrates that whole-field digital microscopy can be used successfully to quantify microvascular change in SSc skin biopsies. It emphasizes that interstitial edema (vascular leak) was present in all SSc biopsies, and that the presence of finger/hand edema clinically was negatively associated to fibrosis. Endothelial cell changes, presence of perivascular mast cells, and CD34+ MVD may be helpful in establishing an earlier diagnosis of SSc than can be done by relying on the clinical recognition of fibrosis, and may be useful in gauging therapeutic response. Further research is needed to determine the usefulness of whole-field digital microscopy in defining disease mechanisms of pathogenesis and to determine whether SSc is indeed an intrinsic disease of the endothelium.
ACKNOWLEDGMENT
The authors thank the staff of the LDS Hospital Digital Imaging Facility.
Supported by the Skaggs Family Foundation, CTSA K Program, and the Department of Defense.
Appendix
Appendix 1. Main histopathological features of healthy controls compared to patients with systemic sclerosis (SSc). EM: electron microscopy; BL: basal lamina; HC: immunohistochemistry; WF: whole-field digital microscopy.
Footnotes
T.M. Frech, MD, MS, Division of Rheumatology, Department of Internal Medicine; M.P. Revelo, MD, Department of Pathology and Laboratory Medicine; S.G. Drakos, MD, Molecular Medicine Program and Division of Cardiology; M.A. Murtaugh, PhD, Division of Epidemiology, Department of Internal Medicine; B.A. Markewitz, MD, Division of Respiratory, Critical Care and Occupational Pulmonary Medicine, Department of Internal Medicine; A.D. Sawitzke, MD, Division of Rheumatology, Department of Internal Medicine; D.Y. Li, MD, PhD, Molecular Medicine Program and Division of Cardiology, University of Utah.
REFERENCES
- 1.Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10:276–81. doi: 10.1016/j.autrev.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol. 2010;37:11–25. doi: 10.1111/j.1346-8138.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 3.Krieg T, Takehara K. Skin disease: A cardinal feature of systemic sclerosis. Rheumatology. 2009;48(Suppl 3):iii14–8. doi: 10.1093/rheumatology/kep108. [DOI] [PubMed] [Google Scholar]
- 4.Pannu J, Trojanowska M. Recent advances in fibroblast signaling and biology in scleroderma. Curr Opin Rheumatol. 2004;16:739–45. doi: 10.1097/01.bor.0000137894.63091.1a. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez SA, Hitraya E, Varga J. Pathogenesis of scleroderma. Collagen. Rheum Dis Clin North Am. 1996;22:647–74. doi: 10.1016/s0889-857x(05)70294-5. [DOI] [PubMed] [Google Scholar]
- 6.Tuffanelli DL, Winkelmann RK. Systemic scleroderma. A clinical study of 727 cases. Arch Dermatol. 1961;84:359–71. doi: 10.1001/archderm.1961.01580150005001. [DOI] [PubMed] [Google Scholar]
- 7.Hesselstrand R, Scheja A, Shen GQ, Wiik A, Akesson A. The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis. Rheumatology. 2003;42:534–40. doi: 10.1093/rheumatology/keg170. [DOI] [PubMed] [Google Scholar]
- 8.Guiducci S, Distler O, Distler JH, Matucci-Cerinic M. Mechanisms of vascular damage in SSc — Implications for vascular treatment strategies. Rheumatology. 2008;47(Suppl 5):v18–20. doi: 10.1093/rheumatology/ken267. [DOI] [PubMed] [Google Scholar]
- 9.Maricq HR, Harper FE, Khan MM, Tan EM, LeRoy EC. Microvascular abnormalities as possible predictors of disease subsets in Raynaud phenomenon and early connective tissue disease. Clin Exp Rheumatol. 1983;1:195–205. [PubMed] [Google Scholar]
- 10.Cutolo M, Matucci Cerinic M. Nailfold capillaroscopy and classification criteria for systemic sclerosis. Clin Exp Rheumatol. 2007;25:663–5. [PubMed] [Google Scholar]
- 11.Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis). Postgrad Med J. 1988;64:121–5. doi: 10.1136/pgmj.64.748.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–6. [PubMed] [Google Scholar]
- 13.Cutolo M, Pizzorni C, Tuccio M, Burroni A, Craviotto C, Basso M, et al. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology. 2004;43:719–26. doi: 10.1093/rheumatology/keh156. [DOI] [PubMed] [Google Scholar]
- 14.Cutolo M, Sulli A, Secchi ME, Paolino S, Pizzorni C. Nailfold capillaroscopy is useful for the diagnosis and follow-up of autoimmune rheumatic diseases. A future tool for the analysis of microvascular heart involvement? Rheumatology. 2006;45(Suppl 4):iv43–6. doi: 10.1093/rheumatology/kel310. [DOI] [PubMed] [Google Scholar]
- 15.Poormoghim H, Lucas M, Fertig N, Medsger TA., Jr Systemic sclerosis sine scleroderma: Demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444–51. doi: 10.1002/1529-0131(200002)43:2<444::AID-ANR27>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Rodnan GP, Fennell RH., Jr Progressive systemic sclerosis sine scleroderma. JAMA. 1962;180:665–70. doi: 10.1001/jama.1962.03050210027006. [DOI] [PubMed] [Google Scholar]
- 17.Della Rossa A, Valentini G, Bombardieri S, Bencivelli W, Silman AJ, D'Angelo S, et al. European multicentre study to define disease activity criteria for systemic sclerosis. I. Clinical and epidemiological features of 290 patients from 19 centres. Ann Rheum Dis. 2001;60:585–91. doi: 10.1136/ard.60.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steen VD, Medsger TA, Jr, Rodnan GP. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): A retrospective analysis. Ann Intern Med. 1982;97:652–9. doi: 10.7326/0003-4819-97-5-652. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann K, Heckmann M, Kulozik M, Haustein UF, Krieg T. Steady-state mRNA levels of collagens I, III, fibronectin, and collagenase in skin biopsies of systemic sclerosis patients. J Invest Dermatol. 1991;97:219–22. doi: 10.1111/1523-1747.ep12480157. [DOI] [PubMed] [Google Scholar]
- 20.Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum. 1979;22:130–40. doi: 10.1002/art.1780220205. [DOI] [PubMed] [Google Scholar]
- 21.Varga J, Abraham D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furst DE, Clements PJ, Steen VD, Medsger TA, Jr, Masi AT, D'Angelo WA, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol. 1998;25:84–8. [PubMed] [Google Scholar]
- 23.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 24.Mahoney WM, Jr, Fleming JN, Schwartz SM. A unifying hypothesis for scleroderma: Identifying a target cell for scleroderma. Curr Rheumatol Rep. 2011;13:28–36. doi: 10.1007/s11926-010-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996;98:785–92. doi: 10.1172/JCI118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norton WL, Hurd ER, Lewis DC, Ziff M. Evidence of microvascular injury in scleroderma and systemic lupus erythematosus: Quantitative study of the microvascular bed. J Lab Clin Med. 1968;71:919–33. [PubMed] [Google Scholar]
- 27.Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–63. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 28.Hesselstrand R, Scheja A, Wildt M, Akesson A. High-frequency ultrasound of skin involvement in systemic sclerosis reflects oedema, extension and severity in early disease. Rheumatology. 2008;47:84–7. doi: 10.1093/rheumatology/kem307. [DOI] [PubMed] [Google Scholar]
- 29.Englert H, Low S. Pitting oedema in early diffuse systemic scleroderma. Ann Rheum Dis. 2001;60:1079–80. doi: 10.1136/ard.60.11.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pope J, McBain D, Petrlich L, Watson S, Vanderhoek L, de Leon F, et al. Imatinib in active diffuse cutaneous systemic sclerosis: Results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis Rheum. 2011;63:3547–51. doi: 10.1002/art.30549. [DOI] [PubMed] [Google Scholar]
- 31.Brazdziute E, Laurinavicius A. Digital pathology evaluation of complement C4d component deposition in the kidney allograft biopsies is a useful tool to improve reproducibility of the scoring. Diagn Pathol. 2011;6(Suppl 1):S5. doi: 10.1186/1746-1596-6-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drakos SG, Kfoury AG, Hammond EH, Reid BB, Revelo MP, Rasmusson BY, et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol. 2010;56:382–91. doi: 10.1016/j.jacc.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilbur DC. Digital cytology: Current state of the art and prospects for the future. Acta Cytol. 2011;55:227–38. doi: 10.1159/000324734. [DOI] [PubMed] [Google Scholar]
- 34.Pots S. Angiogenesis measurement using digital pathology. Lab Medicine. 2008;39:265–71. [Google Scholar]
- 35.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 36.Hachulla E, Launay D. Diagnosis and classification of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40:78–83. doi: 10.1007/s12016-010-8198-y. [DOI] [PubMed] [Google Scholar]
- 37.Oteiza A, Li R, McCuskey RS, Smedsrod B, Sorensen KK. Effects of oxidized low-density lipoproteins on the hepatic microvasculature. Am J Physiol Gastrointest Liver Physiol. 2011;301:G684–93. doi: 10.1152/ajpgi.00347.2010. [DOI] [PubMed] [Google Scholar]
- 38.Freemont AJ, Hoyland J, Fielding P, Hodson N, Jayson MI. Studies of the microvascular endothelium in uninvolved skin of patients with systemic sclerosis: Direct evidence for a generalized microangiopathy. Br J Dermatol. 1992;126:561–8. doi: 10.1111/j.1365-2133.1992.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 39.Frieri M. Systemic sclerosis. The role of the mast cell and cytokines. Ann Allergy. 1992;69:385–92. 395–6. [PubMed] [Google Scholar]
- 40.Claman HN. Mast cell changes in a case of rapidly progressive scleroderma — Ultrastructural analysis. J Invest Dermatol. 1989;92:290–5. doi: 10.1111/1523-1747.ep12276876. [DOI] [PubMed] [Google Scholar]
- 41.Krause DS, Fackler MJ, Civin CI, May WS. CD34: Structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 42.Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121:3683–92. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 43.Cotran RS, Pober JS, Gimbrone MA, Jr, Springer TA, Wiebke EA, Gaspari AA, et al. Endothelial activation during interleukin 2 immunotherapy. A possible mechanism for the vascular leak syndrome. J Immunol. 1988;140:1883–8. [PubMed] [Google Scholar]
- 44.von Bierbrauer A, Barth P, Willert J, Baerwald C, Mennel HD, Schmidt JA. Electron microscopy and capillaroscopically guided nailfold biopsy in connective tissue diseases: Detection of ultrastructural changes of the microcirculatory vessels. Br J Rheumatol. 1998;37:1272–8. doi: 10.1093/rheumatology/37.12.1272. [DOI] [PubMed] [Google Scholar]
- 45.Kahaleh B. Vascular disease in scleroderma: Mechanisms of vascular injury. Rheum Dis Clin North Am. 2008;34:57–71. vi. doi: 10.1016/j.rdc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–91. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawkins RA, Claman HN, Clark RA, Steigerwald JC. Increased dermal mast cell populations in progressive systemic sclerosis: A link in chronic fibrosis? Ann Intern Med. 1985;102:182–6. doi: 10.7326/0003-4819-102-2-182. [DOI] [PubMed] [Google Scholar]