Abstract
Structural, volumetric, and microstructural abnormalities have been reported in the white matter of the brain in individuals with phenylketonuria (PKU). Very little research, however, has been conducted to investigate the development of white matter in children with PKU, and the developmental trajectory of their white matter microstructure is unknown. In the current study, diffusion tensor imaging (DTI) was used to examine the development of the microstructural integrity of white matter across six regions of the corpus callosum in 34 children (7–18 years of age) with early- and continuously-treated PKU. Comparison was made with 61 demographically-matched healthy control children. Two DTI variables were examined: mean diffusivity (MD) and relative anisotropy (RA). RA was comparable to that of controls across all six regions of the corpus callosum. In contrast, MD was restricted for children with PKU in anterior (i.e., genu, rostral body, anterior midbody) but not posterior (posterior midbody, isthmus, splenium) regions of the corpus callosum. In addition, MD restriction became more pronounced with increasing age in children with PKU in the two most anterior regions of the corpus callosum (i.e., genu, rostral body). These findings point to an age-related decrement in the microstructural integrity of the anterior white matter of the corpus callosum in children with PKU.
Keywords: Phenylketonuria, White matter, Corpus callosum, Diffusion tensor imaging, Children
Introduction
Phenylketonuria (PKU1; OMIM 261600 and 261630) is an autosomal recessive disorder characterized by disrupted metabolism of phenylalanine (Phe), which ultimately results in dopamine deficiency [1,2]. This deficiency has been used to explain the impairments in cognition associated with PKU, particularly the deficits in frontally-mediated executive abilities [3,4]. In recent years, however, additional brain abnormalities, especially in white matter, have been increasingly recognized. Because white matter permits interactions among brain regions, it is likely that white matter abnormalities also contribute to the cognitive deficits associated with PKU. This may be particularly true for executive abilities subserved by the frontal lobes, as this brain region is extensively inter-connected with other brain regions via white matter pathways.
In terms of gross white matter structure, MRI has identified hyperintensities in the white matter of individuals with PKU (even in patients diagnosed early and treated continuously), with the most pronounced findings adjacent to the lateral ventricles [5-15]. In the past, the importance of these abnormalities was discounted due to the lack of association with clinical outcomes and the existence of the dopamine hypothesis of brain dysfunction in PKU [16]. More recently, however, research has revealed relationships between white matter abnormalities, clinical outcomes, and cognition. For example, higher Phe levels [5,8,10,12,15,17] and poorer cognitive performance [5,17] have been associated with white matter hyperintensities, which are at least partially reversible when Phe levels are better controlled [8]. Decreases in gray and white matter volume have also been reported [18,19], with one study citing a 10% reduction in the volume of the corpus callosum [19].
Of particular relevance to the current study, a relatively new MRI technique, diffusion tensor imaging (DTI), has also been used to investigate the white matter of individuals with PKU. DTI can evaluate the microstructural integrity of white matter that otherwise appears normal. Two DTI measures are commonly reported. First, the mean diffusivity (MD) reflects the rate at which water diffuses through white matter (equivalent to the apparent diffusion coefficient (ADC) cited in some studies). Second, relative anisotropy (RA) reflects the asymmetry of water diffusion through white matter (comparable to the fractional anisotropy (FA) cited in some studies). Abnormalities in MD and/or RA have been identified in a variety of populations with metabolic disorders and leukodystrophies [20,21].
In individuals with PKU, the majority of DTI studies have shown that MD is restricted (i.e., abnormally low), whereas RA is comparable to that of healthy controls [9-15,22-24]. In addition, greater restriction in MD has been associated with higher Phe levels [10,12,15]. Of particular interest, Vermathen et al. [15] reported MD restriction in the splenium of the corpus callosum in adults with PKU, with greater restriction when Phe levels were higher. Unlike the current study, however, Vermathen et al. [15] examined only the splenium rather than the entire corpus callosum.
Most neuroimaging research in PKU has been conducted in adults; individual cases; or mixed groups of children, adolescents, and adults, but there are a handful of studies focusing on children. Anderson et al. [5,17] identified white matter hyperintensities in children and adolescents with PKU that are similar to those observed in adults. In terms of the microstructural integrity of white matter, using DTI Peng et al. [24] found that RA was lower for infants and children with PKU in comparison with healthy controls, whereas MD was comparable. It should be noted, however, that the findings of Peng et al. [24] are markedly different from those of other DTI studies in PKU. As noted earlier, the other studies using DTI (even those including children) reported the reverse pattern, with restriction in MD and normal RA. Until further research is conducted, it may be prudent to view the results of Peng et al. [24] with caution.
In the current study, DTI was used to examine the microstructural integrity of the white matter comprising the corpus callosum in children with early- and continuously-treated PKU compared with healthy control children. Specific regions of the corpus callosum underlie interactions among specific cortical regions; anterior regions interconnect anterior cortical regions, whereas posterior regions interconnect posterior cortical regions [25]. Thus, the corpus callosum was divided into six regions of interest (ROIs: genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium) to determine whether PKU is associated with MD and/or RA abnormalities in anterior versus posterior regions of the corpus callosum. In addition, it was important to examine the developmental trajectory of the white matter microstructure. During typical development, limited changes in MD or RA occur beyond middle childhood [26-28], but in children with PKU the developmental trajectories are unknown. As such, a broad age range (7–18 years) was used.
Materials and methods
Participants
Children with PKU (n = 34) were recruited through the Division of Genetics and Genomic Medicine in the Department of Pediatrics at St. Louis Children’s Hospital and Washington University in St. Louis, Missouri (WU) and through the Metabolic Clinic at the Child Development and Rehabilitation Center at Doernbecher Children’s Hospital at Oregon Health & Science University in Portland, Oregon (OHSU). All children were diagnosed with PKU soon after birth and were treated early and continuously through dietary management to limit Phe intake. Blood Phe level obtained closest to the time of neuroimaging (typically on the day of neuroimaging) ranged from 1.4 to 26.0 mg/dL (M = 8.9 mg/dL, SD = 5.8 mg/dL).
The data of children with PKU were compared with those of healthy control children (n = 61) recruited from the St. Louis and Portland communities. All children in the PKU and control groups were recruited in conjunction with an NIH-funded longitudinal study (R01 HD0449901). Children in both groups ranged from 7 to 18 years of age (PKU: M = 12.2, SD = 4.0; Control: M = 12.4, SD = 3.3). Education ranged from 1 to 13 years for the PKU group and 1 to 14 years for the control group (PKU: M = 6.4, SD = 3.7; Control: M = 6.4, SD = 3.4). There were no significant between-group differences in either age or education (p > 0.05 in both instances). In terms of race/ethnicity, 6% and 25% of the PKU and control groups, respectively, comprised individuals from minority populations. Although the PKU group included significantly fewer individuals from minority populations than the control group (χ2 (1, N = 95) = 5.20, p < 0.05), the pattern of findings from neuroimaging analyses did not differ when race/ethnicity was controlled. As such, the reported analyses do not include race/ethnicity. Intelligence quotient (IQ) ranged from 85 to 139 for the PKU group and 82 to 143 for the control group (PKU: M = 105.9, SD = 11.6; Control: M = 113.8, SD = 15.0). IQ was significantly lower for the PKU than control group (t(93) = 2.66, p < 0.01), but there were no significant correlations between IQ and DTI variables. In addition, because differences in IQ may be attributable to differences in brain function rather than the reverse, IQ was not controlled in neuroimaging analyses. No child had a history of mental retardation, learning disorder, or major medical disorder unrelated to PKU.
Procedure
Approval to conduct the study was obtained from the Human Research Protection Offices at WU and OHSU.
Children were scanned with a 3.0T Siemens Trio at OHSU and with a 1.5T Siemens Sonata (Erlangen, Germany) at WU. Structural scans included a T1-weighted (T1W) sagittal, magnetization-prepared rapid gradient echo (MP-RAGE; repetition time (TR) = 1580 ms (OHSU) and 1900 ms (WU), echo time (TE) = 3.93 ms, flip angle = 8° (OHSU) and 15° (WU), 1 × 1 × 1 mm (OHSU) and 1 × 1 × 1.25 mm (WU) voxels) and a T2-weighted (T2W) fast spin echo (TR = 3500 ms (OHSU) and 10,000 ms (WU), TE = 106 ms (OHSU) and 94 ms (WU), 1 × 1 × 2 mm (OHSU) and 1 × 1 × 3 mm (WU)). DTI was acquired using an echo planar imaging (EPI) sequence (TR = 9000 ms, TE = 84 ms (OHSU) and 78 ms (WU), 2.5 mm (OHSU) and 3.0 mm (WU) isotropic voxels, conventional hexahedral (6 direction) encoding with diffusion sensitization of b-values = 0 and 1000 s/mm2). Four complete DTI datasets were acquired for each participant. Total imaging time was approximately 1 h.
The first image-processing step was to define the spatial relationships between all images in terms of affine transforms computed by image registration. Multi-modality (e.g., T2W → T1W) image registration was accomplished using vector gradient measure (VGM) maximization [29]. The first acquired, unsensitized (b = ~0 s/mm2; I0) DTI volume was registered to the T2W image; stretch and shear were enabled (12 parameter affine transform) to partially compensate for EPI distortion. Atlas transformation was computed via the T1W image, which itself was registered to an atlas representative target produced by mutual co-registration of MP-RAGE images from 12 normal, young adults. The atlas target conformed to the Talairach system as implemented by Lancaster et al. [30].
T2W images were visually inspected to ensure that measurements were performed in normal-appearing white matter without hyperintensities. ROIs of the corpus callosum were defined in a manner similar to that used by Ota et al. [31] and Wilde et al. [32]. ROIs were drawn semi-automatically on color-coded directional diffusion (RGB) maps using the image processing package Analyze (Mayo Clinic Foundation, Rochester, MN) by tracing the corpus callosum on sagittal slices 82–93 out of 176. After defining a single ROI for the corpus callosum, it was split into four equal parts along its anterior to posterior direction. This was followed by vertically dividing the most anterior and most posterior of the four resulting ROIs into equal halves. The final result was six ROIs corresponding to the genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium of the corpus callosum.
The diffusion tensor and its three eigenvalues were calculated using log-linear regression in each voxel [33] for each ROI. Using standard methods, MD was computed as the average of the three eigenvalues. As a quantitative measure of anisotropy, RA was used [34]. Diffusion parameter values were measured in individuals by averaging over voxels within each of the six ROIs. An example of the images obtained and ROIs within the corpus callosum is provided in Fig. 1.
Fig. 1.
Sample MD (RBG) and RA maps for a participant with PKU.
Results
MD and RA were examined separately using repeated measures analysis of variance (ANOVA), with group (PKU, control) as the between-subjects variable and ROI of the corpus callosum (genu, rostral body, anterior midbody, posterior midbody, isthmus, splenium) as the within-subjects variable. Because the purpose of these analyses was to determine whether group differentially affected MD or RA, post hoc analyses were conducted only when the main effect of group and/or the interaction between group and ROI in the omnibus ANOVA was significant (p < 0.05). In these instances, post hoc analyses were conducted for each of the six ROIs of the corpus callosum using the Bonferroni correction for multiple comparisons, with the α value set at 0.008. It was expected that the main effect of ROI would be significant, because regions of the corpus callosum typically vary in MD and RA [25]. For this reason, post hoc analyses were not performed when only the main effect of ROI was significant.
When the omnibus ANOVAs described above resulted in a significant main effect of group and/or a significant interaction between group and ROI, the effect of age on RA and MD was also examined for each ROI of the corpus callosum using hierarchical regression analysis. Age was entered in the first step, group (PKU, control) was entered in the second step, and the interaction between age and group was entered in the final step.
For RA, repeated measures ANOVA revealed the expected significant main effect of ROI (F(5, 465) = 285.55, p < 0.001). Neither the main effect of group nor the interaction between group and ROI was significant (p > 0.05 in both instances), and no further analyses were conducted for RA.
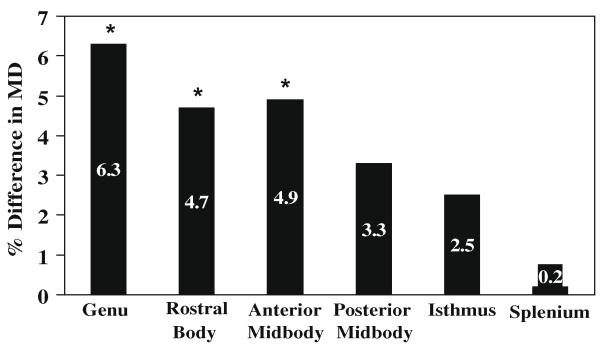
In contrast, for MD, repeated measures ANOVA revealed a significant main effect of group (F(1, 93) = 10.86, p < 0.001) and the expected significant main effect of ROI (F(5, 465) = 10.86, p < 0.001). The interaction between group and ROI was also significant (F(5, 465) = 2.98, p < 0.02). As shown in Table 1, post hoc analyses indicated that MD was significantly restricted in the PKU group compared with the control group in more anterior regions of the corpus callosum (i.e., genu (t(93) = 5.66, p < 0.001), rostral body (t(93) = 3.24, p < 0.002), anterior midbody (t(93) = 2.89, p < 0.005)). No significant between-group differences were observed for MD in more posterior regions of the corpus callosum (i.e., posterior midbody, isthmus, splenium (p > 0.008 in all instances)). As shown in Fig. 2, the percent difference in MD between the PKU and control groups was greatest in the anterior regions of the corpus callosum.
Table 1.
Mean (SD) for RA and MD for ROIs of the corpus callosum
| ROI | RA |
MD |
||
|---|---|---|---|---|
| Control | PKU | Control | PKU | |
| Genu | 600 (35) | 598 (34) | 838 (32) | 785 (60)* |
| Rostral body | 520 (44) | 525 (51) | 858 (48) | 818 (73)* |
| Anterior midbody | 471 (53) | 500 (65) | 892 (65) | 848 (79)* |
| Posterior midbody | 433 (66) | 451 (83) | 981 (89) | 950 (112) |
| Isthmus | 583 (38) | 593 (39) | 844 (40) | 823 (60) |
| Splenium | 612 (43) | 629 (41) | 813 (47) | 811 (60) |
MD significantly restricted in PKU group compared with control group.
Fig. 2.
Percent difference in MD between PKU and control groups for ROIs of the corpus callosum. *MD significantly restricted in PKU group compared with control group.
Because the omnibus repeated measures ANOVA for MD revealed a significant main effect of group and a significant interaction between group and ROI, the effect of age on MD for each ROI of the corpus callosum was examined using hierarchical regression analysis. As shown in Table 2, age accounted for a significant proportion of the variance in MD across all ROIs of the corpus callosum. Consistent with results from the earlier ANOVAs, group accounted for a significant proportion of the variance in MD in the genu, rostral body, and anterior midbody (a finding for the isthmus should be disregarded because in earlier post hoc analyses the isthmus was not significantly different between the groups after the Bonferonni correction).
Table 2.
Significant results from regressions examining MD as a function of age across six ROIs of the corpus callosum for PKU and control groups
| ROI | Age |
Group |
Age × group |
||||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 | F | P | Δ R 2 | Δ F | P | Δ R 2 | Δ F | P | |
| Genu | 0.13 | 13.67 | 0.001 | 0.26 | 39.93 | 0.001 | 0.04 | 7.13 | 0.009 |
| Rostral body | 0.07 | 6.50 | 0.02 | 0.11 | 11.61 | 0.001 | 0.07 | 8.20 | 0.005 |
| Anterior midbody | 0.13 | 13.87 | 0.001 | 0.09 | 10.15 | 0.001 | NS | ||
| Posterior midbody | 0.04 | 4.12 | 0.05 | NS | NS | ||||
| Isthmus | 0.14 | 15.61 | 0.001 | 0.05 | 5.46 | 0.03 | NS | ||
| Splenium | 0.12 | 13.13 | 0.001 | NS | NS | ||||
Degrees of freedom: age (1,93), group (1, 92), age × group (1,91).
NS, not significant.
Of greatest interest, the interaction between age and group accounted for a significant proportion of variance in MD in the two most anterior ROIs of the corpus callosum (i.e., genu, rostral body). For the genu, MD was more restricted as a function of increasing age for the PKU group (r = −0.57, p < 0.001) than control group (r = −0.28, p < 0.04). For the rostral body, MD was more restricted as a function of increasing age for the PKU group (r = −0.52, p < 0.002) but not for the control group (r = −0.04, p > 0.05).
We also examined possible relationships between MD and Phe or IQ. Phe closest to the time of neuroimaging increased significantly as a function of age in the PKU group (r = −0.48, p < 0.005), but no significant correlations were identified between Phe and MD for any ROI of the corpus callosum (correlations ranged from r = −0.08 in the splenium to r = −0.27 in the genu). Similarly, no significant correlations were identified between IQ and MD for any ROI.
Discussion
Using DTI, we identified an age-related decrement in the microstructural integrity of the anterior white matter of the corpus callosum in children with early- and continuously-treated PKU. None of the participants with PKU in our study had white matter hyperintensities in the corpus callosum, and thus our study focused on normal-appearing white matter. Consistent with past research [9-15,22,23], we found that MD was restricted in our PKU group, but we found no difference between PKU and control groups in RA. More specifically, MD restriction was evident in anterior (i.e., genu, rostral body, anterior body) but not posterior regions of the corpus callosum. Furthermore, the restriction in MD became increasingly pronounced with age in the two most anterior regions (i.e., genu, rostral body) of the corpus callosum. Thus, in addition to general differences between children with PKU and controls, the developmental trajectory of MD was different between the groups in anterior regions of the corpus callosum.
In healthy children without PKU, a distinct developmental pattern has been identified in which MD is high after birth then decreases during early childhood, whereas RA is low after birth then increases during early childhood. By middle childhood little additional change occurs, with MD and RA remaining relatively stable [26-28]. Our results show that in children with PKU this developmental pattern is disrupted, at least for MD in anterior regions of the corpus callosum. Recall that MD reflects the rate of water diffusion through the white matter, whereas RA reflects the asymmetry of water diffusion. As such, our findings indicate that the rate of water diffusion in the anterior corpus callosum is abnormal in children with PKU, whereas the microscopic architecture of the corpus callosum is preserved.
Although further research is needed to elucidate the precise mechanism(s) underlying MD restriction in PKU, prior research has suggested status spongiosis and/or damage to myelin sheaths with rapid myelin turnover [13]. The accumulation of intracellular debris produced as a byproduct of inadequate Phe metabolism has also been suggested as a mechanism of restricted MD [11]. At an even broader level, further research is needed to determine whether the physiological mechanisms underlying dopamine deficiency, white matter hyperintensities, and compromises in the microstructural integrity of white matter are interrelated or separable in PKU.
Turning to cognition, white matter permits interactions among brain regions that subserve various cognitive abilities. Anderson et al. [5,17] used MRI to identify an association between gross structural white matter hyperintensities and intelligence, academic skills, attention, processing speed, memory, and executive abilities in children with PKU. It is also possible that compromises in the microstructural integrity of white matter, occurring within the context of normal-appearing white matter (i.e., in the absence of hyperintensities), have negative effects on cognition. In our study, we did not find a significant relationship between MD restriction and intelligence. It should be kept in mind, however, that intelligence is a very broad and nonspecific construct that essentially averages across a range of cognitive abilities.
MD restriction in the anterior corpus callosum could be associated with impairments in specific rather then general aspects of cognition. Given that the anterior regions of the corpus callosum interconnect anterior regions of the cerebral cortex [25], frontally-mediated executive abilities may be especially vulnerable. In support of this hypothesis, relationships have been identified between executive abilities and the integrity of the genu of the corpus callosum in healthy young and older adults [35,36]. In addition, there are reports of disrupted interhemispheric transfer of information in children with PKU [37,38]. There is also evidence of age-related decrements in executive abilities in children with PKU [39,40], which could map onto the age-related restriction in MD that we identified in the current study. Examination of the hypothesis that compromises in the microstructural integrity of the anterior corpus callosum are associated with deficits in executive abilities is beyond the scope of the current investigation, but this is clearly an important avenue for future research.
Another avenue for future research is exploration of the relative contributions of dopamine deficiency, gross structural white matter hyperintensities, and compromises in the microstructural integrity of white matter to the cognitive impairments associated with PKU. Until fairly recently, dopamine deficiency was hypothesized as the primary (if not exclusive) neural mechanism of PKU-related impairments in cognition. It is now obvious that we must examine the individual and synergistic contributions of several neural abnormalities if we are to fully understand the mechanisms underlying cognitive impairments in PKU.
In this vein, we did not identify correlations between Phe levels obtained closest to neuroimaging (i.e., concurrent Phe levels) and MD restriction in the anterior corpus callosum. This could be due to a restricted range of Phe levels within our sample, but it is also possible that this indirectly reflects a dissociation between dopamine deficiency and compromises in the microstructural integrity of white matter. Another possibility is that lifetime Phe levels (which were not available for analysis) are associated with restricted MD, although concurrent Phe levels are not. Comprehensive research examining specific cognitive abilities within the context of findings from neuroimaging techniques such as spectroscopy, structural MRI, functional MRI, and DTI within the same sample of individuals with PKU may be particularly informative in determining the relative contributions of dopamine deficiency, white matter hyperintensities, and compromises in the microstructural integrity of white matter to cognitive impairments in individuals with PKU.
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development (R01 HD0449901 and K23 HD053212) and the National Institute of Neurologic Disorders and Stroke (P30 NS048056). We thank Carla Coleman, Laurie Sprietsma, Jeri Janowski, Yvonne Timmermann, Tina Marrone, Sarah Kachan, and Jean-Baptiste Roullet for their assistance in participant recruitment and data collection.
Footnotes
References to electronic databases: Phenylketonuria, OMIM 261600 and 261630.
Abbreviation used: PKU, phenylketonuria; DTI, diffusion tensor imaging; MD, mean diffusivity; ADC, apparent diffusion coefficient; RA, relative anisotropy; FA, fractional anisotropy; ROI, region of interest; IQ, intelligence quotient; EPI, echo planar imaging; MP-RAGE, magnetization-prepared rapid gradient echo; VGM, vector gradient measure; ANOVA, analysis of variance.
Financial disclosures: Desiree A. White and Dorothy K. Grange serve as consultants for BioMarin Pharmaceutical Inc.; Robert D. Steiner served as a consultant for BioMarin Pharmaceutical Inc. in the past; Lisa Tabor Connor, Binyam Nardos, Joshua S. Shimony, Asif Moinuddin, Abraham Z. Snyder, Rebecca Archer, and Robert C. McKinstry reported no biomedical financial interests or potential conflicts of interest.
References
- [1].Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Hum. Mutat. 2007;28:831–845. doi: 10.1002/humu.20526. [DOI] [PubMed] [Google Scholar]
- [2].Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly W, Valle D, Childs B, Vogelstein B, editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 2001. pp. 1667–1724. [Google Scholar]
- [3].DeRoche K, Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: intelligence and executive function. Dev. Neuropsychol. 2008;33:474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- [4].Christ SE, Huijbregts SCJ, de Sonnerville LMJ, White DA. Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol. Genet. Metab. 2009;99:S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [5].Anderson PJ, Wood SJ, Francis DE, Coleman L, Warwick L, Casanelia S, Anderson VA, Boneh A. Neuropsychological functioning in children with early-treated phenylketonuria: impact of white matter abnormalities. Dev. Med. Child Neurol. 2004;46:230–238. doi: 10.1017/s0012162204000386. [DOI] [PubMed] [Google Scholar]
- [6].Bick U, Ullrich K, Stober U, Moller H, Schuierer G, Ludolph AC, Oberwittler C, Weglage J, Wendel U. White matter abnormalities in patients with treated hyperphenylalaninemia: magnetic resonance relaxometry and proton spectroscopy findings. Eur. J. Pediatr. 1993;152:1012–1020. doi: 10.1007/BF01957228. [DOI] [PubMed] [Google Scholar]
- [7].Cleary MA, Walter JH, Wraith JE, Jenkins JP, Alani SM, Tyler K, Whittle D. Magnetic resonance imaging of the brain in phenylketonuria. Lancet. 1994;344:87–90. doi: 10.1016/s0140-6736(94)91281-5. [DOI] [PubMed] [Google Scholar]
- [8].Cleary MA, Walter JH, Wraith JE, White F, Tyler K, Jenkins PR. Magnetic resonance imaging in phenylketonuria: reversal of cerebral white matter change. J. Pediatr. 1995;127:251–255. doi: 10.1016/s0022-3476(95)70303-9. [DOI] [PubMed] [Google Scholar]
- [9].Dezortova M, Jajek M, Tintera J, Hejcmanova L, Sykova E. MR in phenylketonuria-related brain lesions. Acta Radiol. 2001;42:459–466. doi: 10.1080/028418501127347179. [DOI] [PubMed] [Google Scholar]
- [10].Kono K, Okano Y, Nakayama K, Hase Y, Minamkiawa S, Ozawa N, Yokote H, Inoue Y. Diffusion-weighted MR imaging in patients with phenylketonuria: relationship between serum phenylalanine levels and ADC values in cerebral white matter. Radiology. 2005;236:630–636. doi: 10.1148/radiol.2362040611. [DOI] [PubMed] [Google Scholar]
- [11].Leuzzi V, Tosetti M, Montanaro D, Carducci C, Artiola C, Carducci C, Antonozzi I, Burroni M, Carnevale F, Chiarotti F, Popolizio T, Giannatempo GM, D’Alesio B, Scarabino T. The pathogenesis of the white matter abnormalities in phenylketonuria. A multimodal 3.0 T MRI and magnetic resonance spectroscopy (1H MRS) study. J. Inherit. Metab. Dis. 2007;30:209–216. doi: 10.1007/s10545-006-0399-4. [DOI] [PubMed] [Google Scholar]
- [12].Manara R, Burlina AP, Citton V, Ermani M, Vespignani F, Carollo C, Burlina AB. Brain MRI diffusion-weighted imaing in patients with classical phenylketonuria. Neuroradiology. 2009 doi: 10.1007/s00234-009-0574-z. EPub ahead of print. [DOI] [PubMed] [Google Scholar]
- [13].Phillips MD, McGraw P, Lowe MJ, Mathews VP, Hainline BE. Diffusion-weighted imaging of white matter abnormalities in patient with phenylketonuria. Am. J. Neuroradiol. 2001;22:1583–1586. [PMC free article] [PubMed] [Google Scholar]
- [14].Scarabino T, Popolizio T, Tosetti M, Montanaro D, Giannatempo GM, Terlizzi R, Pollice S, Maiorana A, Maggialetti N, Carriero A, Leuzzi V, Salvolini U. Phenylketonuria: white matter changes assessed by 3.0-T magnetic resonance (MR) imaging, MR spectroscopy and MR diffusion. Radiol. Med. 2009;114:461–474. doi: 10.1007/s11547-009-0365-y. [DOI] [PubMed] [Google Scholar]
- [15].Vermathen P, Robert-Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magn. Reson. Med. 2007;58:1145–1156. doi: 10.1002/mrm.21422. [DOI] [PubMed] [Google Scholar]
- [16].Diamond A. Evidence for the importance of dopamine for prefrontal functions early in life. In: Roberts A, Robbins T, editors. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford University Press; New York: 1998. pp. 144–164. [Google Scholar]
- [17].Anderson PJ, Wood SJ, Francis DE, Coleman L, Anderson V, Boneh A. Are neuropsychological impairments in children with early-treated phenylketonuria (PKU) related to white matter abnormalities or elevated phenylalanine levels? Dev Neuropsychol. 2007;32:645–668. doi: 10.1080/87565640701375963. [DOI] [PubMed] [Google Scholar]
- [18].Pérez-Dueñas B, Pujol J, Soriano-Mas C, Ortiz H, Artuch R, Vilaseca MA, Campistol J. Global and regional volume changes in the brains of patients with phenylketonuria. Neurology. 2006;66:1074–1078. doi: 10.1212/01.wnl.0000204415.39853.4a. [DOI] [PubMed] [Google Scholar]
- [19].Pfaendner NH, Reuner G, Pietz J, Jost G, Rating D, Magnotta VA, Mohr A, Kress B, Sartor K, Hahnel S. MR imaging-based volumetry in patients with early-treated phenylketonuria. Am. J. Neuroradiol. 2005;26:1681–1685. [PMC free article] [PubMed] [Google Scholar]
- [20].Engelbrecht V, Scherer A, Rassek M, Witsack HJ, Modder U. Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiology. 2002;222:410–418. doi: 10.1148/radiol.2222010492. [DOI] [PubMed] [Google Scholar]
- [21].Sener RN. Diffusion magnetic resonance imaging patterns in metabolic and toxic brain disorders. Acta Radiol. 2004;45:561–570. doi: 10.1080/02841850410006128. [DOI] [PubMed] [Google Scholar]
- [22].Ding X, Fiehler J, Kohlschutter B, Wittkugel O, Grzyska U, Zeumer H, Ullrich K. MRI abnormalities in normal-appearing brain tissue of treated adult PKU patients. J. Magn. Reson. Imaging. 2008;27:998–1004. doi: 10.1002/jmri.21289. [DOI] [PubMed] [Google Scholar]
- [23].Sener RN. Phenylketonuria: diffusion magnetic resonance imaging and proton magnetic resonance spectroscopy. J. Comput. Assist. Tomogr. 2003;27:541–543. doi: 10.1097/00004728-200307000-00016. [DOI] [PubMed] [Google Scholar]
- [24].Peng SS, Tseng WY, Chien YH, Hwu WL, Liu HM. Diffusion tensor images in children with early-treated, chronic, malignant phenylketonuric: correlation with intelligence assessment. Am. J. Neuroradiol. 2004;25:1569–1574. [PMC free article] [PubMed] [Google Scholar]
- [25].Hofer S, Frahm J. Topography of the human corpus callosum revisited-comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- [26].Miller JH, McKinstry RC, Philip JV, Mukherjee P, Neil JJ. Diffusion-tensor MR imaging of normal brain maturation: a guide to structural development and myelination. Am. J. Roentgenol. 2003;180:851–859. doi: 10.2214/ajr.180.3.1800851. [DOI] [PubMed] [Google Scholar]
- [27].Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- [28].Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin. N. Am. 2006;16:19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- [29].Rowland DJ, Garbow JR, Laforest R, Snyder AZ. Registration of [18F]FDG microPET and small-animal MRI. Nucl. Med. Biol. 2005;32:567–572. doi: 10.1016/j.nucmedbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [30].Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum. Brain Mapp. 1995;3:209–223. [Google Scholar]
- [31].Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- [32].Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2006;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- [33].Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- [34].Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- [35].Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J. Cogn. Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Banich MT, Passarotti AM, White DA, Nortz MJ, Steiner RD. Interhemispheric interaction during childhood: II. Children with early-treated phenylketonuria. Dev. Neuropsychol. 2000;18:53–71. doi: 10.1207/S15326942DN1801_4. [DOI] [PubMed] [Google Scholar]
- [38].Gourevitch ML, Craft S, Dowton SB, Ambrose P, Sparta S. Interhemispheric transfer in children with early-treated phenylketonuria. J. Clin. Exp. Neuropsychol. 1994;16:393–404. doi: 10.1080/01688639408402650. [DOI] [PubMed] [Google Scholar]
- [39].White DA, Nortz MJ, Mandernach T, Huntington K, Steiner RD. Deficits in memory strategy use related to prefrontal dysfunction during early development: evidence from children with phenylketonuria. Neuropsychology. 2001;15:221–229. doi: 10.1037//0894-4105.15.2.221. [DOI] [PubMed] [Google Scholar]
- [40].White DA, Nortz MJ, Mandernach T, Huntington K, Steiner RD. Age-related working memory impairments in children with prefrontal dysfunction associated with phenylketonuria. J. Int. Neuropsychol. Soc. 2002;8:1–11. [PubMed] [Google Scholar]