Abstract
Objective
Prenatal maternal anxiety has detrimental effects on the resulting offspring’s neurocognitive development, including impaired attentional function. Antidepressants are commonly utilized during pregnancy, yet their impact on offspring attention and their interaction with maternal anxiety has not been assessed. Using P50 auditory sensory gating, a putative marker of early attentional processes measurable in young infants, the impact of maternal anxiety and antidepressant use are explored.
Method
Two hundred forty-two mother-infant dyads were classified relative to maternal history of anxiety and maternal prenatal antidepressant use. Infant P50 auditory sensory gating was recorded during active sleep at a mean± standard deviation of 76 ± 38 days of age.
Results
In the absence of prenatal antidepressant exposure, infants with mothers with a history of anxiety diagnoses had diminished P50 sensory gating (p<.001). Prenatal antidepressants mitigated the effect of anxiety (uncorrected p=.041). The effect of maternal anxiety was limited to amplitude of response to the second stimulus while antidepressants impacted the amplitude or response to both the first and second stimulus.
Conclusion
Maternal anxiety disorders are associated less inhibition during infant sensory gating, a performance deficit mitigated by prenatal antidepressant use. This effect may be important in considering the risks and benefits of prenatal antidepressant treatment. Cholinergic mechanisms are hypothesized for both anxiety and antidepressant effects; however the cholinergic receptors involved are likely different for anxiety and antidepressant effects. Additional work focused on understanding how treatment impacts the relationship between maternal prenatal illness and offspring neurocognitive development is indicated.
At least 8% of all pregnancies in the United States include treatment with antidepressants (1), primarily for the treatment of anxiety and mood disorders. Yet, the use of antidepressants remains controversial. While there have been no randomized controlled trials of antidepressants in pregnant women, cessation of ongoing treatment is associated with psychiatric illness relapse (2), and risks and benefits for the pregnant woman are similar to those found in non-pregnant populations (3). Concerns arise because of a lack of knowledge about the potential effect of antidepressant exposure on the developing fetus.
In utero exposure to elevated maternal stress is generally accepted as an experience with lifelong ramifications. Children with a history of in utero exposure to maternal anxiety, depression, and other forms of prenatal stress have increased risk for a variety of neuropsychiatric conditions including general psychopathology such as increased internalizing and externalizing disorders (4), specific psychopathological symptoms such as those found in attention deficit hyperactivity disorder, anxiety, and depression (5–9), and generally lower neurocognitive performance particularly in areas of attention and memory (10–12). The impact of maternal stress on offspring outcome has been demonstrated, even when controlling for confounding factors such as tobacco exposure, socioeconomic status, and later maternal psychopathology. Epidemiological studies, which often focus on a specific diagnosis, have found that in utero exposure to elevated maternal stress is associated with increased risk for autism (13) and schizophrenia (14). It has been estimated that as much as 15% of childhood emotional problems may be attributable to prenatal exposure to maternal psychopathology (15). At least some of the negative impact of in utero exposure remains even with postnatal treatment of the mother (16); whether effective prenatal treatment of the mother lowers the risk for her infant remains unknown.
Antidepressant treatment during pregnancy may be associated with a small increased risk of negative birth outcomes including spontaneous abortion (17), prematurity (18), small for gestational age at birth (19), persistent pulmonary hypertension of the newborn (20), and congenital heart defects (20–22). Antidepressants cross both placental and blood brain barriers, yet there are few studies examining brain and/or behavioral outcomes. In general, other than a slight delay in motor development (23), no significant effects of in utero antidepressant exposure on brain and behavior measures have been identified; however, sample sizes have been small, outcome measures have been nonspecific such general intelligence scores, and only a limited number of studies control for maternal psychopathology (18;23) in order to distinguish the neuropsychiatric effects of antidepressant exposure from the underlying maternal pathology leading to antidepressant use (20). Attentional deficits are one of the most commonly identified deficits associated with in utero exposure to maternal stress; however many measures of attention, such as cognitive tasks, can only be assessed later in childhood, when the child’s rearing by an ill parent may have its own effects (24). This study therefore, soon after birth, assessed the development of an evoked potential infant correlate of attentional function, P50 sensory gating, in infants born to women with and women without anxiety diagnoses, some of who were treated with antidepressants.
Repeated stimuli, such as auditory clicks delivered in pairs, engage both excitatory and inhibitory cerebral mechanisms. Diminished response to the second stimulus of the pair occurs because of inhibitory mechanisms activated during the response to the first stimulus. In adults, P50 sensory gating is impaired in a number of psychiatric disorders characterized by attentional dysfunction, including schizophrenia (25), bipolar disorder (26), attention deficit-hyperactivity disorder (27), post-traumatic stress disorder (28–31), and Parkinson’s Disease (32). In families with high rates of P50 sensory gating dysfunction, such as families of individuals with schizophrenia, P50 sensory gating deficits and attentional dysfunction cosegregate (33). P50 sensory gating is also correlated with attentional functioning within individuals, whether attention is assessed via neurocognitive testing (34) or based on self-reported ability to selectively attend (35).
The P50 auditory evoked potential is not fully developed in infants, occuring about 70 ms after the stimulus in infants rather than the 50 ms generally seen in adult populations. The infant response is also somewhat broader than the adult P50. However, its inhibition to the second of paired stimuli occurs and to keep terminology consistent with adult literature, this inhibition is referred to as P50 sensory gating (36). The infants are recorded in active sleep, a REM-like state in which they spend a majority of their sleeping time. Adults recorded in REM sleep show sensory gating inhibition and deficits similar to those seen in the awake state (37;38). P50 sensory of 14 children assessed at age 14 weeks correlates with P50 sensory gating at 47 months (r=0.75; p=.002) suggesting sensory gating is stable across early childhood (39). Elevated ratios (impaired sensory gating) in infancy are also associated with factors that suggest increased vulnerability to attentional deficits such as increased genetic vulnerability from having a parent with psychosis and in utero exposure to nicotine, an environmental risk factor (40).
The most common maternal psychiatric illnesses involve anxiety and depression, which are often comorbid. There is some indication that maternal anxiety may be the more significant risk factor for the fetus (41–43). The object of this study was to assess the effects of antidepressant exposure on the development of inhibitory brain function in the fetus while accounting for the independent effects of maternal anxiety.
Method
Participants
Initial recruitment screening included exclusionary criteria of known birth defect, chromosomal abnormality, or infant major neurological disorder. Three-hundred thirteen (313) dyads completed both an infant physiological recording and a maternal structured diagnostic psychiatric instrument. Prenatal exposure to nicotine, non-nicotine substance use, and parental psychotic illness are associated with impaired infant sensory gating (40;44); seventy-one dyads were excluded for presence of at least one of these exposures (56 for tobacco use, 16 for non-nicotine substance use disorder, and 16 for a parental psychotic diagnosis). Of the remaining 242 dyads, one-hundred thirty three (55%) were recruited via a state birth registry; one-hundred two (42%) were recruited from local obstetrics clinics; four (2%) self-referred themselves to the research program, and three (1%) were referred from a local infant care treatment program. Written informed consent was obtained from mothers and participating fathers as monitored by a local institutional review board. Information on a subset of the mother-infant pairs has previously been reported (40).
Maternal Diagnosis
Mothers’ psychiatric histories were elicited via the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)(45). All interviews were completed by an experienced psychiatric clinician (MD, DO, or MSW) with translation services when necessary. Mothers were considered to have an anxiety disorder with any history of chronic or sustained anxiety diagnosis, including agoraphobia, generalized anxiety disorder, obsessive compulsive disorder, panic disorder, and post-traumatic stress disorder. Mild or intermittent anxiety disorders that could generally be avoided by behavioral changes, such as specific phobia and social phobia, were not considered positive. Current psychiatric symptoms were defined as a mother having sufficient symptoms to meet criteria for an anxiety or depression diagnosis during her pregnancy or an illness with onset prior to pregnancy with continued symptoms causing impairment during pregnancy. Mothers with previous or chronic illness who were not impaired during pregnancy, regardless of treatment, were considered not to have current anxiety symptoms.
Auditory Sensory Gating
Details of the recording procedures have been reported previously (36). EEG (electroencephalogram) at site Cz, bipolar EOG (electrooculagram), submental EMG (electromyogram), and respiration were continuously recorded while infants slept. Paired clicks were presented through two speakers positioned at either side of the infant’s head, approximately 0.5m from each ear. Auditory clicks were 85 dB sound pressure level at the ear. Recording continued for as long as the infant remained asleep.
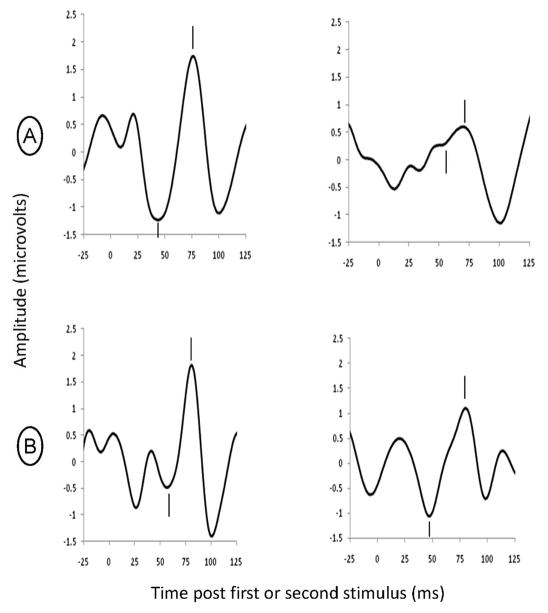
Sleep state was identified offline and average waveforms were computed from the first 15 minutes of usable active sleep data. The amplitude and latency of the largest positive peak (P1) between 50 and 100 ms following a click and preceded by a negative trough was determined by an investigator who was blinded maternal diagnosis and antidepressant treatment. Sensory gating was measured by divided the average amplitude of P1 evoked by the second click to the average amplitude of the first click, yielding a P50 sensory gating ratio (see Figure 1). The test/retest reliability of this measure is 0.86 in infants (46).
Figure 1.
Individual examples of P50 sensory gating responses during active sleep. Clicks are presented 500 ms apart; the P50 response is noted by hashmarks. The positive P50 peak (hashmark above) was measured relative to the preceding negative trough (hashmark below). (a) An example of intact sensory gating in an infant at 44 weeks post last menstrual period (approximately 4-weeks of age). Note that the response test stimulus (on the right) is suppressed in comparison to the conditioning stimulus (on the left) for a P50 sensory gating ratio of 0.11. (b) An example of an infant also at 44 weeks post last menstrual period (approximately 4-weeks of age) with decreased sensory gating. This infant’s P50 response to the test stimulus (on the right) is similar to that for the conditioning stimulus (on the left), demonstrating lack of response suppression with a sensory gating ratio = 0.94.
Statistical Methods
Maternal and infant demographics are reported in Table 1. Continuous variables are reported as means ± standard deviations and compared by students t tests. Categorical variables are reported as percents and compared by chi-squared tests. For infants, means and standard deviations for the amplitudes of the P50 response to the first and second stimuli and for P50 sensory gating ratios are reported in Table 2 by maternal anxiety disorder status by maternal prenatal antidepressant use.
Table 1.
Demographic Characteristics of Mothers and Infants
| Total | No Maternal Anxiety Disorder History | Maternal Anxiety Disorder History | Statistic | p | |
|---|---|---|---|---|---|
|
| |||||
| N=242 | N=182 | N=60 | |||
|
| |||||
| Maternal Demographics
| |||||
| Gestational age at birth (days±S.D.) | 273±12 | 273±11 | 271±15 | t=1.1 | .29 |
|
| |||||
| Maternal age at birth (yrs±S.D.) | 30±5 | 30±5 | 27±6 | t=3.9 | <.001 |
|
| |||||
| Maternal education (yrs±S.D.) | 15±3 | 15±3 | 14±3 | t=3.3 | .002 |
|
| |||||
| Maternal SEIa( ±S.D.) | 46±23 | 48±24 | 39±19 | t=2.5 | .014 |
| N# (%) ≤ 34 | 109 (45%) | 78 (43%) | 31 (52%) | ||
| N# (%) 35–60 | 54 (22%) | 37 (20%) | 17 (28%) | ||
| N# (%) ≥ 61 | 76 (31%) | 65 (36%) | 11 (18%) | ||
| unknown | 3 (1%) | 2 (1%) | 1 (2%) | ||
|
| |||||
| Active illness during pregnancyb | |||||
| anxiety | 38 (16%) | 0 (0%) | 38 (63%) | chi2=136.7 | <.001 |
| depression | 22 (9%) | 8 (4%) | 14 (23%) | chi2=19.6 | <.001 |
| anxiety or depression | 49 (20%) | 8 (4%) | 41 (68%) | chi2=114.2 | <.001 |
|
| |||||
| N# (%) with antidepressant use during pregnancy | 27 (11%) | 13 (7%) | 14 (24%) | chi2=11.9 | .001 |
| sertraline | 11 (5%) | ||||
| fluoxetine | 4 (2%) | ||||
| citalopram/escitalopram | 4 (2%) | ||||
| bupropion | 4 (2%) | ||||
| other | 4 (2%) | ||||
|
| |||||
| Infant Demographics
| |||||
| N# (%) female | 127 (53%) | 89 (49%) | 38 (63%) | chi2=3.8 | .052 |
|
| |||||
| Infant race/ethnicity N# (percent) | |||||
| Caucasian non-Hispanic | 125 (52%) | 96 (53%) | 29 (49%) | chi2=0.6 | .75 |
| Caucasian Hispanic | 72 (30%) | 54 (30%) | 18 (30%) | ||
| Other | 45 (19%) | 32 (18%) | 13 (22%) | ||
|
| |||||
| N# (%) lives with both biological parents | 198 (82%) | 153 (84%) | 45 (75%) | chi2=2.5 | .11 |
|
| |||||
| Chronological age at P50 recording in days (mean±S.D.) | 76±38 | 76±40 | 73±33 | t=0.5 | .63 |
The Socio-economic Index (SEI) is based on The Socioeconomic Index of Occupations (76). 503 occupations are included and are scored in a potential range of 0–100. Managerial and professional occupations generally have scores above 60; technical, sales, and administrative support occupations generally score between 35 and 60; service, agricultural, and labor occupations generally have scores below 35. Scores are based on the highest occupation value achieved across an individual’s life. A single individual had never been employed as was assigned an SEI score of 0.
Active psychiatric illness is defined as having sufficient active symptoms during pregnancy to meet DSM-IV criteria for an Anxiety or Depressive diagnosis, or having active illness prior to pregnancy with continued symptoms during pregnancy sufficient to cause impairment. Individuals with chronic illness who were treated to a level where symptoms were not impairing during pregnancy were not considered to have active illness.
Table 2.
Amplitude of the evoked P50 response to the first and second stimulus and the P50 sensory gating ratio in infants who mothers were positive or negative for an anxiety disorder and who did or did not utilize antidepressants during pregnancy.
| No prenatal antidepressant use | Prenatal antidepressant use | |||||||
|---|---|---|---|---|---|---|---|---|
| N | First stimulus P50 amplitude (μv) mean±S.D. | Second stimulus P50 amplitude (μv) mean±S.D. | P50 sensory gating ratio mean±S.D. | N | First stimulus P50 amplitude (μv) mean±S.D. | Second stimulus P50 amplitude (μv) mean±S.D. | P50 sensory gating ration mean±S.D. | |
| Anxiety disorder negative | 169 | 2.44±1.32 | 1.05±.96 | .425±.284 | 13 | 1.90±.78 | .74±.64 | .366±.254 |
| Anxiety disorder positive | 46 | 2.58±1.15 | 1.46±.71 | .607±.289 | 14 | 2.00±.75 | .86±.55 | .431±.207 |
Because recruitment was not stratified and assignment to antidepressants not randomized, the 2-factor maternal anxiety and maternal antidepressant use analysis had sample sizes ranging from 13 to 169 subjects per cell. The small number of subjects in some cells resulted in relatively low power to test interactions; for an analysis of variance with an alpha = .05, 80% power was achievable only for large interaction effect sizes of 1.18 standard deviations or greater. Insufficient power increases risk of an interaction effect Type II error which decreases confidence in main effect statistical results. In order to avoid problems associated with interpretation of main effects in the presence of a potential interaction that cannot be detected because of low power, analysis of variance was used to compare the four group cell means, as is common practice when it is known that interaction is present. Each dependent measure—P50 gating ratio and amplitudes of responses to the first and second stimuli—were assessed with separate analyses of variance comparing post-hoc differences of least squares group means. The Tukey-Kramer method of multiple comparisons was used where appropriate.
Results
Clinical and demographic characteristics
Sixty (60) of the 242 mothers (25%) had a history of anxiety disorder. Mothers with a history of anxiety disorder were more likely than mothers without to meet diagnostic criteria for current or residual anxiety (63% versus 0%; chi2 = 136.7; p <.001) and depressive disorders (23% versus 4%; chi2 = 16.7; p <.001) and to utilize antidepressants (24% versus 7%; chi2 = 11.9; p =.001) during pregnancy. Mothers with a history of anxiety disorder were also younger, less educated, of lower socio-economic status, and had a non-significant trend towards increased frequency of having a female infant. Infants exposed to maternal anxiety disorder did not significantly differ from those not exposed on race/ethnicity, frequency of living with both biological parents, gestational age at birth, or chronological age at time of physiological recording. Demographic information for the 242 dyads is summarized in Table 1.
Twenty-seven (11%) women utilized an antidepressant during pregnancy including sertraline (n=11), fluoxetine (n=4), escitalopram/citalopram (n=4), bupropion (n=4), and other (n=4). Treatment occurred in first trimester only (n=4; 15% of those utilizing antidepressants), second trimester only (n=1; 4%), third trimester only (n=6; 22%), and during more than one trimester (n=16; 59%). Nineteen (70% of those exposed during pregnancy) mothers continued on antidepressants after birth. Of these, sixteen infants (59% of those exposed prenatally) were exposed to antidepressants postnatally through breastmilk.
Twenty-six of the twenty-seven women (96%) who used antidepressants during pregnancy had a lifetime history of either a mood or anxiety disorder; however, only twenty-six of 118 women (22%) with a lifetime history of a mood or anxiety disorder utilized antidepressants during pregnancy. Of those with a lifetime history of a mood or anxiety disorder, antidepressant use was associated with a non-significant elevation in age at delivery (30.9 versus 28.6 years, students t = 1.85, d.f. = 116, p=.067), and with no significant associations with maternal education, socioeconomic status, minority racial or ethnic status, marital status, duration of gestation, or gender of the fetus (all p’s>.20).
Effects on Infant Sensory Gating
Age at child’s birth, years of education, and socioeconomic status differed between women with and without a history of anxiety disorder:. All analyses were conducted both with and without these covariates with no notable difference in results. Analyses without the covariates are reported here.
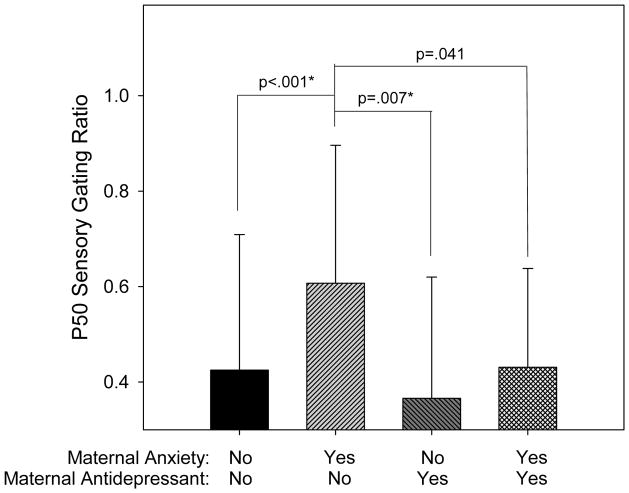
Table 2 summarizes the P50 sensory gating results. For infant P50 sensory gating ratios, there was an overall significant analysis of variance among the four groups formed by the presence or absence of a history of maternal anxiety disorder and by the presence or absence of prenatal antidepressant treatment (differences between the four means; F=5.60, ndf=3, ddf=238, p=.001). In the absence of antidepressants, maternal anxiety disorder was associated with increased P50 sensory gating (Tukey-Kramer p<.001; Figure 2). Infants of mothers treated with antidepressants, either with or without maternal anxiety disorder, had mean P50 sensory gating ratios that were less than the mean for infants from untreated mothers with anxiety disorder (Tukey-Kramer p=.007 for those without maternal anxiety disorder, p=.041 (uncorrected) for those with maternal anxiety disorder).
Figure 2.
Infant P50 ratios (mean ± SD) by group. Group membership is determined by the presence or absence of a maternal history of anxiety disorder and maternal prenatal antidepressant use. There is a significant effect of group membership (F=5.60, ndf=3, ddf=238, p=.001). Infants with a maternal history of an anxiety disorder but without prenatal exposure to antidepressants have P50 ratios significantly elevated compared to each of the other groups. The other groups do not significantly differ from each other. Differences that remain significant after Tukey-Kramer adjustment for multiple comparisons are denoted by an asterisk.
The limited sample sizes in the antidepressant exposed groups restrict additional subgroup analyses. Exploratory analyses did not identify P50 sensory gating differences between infants with pre and post-natal antidepressant exposure compared to infants with only prenatal exposure (students t =0.42, df=25; p = 0.678) or between infants exposed to selective serotonin reuptake inhibitors compared to infants exposed to other antidepressants (students t=1.40; df=25, p=.175).
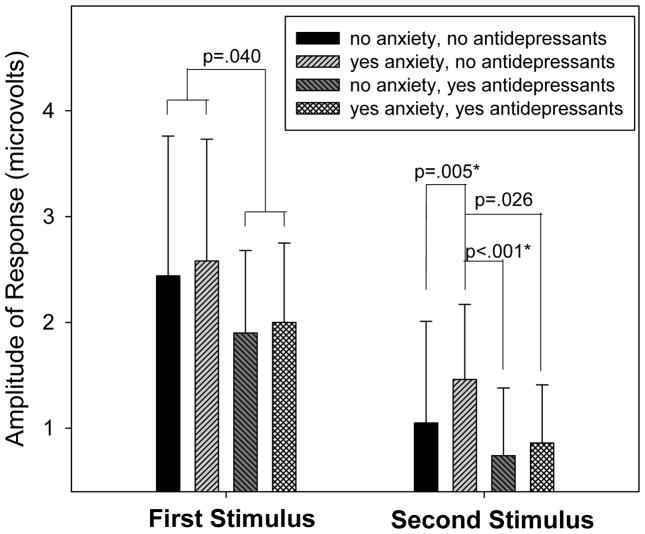
P50 sensory gating is calculated as a ratio: amplitude of response to the second stimulus/ amplitude of response to the first stimulus. Ratios can be altered by altering the numerator, denominator, or both. For all four groups of subjects, the amplitude of response to the second stimulus was significantly lower than the amplitude in response to the first stimulus (all p’s<.001) demonstrating the presence of sensory gating in all groups. To further explore the impact of group membership on P50 amplitude, two separate ANOVAs were conducted, one to explore the impact of group on amplitude of response to the first stimulus and the other to explore the impact of group on the second stimulus. There was no significant effect of group on P50 amplitude in response to the first stimulus (F=1.57, ndf=3, ddf=238, p=.197); however, when groups were collapsed across anxiety status, there was a significant effect of antidepressant exposure (t=2.06, df=240, p=.040) (Figure 3). An overall significant effect of group membership was identified for amplitude of response to the second stimulus (F=3.83, ndf=3, ddf=238, p=.010; Figure 3). Anxiety disorder (in the absence of antidepressant use) significantly increased the amplitude of infant P50 response to the second stimulus (Tukey-Kramer p=.005). Antidepressants (in the presence of anxiety) reduced amplitude to levels similar to those found in infants prenatally exposed to neither maternal anxiety nor maternal antidepressant use. Compared to infants of untreated mothers with anxiety, antidepressants in mothers without anxiety disorder decreased the P1 amplitude in infant response to the second stimulus (Tukey-Kramer p<.001) and there was indication of the same effect in infants of mothers with anxiety disorder (p=.026 uncorrected).
Figure 3.
Infant P50 amplitudes in response to the first and second stimuli presented 500 ms apart. Values are means ± standard deviations. For amplitude of P50 response to the first stimulus, the effect of group is not significant (F=1.57, ndf=3, ddf=238, p=.197); however, when groups are collapsed across anxiety status, there is a significant effect of antidepressant exposure (t=2.06, df=240, p=.040). For amplitude of P50 response to the second stimulus, there is a significant effect of group (F=3.83, ndf=3, ddf=.010). For infants without prenatal antidepressant exposure, having a mother with a history of an anxiety disorder is associated with an elevated P50 amplitude in response to the second stimulus, an effect which is at least partially mitigated by prenatal exposure to antidepressants. Differences that remain significant after adjustment for multiple comparisons are denoted by an asterisk.
Discussion
In the absence of prenatal antidepressant exposure, infants born to a mother with an anxiety disorder had more impaired P50 sensory gating than infants born to a mother with no identified anxiety disorders. For infants with a maternal anxiety disorder, prenatal antidepressant treatment improved sensory gating. Because the effect’s significance in mothers with anxiety disorders, p=0.041, was not verified by rigorous correction for multiple testing, the effect of maternal disorder anxiety should be considered mitigated, but not fully reversed by antidepressant treatment. Two-thirds of the mothers with anxiety disorder had continuing symptoms despite treatment, which may explain why treatment was only partially effective. Although tests of equivalence were not conducted, the P50 sensory gating ratios of infants whose mothers took antidepressants, regardless of diagnosis, were within the range for P50 ratios in infants of untreated mothers with no history of anxiety disorders. The limited sample size prevents firm conclusions on the relative effect sizes of maternal anxiety versus maternal antidepressant use; however, the effects are in opposite directions and suggest that antidepressant use at least partially mitigates the effect of anxiety. To our knowledge, this is the first report of antidepressant use protecting against adverse effects on fetal brain development of a maternal history of anxiety.
The relationship between prenatal exposure to maternal anxiety disorder and impaired P50 sensory gating is consistent with previous reports that attentional dysfunction is elevated in children prenatally exposed to maternal anxiety (47;48). The mechanism by which maternal stress impacts fetal brain development is unclear, but several possibilities exist. In older children and adults, anxiety and attentional dysfunction are often comorbid (49–51) and shared genetic risk between maternal anxiety and offspring attentional dysfunction is a possibility. Maternal trait anxiety may be associated with alterations in maternal cortisol (52) and both state and trait anxiety may increase the percentage of maternal cortisol which crosses the placenta and reaches the fetus (53); A deleterious direct effect of cortisol on the developing brain is thus a second possibility. Other possibilities include effects of estrogen and other hormones, which affect other measures of sensory gating (54).
The increased impairment in sensory gating seen in infant offspring of mothers with maternal anxiety disorders is primarily due to an increase in response to the second stimulus, with little to no change in response to the first stimulus. The normal inhibition seen in response to the second stimulus is mediated by a local inhibitory neurocircuit involving GABAergic interneurons (55). Stimulation during early development of α7 nicotinic cholinergic receptors on the interneurons appears critical for normal development of the inhibitory neurocircuit and elements which interfere with adequate early development, including both genetically-mediated decreases in α7 nicotinic receptor density (56;57) and decreases in the prenatal ligand choline (58), permanently impair inhibition to the second stimulus, increasing the resulting P50 sensory gating ratio. Stress leads to decreased serum choline levels (59–62), primarily due to corticosteroid-mediated increased sequestration of choline in the liver (63). Maternal anxiety disorders may result in a cortisol-mediated drop in maternal and fetal serum choline levels resulting in decreased fetal brain α7 nicotinic receptor stimulation, diminished development of inhibitory neurocircuits, and resultant impairment in the ability to gate the response to repetitive auditory stimuli.
Although it did not survive rigorous adjustment for multiple testing, the current study suggests that antidepressant exposure may be associated with improved P50 sensory gating. In adults, P50 sensory gating is impaired in adults with a history of mood disorder, but is not correlated with current mood state (64;65). Antidepressant usage in adults is associated with normalization of sensory gating even when mood symptoms are not fully treated in some but not all studies (65;66). One possibility is that antidepressant-exposure improvement in infant P50 sensory gating is an acute effect of current antidepressant exposure; however, infants with pre- and post-natal antidepressant exposure did not differ on P50 sensory gating ratios from infants with pre-natal exposure only. Thus, post-natal exposure effects seem unlikely.
The mechanisms by which antidepressants could exert an effect in the fetal brain are unknown, but could include reduction in duration or severity of mothers’ symptoms or direct protective effects on the fetal brain. Antidepressant exposure was associated with reductions in evoked P50 response amplitude in response to both the first and second stimuli. As discussed above, response to the second stimulus is associated with α7 nicotinic receptor function; response to the first stimulus appears more closely related to α4β2 nicotinic receptor activity (67), at least in adult animal models. A pharmacological feature common to most antidepressants—including selective serotonin uptake inhibitors, tricyclics, bupropion, and venlafaxine—is non-competitive inhibition of a broad range of nicotinic receptors, including α7 and α4β2 (68–75). Awareness that both α7 and α4β2 nicotinic receptors play a role in local inhibitory neurocircuit formation and function and that antidepressants are noncompetitive inhibitors of both receptor types suggests one avenue for additional research.
Limitations
Only 27 women (11% of the sample), took antidepressants during pregnancy. When combined with an anxiety history classification, some subgroups became as small as 13 participants, limiting power. In addition, while antidepressant use was almost completely limited to women with a history of either a mood or anxiety disorder, only a minority of women with such a history used antidepressants and use of antidepressants was not random. Among women who had such a history of mood and anxiety disorders, there was no difference between those who did and did not take antidepressants on several demographic variables; however, there are likely other factors which influence the individual’s choice around pharmacological treatment, factors which were not identified or controlled for in this report. Additional efforts to replicate and extend the current findings should account for other potential confounding factors to establish, for the fetus, the risk and benefit of maternal antidepressant treatment.
Acknowledgments
Supported in part by National Institutes of Health grants R25MH080859, P50MH086383, T32MH015442, R01MH056539, and K12HD001271.
Footnotes
Dr Ross has equity interest in Johnson and Johnson Pharmaceuticals. Dr. Zerbe has equity interest in Abbott Laboratories, Johnson and Johnson Pharmaceuticals, Merck Pharmaceuticals, and Pfizer. Dr. Zerbe also has a contract with Merck Pharmaceuticals as a statistician in a study of potential benefits of a booster dose of vaccine for varicella zoster.
All other authors report no financial conflicts of interest.
Reference List
- 1.Alwan S, Reefhuis J, Rasmussen SA, Friedman JM. National Birth Defects Prevention Study. Patterns of Antidepressant Medication Use Among Pregnant Women in a United States Population. The Journal of Clinical Pharmacology. 2011 Feb 1;51(2):264–270. doi: 10.1177/0091270010373928. [DOI] [PubMed] [Google Scholar]
- 2.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of Major Depression During Pregnancy in Women Who Maintain or Discontinue Antidepressant Treatment. JAMA: The Journal of the American Medical Association. 2006 Feb 1;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 3.Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. General Hospital Psychiatry. 2009;31(5):403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioral/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180(502):508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 5.Huizink AC, Dick DM, Sihvola E, Pulkkinnen L, Rose RJ, Kaprio J. Chernobyl exposure as a stressor during pregnancy and behaviour in adolescent offspring. Acta Psychiatr Scand. 2007;116(6):438–446. doi: 10.1111/j.1600-0447.2007.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Bergh BR, Van Calster B, Mits ST, Van Hufel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33(3):536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 7.Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75(4):4085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children. Journal of Child Psychology and Psychiatry. 2005;46(3):246–254. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 9.Halligan SL, Murray L, Martins C, Cooper PJ. Maternal depression and psychiatric outcomes in adolescent offspring: A 13-year longitudinal study. Journal of Affective Disorders. 2007;97(1–3):145–154. doi: 10.1016/j.jad.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Gutteling BM, de Weerth D, Zandbelt N, Mulder EJH, Viser GHA, Buitelaar JK. Does maternal prenatal stress adversely affect the Child’s learning and memory at age six. J Abnorm Child Psychol. 2006;34:789–798. doi: 10.1007/s10802-006-9054-7. [DOI] [PubMed] [Google Scholar]
- 11.Laplante DP, Barr RG, Brunet A, Dalbaud Du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56(3):400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- 12.Van den Bergh BRH, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, Lagae L. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neuroscience and Biobehavioral Reviews. 2005;29:259–269. doi: 10.1016/j.neubiorev.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008;38(3):481–488. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- 14.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65(2):146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 15.Oberlander TF, Gingrich JA, Ansorge MS. Clin Pharmacol Ther. 6. Vol. 86. American Society of Clinical Pharmacology and Therapeutics; Nov 4, 2009. Sustained Neurobehavioral Effects of Exposure to SSRI Antidepressants During Development: Molecular to Clinical Evidence; pp. 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunlicks ML, Weissmann MM. Change in child psychopathology with improvement in parental depression: A systematic review. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(4):379–389. doi: 10.1097/CHI.0b013e3181640805. [DOI] [PubMed] [Google Scholar]
- 17.Nakhai-Pour HR, Broy P, B+¬rard A. Use of antidepressants during pregnancy and the risk of spontaneous abortion. Canadian Medical Association Journal. 2010 Jul 13;182(10):1031–1037. doi: 10.1503/cmaj.091208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisner MD, Sit MD, Hanusa P, Moses-Kolko MD, Bogen MD, Hunker RN, Perel P, Jones-Ivy MD, Bodnar P, Singer P. Major depression and antidepressant treatment: Impact on pregnancy and neonatal outcomes. American Journal of Psychiatry. 2009 May 1;166(5):557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toh S, Mitchell AA, Louik C, Werler MM, Chambers CD, Hernádez-Diaz S. Antidepressant use during pregnancy and the risk fo preterm delivery and fetal growth restriction. Journal of Clinical Psychopharmacology. 2009;29(6):555–560. doi: 10.1097/JCP.0b013e3181bf344c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reis M, Källén B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychological Medicine. 2010;40(10):1723–1733. doi: 10.1017/S0033291709992194. Cambridge Journals Online. [DOI] [PubMed] [Google Scholar]
- 21.Diav-Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, Di Gianantonio E, Clementi M, Weber-Schoendorfer C, Schaefer C, Ornoy A. British Journal of Clinical Pharmacology. 5. Vol. 66. Blackwell Publishing Ltd; 2008. Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study; pp. 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakker MK, Kerstjens-Frederikse WS, Buys CHCM, de Walle HEK, de Jong-van den Berg L. Birth Defects Research Part A: Clinical and Molecular Teratology. 2. Vol. 88. Wiley Subscription Services, Inc., A Wiley Company; 2010. First-trimester use of paroxetine and congenital heart defects: A population-based case-control study; pp. 94–100. [DOI] [PubMed] [Google Scholar]
- 23.Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. The Journal of Pediatrics. 2003;142(4):402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- 24.Murray L, Arteche A, Fearon P, Halligan S, Croudace T, Cooper P. Journal of Child Psychology and Psychiatry. 10. Vol. 51. Blackwell Publishing Ltd; 2010. The effects of maternal postnatal depression and child sex on academic performance at age 16 years: a developmental approach; pp. 1150–1159. [DOI] [PubMed] [Google Scholar]
- 25.Adler LE, Pachtman E, Franks R, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 26.Martin LF, Mei-Hua H, Ross RG, Zerbe G, Freedman R, Olincy A. Physiology of schizophrenia, bipolar disorder and schizoaffective disorder. Am J Psychiatry. 2007;164(12):1900–1906. doi: 10.1176/appi.ajp.2007.06010017. [DOI] [PubMed] [Google Scholar]
- 27.Olincy A, Ross RG, Harris JG, Freedman R. Neurophsyiological studies of the P50 auditory evoked potential in adult attention deficit disorder: comparison with schizophrenia. Schizo Res. 1999;36:257. doi: 10.1016/s0006-3223(00)00239-0. [DOI] [PubMed] [Google Scholar]
- 28.Stewart LP, White PM. Sensory filtering phenomenology in PTSD. Depression and anxiety. 2008;25(1):38–45. doi: 10.1002/da.20255. [DOI] [PubMed] [Google Scholar]
- 29.Ghisolfi ES, Margis R, Becker J, Zanardo AP, Strimitzer IM, Lara DR. Impaired P50 sensory gating in post-traumatic stress disorder secondary to urban violence. Int J Psychophysiol. 2004;51(3):209–214. doi: 10.1016/j.ijpsycho.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Neylan TC, Fletcher DJ, Lenoci M, Mcallin K, Eiss WDS, Schoenfeld FB, Marmar CR, Fein G. Sensory gating in chronic posttraumatic stress disorder: reduced auditory P50 suppression in combat veterans. Biol Psychiatry. 1999;46(12):1656–1664. doi: 10.1016/s0006-3223(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 31.Gillete GM, Skinner RD, Rasco LM, Fielstein LM, Davis EM, Pawelak DH, Freeman JE, Karson TW, Boop CN, Garcia-Rill E. Combat veterans with posttraumatic stress disorder exhibit decreased habituation of the P1 midlatency auditory evoked potential. Life Sci. 1997;61(14):1421–1434. doi: 10.1016/s0024-3205(97)00688-7. [DOI] [PubMed] [Google Scholar]
- 32.Teo C, Rasco L, al-Mefty K, Skinner RD, Boop FA, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson’s disease. Mov Disord. 1997;12(5):655–664. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- 33.Harris JG, Adler LE, Young DA, Cullum CM, Rilling LM, Cicerello A, Intemann PM, Freedman R. Neuropsychological dysfunction in parents of schizophrenics. Schizo Res. 1996;(20):252–260. doi: 10.1016/0920-9964(96)00009-6. [DOI] [PubMed] [Google Scholar]
- 34.Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizo Res. 1993;10(2):131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- 35.Kisley MA, Noecker TL, Guinther PM. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology. 2004;41(4):604–612. doi: 10.1111/j.1469-8986.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 36.Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R. Early postnatal development of sensory gating. Neuroreport. 2003;14(5):693–697. doi: 10.1097/00001756-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisley MA, Olincy A, Freedman R. The effect of state on sensory gating: comparison of waking, REM and non-REM sleep. Clinical Neurophysiology. 2001;112:1154–1165. doi: 10.1016/s1388-2457(01)00578-8. [DOI] [PubMed] [Google Scholar]
- 38.Kisley MA, Olincy A, Robbins E, Polk SD, Adler LE, Waldo MC, Freedman R. Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychophysiology. 2003;40 (1):29–38. doi: 10.1111/1469-8986.00004. [DOI] [PubMed] [Google Scholar]
- 39.Gillow S, Hunter S, Ross R. Stability of P50 sensory gating in preschoolers. Journal of Investigative Medicine. 2010;58(1):154–155. [Google Scholar]
- 40.Hunter SK, Kisley MA, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophrenia Bulletin. 2010 doi: 10.1093/schbul/sbq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connor TG, eron J, lover V. Antenatal Anxiety Predicts Child Behavioral/Emotional Problems Independently of Postnatal Depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(12):1470–1477. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 42.Obel C, Hedegaard M, Brink T, Secher NJ, Olsen J+ Developmental Medicine & Child Neurology. 8. Vol. 45. Blackwell Publishing Ltd; 2003. Psychological factors in pregnancy and mixed-handedness in the offspring; pp. 557–561. [DOI] [PubMed] [Google Scholar]
- 43.Glover V. Journal of Child Psychology and Psychiatry. 4. Vol. 52. Blackwell Publishing Ltd; 2011. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective; pp. 356–367. [DOI] [PubMed] [Google Scholar]
- 44.Herndon A, Hunter S, Ross R. How does auditory gating in newborns with prenatal drug and/or nicotine expsoure differ from newborns with normal pattern exposures? Journal of Investigative Medicine. 2009;57(1):98. [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M, Wingerson DK. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition. New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- 46.Hunter SK, Corral N, Ponicsan H, Ross RG. Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport. 2008;19(1):79–82. doi: 10.1097/WNR.0b013e3282f35823. [DOI] [PubMed] [Google Scholar]
- 47.Clavarino AM, Mamun AA, O’Callaghan M, Aird R, Bor W, O’Callaghan F, Williams GM, Marrington S, Najman JM, Alati R. Maternal Anxiety and Attention Problems in Children at 5 and 14 Years. Journal of Attention Disorders. 2010 May 1;13(6):658–667. doi: 10.1177/1087054709347203. [DOI] [PubMed] [Google Scholar]
- 48.Van den Bergh BRH, Marcoen A. Child Development. 4. Vol. 75. Blackwell Publishing; 2004. High Antenatal Maternal Anxiety Is Related to ADHD Symptoms, Externalizing Problems, and Anxiety in 8- and 9-Year-Olds; pp. 1085–1097. [DOI] [PubMed] [Google Scholar]
- 49.Scholtissen-In de Braek D, Hurks PPM, van Boxtel MPJ, Dijkstra JB, Jolles J. The Identification of Attention Complaints in the General Population and Their Effect on Quality of Life. Journal of Attention Disorders. 2011 Jan 1;15(1):46–55. doi: 10.1177/1087054709347260. [DOI] [PubMed] [Google Scholar]
- 50.Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-Order Genetic and Environmental Structure of Prevalent Forms of Child and Adolescent Psychopathology. Archives of General Psychiatry. 2011 Feb 1;68(2):181–189. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larson K, Russ SA, Kahn RS, Halfon N. Patterns of Comorbidity, Functioning, and Service Use for US Children With ADHD, 2007. Pediatrics. 2011 Mar 1;127(3):462–470. doi: 10.1542/peds.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pluess M, Bolten M, Pirke KM, Hellhammer D. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biological Psychology. 2010;83(3):169–175. doi: 10.1016/j.biopsycho.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Glover V, Bergman K, Sarkar P, O’CONNOR TG. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2009;34(3):430–435. doi: 10.1016/j.psyneuen.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuropsychiatric disorders. Biological Psychiatry. 1997 Feb 15;41(4):452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- 55.Ross RG, Stevens KE, Proctor WR, Leonard S, Kisley MA, Hunter SK, Freedman R, Adams CE. Cholinergic mechanisms, early brain development, and risk for schizophrenia. Journal of Child Psychology and Psychiatry. 2010;51(5):535–549. doi: 10.1111/j.1469-7610.2009.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15(2):152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- 57.Adams CE, Yoncheck JC, Zheng L, Collins AC, Stevens KE. Altered hippocampal circuit function in C3H alpha7 null mutant heterozygous mice. Brain Research. 2008;1194:138–145. doi: 10.1016/j.brainres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens K, Adams CE, Mellot TJ, Robbins E, Kisley MA. Perinatal choline deficiency produces abnormal sensory inhibition in Sprague-Dawley rats. Brain Research. 2008;1237:84–90. doi: 10.1016/j.brainres.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulus IH, Ozyurt G, Korfali E. Decreased serum choline concentrations in humans after surgery, childbirth, and traumatic head injury. Neurochemical Research. 1998;23(5):727–732. doi: 10.1023/a:1022455325657. [DOI] [PubMed] [Google Scholar]
- 60.Ilcol YO, Uncu G, Goren S, Sayan E, Ulus IH. Declines in serum free and bound choline concentrations in humans after three different types of major surgery. Clinical Chemistry and Laboratory Medicine. 2004;42(12):1390–1395. doi: 10.1515/CCLM.2004.259. [DOI] [PubMed] [Google Scholar]
- 61.Ilcol YO, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery, and in newborns. Archives of Physiology an Biochemistry. 2002;110(5):393–399. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 62.Ilcol YO, Ozyurt G, Kilicturgay S, Uncu G, Ulus IH. The decline in serum choline concentration in humans during and after surgery is associated with the elevation of cortisol, adrenocorticotropic hormone, prolactin, and beta-endorphin concentrations. Neruoscience Letters. 2002;324(1):41–44. doi: 10.1016/s0304-3940(02)00171-4. [DOI] [PubMed] [Google Scholar]
- 63.Ilcol YO, Yilmaz Z, Ulus IH. Serum free and phospholipid-bound choline decrease and surgery and methylprednisolone administration in dogs. Neuroscience Letters. 2003;339(3):195–198. doi: 10.1016/s0304-3940(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 64.Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. American Journal of Psychiatry. 2005;162:43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Fang Y-R, Chen X-S, Chen J, Wu Z-G, Yuan C-M, Yi Z-H, Nong W, Zhang C, Cao L. A follow-up study on featuers of sensory gating P50 in treatment-resistant depression patients. Chinese Medical Journal. 2009;122(24):2956–2960. [PubMed] [Google Scholar]
- 66.Fann AV, Preston MA, Bray P, Mamiya N, Williams DK, Skinner RD, Garcia-Rill E. The P50 midlatency auditory evoked potential in patients with chronic low back pain (CLBP) Clinical Neurophysiology. 2005;116:681–689. doi: 10.1016/j.clinph.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 67.Wildeboer KM, Stevens KE. Stimulation of the [alpha]4[beta]2 nicotinic receptor by 5-I A-85380 improves auditory gating in DBA/2 mice. Brain Research. 2008;1224:29–36. doi: 10.1016/j.brainres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arias HR, Feuerbach D, Targowska-Duda KM, Russell M, Jozwiak K. Biochemistry. 27. Vol. 49. American Chemical Society; Jun 9, 2010. Interaction of Selective Serotonin Reuptake Inhibitors with Neuronal Nicotinic Acetylcholine Receptors; pp. 5734–5742. [DOI] [PubMed] [Google Scholar]
- 69.Arias HR, Rosenberg A, Targowska-Duda KM, Feuerbach D, Jozwiak K, Moaddel R, Wainer IW. Tricyclic antidepressants and mecamylamine bind to different sites in the human [alpha]4[beta]2 nicotinic receptor ion channel. The International Journal of Biochemistry & Cell Biology. 2010;42(6):1007–1018. doi: 10.1016/j.biocel.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayer A, Szasz BK, Kiss JP. Inhibitory effect of antidepressants on the NMDA-evoked [3H]noradrenaline release from rat hippocampal slices. Neurochemistry International. 2009;55(6):383–388. doi: 10.1016/j.neuint.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Arias HR. Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? The International Journal of Biochemistry & Cell Biology. 2009;41(11):2098–2108. doi: 10.1016/j.biocel.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 72.Mansvelder HD, Fagen ZM, Chang B, Mitchum R, McGehee DS. Bupropion inhibits the cellular effects of nicotine in the ventral tegmental area. Nicotinic Acetylcholine Receptors as Therapeutic Targets: Emerging Frontiers in Basic Research and Clinical Science. Biochemical Pharmacology. 2007 Oct 15;74(8):1283–1291. doi: 10.1016/j.bcp.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. CNS Drug Reviews. 3–4. Vol. 12. Blackwell Publishing Ltd; 2006. Review of the Pharmacology and Clinical Profile of Bupropion, an Antidepressant and Tobacco Use Cessation Agent; pp. 178–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopez-Valdes HE, Garia-Colunga J, Miledi R. Effects of clomipramine on neuronal nicotinic acetylcholine receptors. European Journal of Pharmacology. 2002 May 24;444(1–2):13–19. doi: 10.1016/s0014-2999(02)01556-x. [DOI] [PubMed] [Google Scholar]
- 75.Slemmer JE, Martin BR, Damaj MI. Bupropion Is a Nicotinic Antagonist. Journal of Pharmacology and Experimental Therapeutics. 2000 Oct 1;295(1):321–327. [PubMed] [Google Scholar]
- 76.Nakao K, Treas J. General Social Survey Methodological Report No 74. Chicago: University of Chicago, National Research Center; 1992. The 1989 socioeconomic index of occupations: construction from the 1989 occupational prestige scores. [Google Scholar]