Abstract
Background
Deficient cerebral inhibition is a pathophysiological brain deficit related to poor sensory gating and attention in schizophrenia and other disorders. Cerebral inhibition develops perinatally, influenced by genetic and in utero factors. Amniotic choline activates fetal α7-nicotinic acetylcholine receptors and facilitates development of cerebral inhibition. Increasing this activation may protect infants from future illness by promoting normal brain development.
Methods
A randomized placebo-controlled clinical trial of dietary phosphatidylcholine supplementation was conducted with 100 healthy pregnant women, who consented to the study at second trimester. Supplementation to twice normal dietary levels for mother or newborn continued through the third postnatal month. All women received dietary advice regardless of treatment. Infants’ electroencephalographic recordings of inhibition of the P50 component of the cerebral evoked response to paired sounds were analyzed. Criterion for inhibition was suppression of the amplitude of the second P50 response by at least half, compared to the first response.
Results
No adverse effects of choline were observed in maternal health and delivery, birth, or infant development. More choline-treated infants (76%) suppressed the P50 response, compared to placebo-treated infants (43%) at the fifth postnatal week (effect size 0.7). There was no difference at the 13th week. A CHRNA7 genotype associated with schizophrenia diminished P50 inhibition in the placebo-treated infants, but not in the choline-treated infants.
Conclusion
Neonatal developmental delay in inhibition is associated with attentional problems as the child matures. Perinatal choline activates timely development of cerebral inhibition, even in the presence of gene mutations that otherwise delay it.
Infants who later develop schizophrenia already have signs of early developmental delay.1–9 Genes associated with schizophrenia, like NRG1, ErbB4, and DISC1, are expressed at high levels in the fetus, where they normally promote synaptogenesis.10 Environmental risk factors associated with later expression of psychotic symptoms in the offspring include maternal malnutrition from famine to micronutrient deficiency, depression, anxiety, infection, and cigarette smoking.11–15 Abnormalities in the development of cerebral inhibitory neurons are a key pathophysiological defect in schizophrenia.16 Cerebral inhibition develops during fetal and early postnatal life.17,18 Newborns whose parents are psychotic or whose mothers had depression or anxiety disorders or smoked cigarettes during pregnancy have diminished inhibition.19 Schizophrenia and related mental illnesses are common disorders and their genetic and perinatal environmental risk factors have high frequency in the general population. Thus, as for other illnesses with early developmental origins, primary prevention must be a safe, population-wide intervention that promotes normal development even in healthy women and infants.
GABA, the inhibitory neurotransmitter of adult life, is excitatory in early fetal brain development. Expression of the membrane chloride transporter KCC2 that switches GABA from excitatory to inhibitory is stimulated by activation of postsynaptic α7-nicotinic acetylcholine receptors.17 Αlpha7-nicotinic receptors are expressed at 10-fold higher levels in fetal hippocampus than in adults, perhaps reflecting this critical developmental function, but these receptors are diminished in the brains of person who had schizophrenia.20,21 Innervation of postsynaptic alpha7-nicotinic receptors by cholinergic axons does not occur until the end of the third trimester.22 Instead, they are activated by the millimolar concentrations of choline in the amniotic fluid.23,24
Several maternal risk factors for schizophrenia are associated with decreased choline availability for the fetus.24 Because of the demands for choline as a lipid component of cell membranes, the pregnant woman has to increase her dietary intake with foods rich in choline, such as meats and eggs. Malnutrition may prevent that increase. The mother may sequester choline in her own liver, depriving the fetus, if she is stressed from trauma, anxiety, or depression. Choline levels are regulated in part by phenylethanolamine methyl transferase. Polymorphisms in its gene PEMT associated with diminished choline levels are also associated with schizophrenia.25
In the inbred mouse strain DBA/2 with genetically diminished expression of hippocampal α7-nicotinic receptors, dietary supplementation of maternal choline from conception through weaning significantly increased cerebral inhibition in the adult offspring.26 We conducted a similar randomized placebo-controlled trial of effects of perinatal choline on the development of cerebral inhibition in human infants. Choline perinatal supplementation is already advocated in the public media because animal models show that it facilitates cognitive function in offspring.27 This is the first randomized human trial conducted under an FDA Investigational New Drug exemption for the purpose of ameliorating a pathophysiological deficit associated with risk for illness. Because the infants in this study were too young to show the effects of the intervention on a wider behavioral repertoire, a group of children who had been recorded electrophysiologically as infants 4 years earlier were studied to assess the behavioral sequelae of diminished infant P50 inhibition.28
Methods
Healthy pregnant women were recruited from Denver obstetrical practices at the beginning of their second trimester. They had no self-reported illicit substance or medical marijuana use in the last 6 months, alcohol dependence, or current nicotine use confirmed by urinary cotinine levels. Exclusions included maternal history of trimethylaminuria, renal disease, liver disease, Parkinson’s disease, maternal history of fetal death, fetal congenital malformation, fetal genetic abnormality, or current multiparous pregnancy. Women were also excluded for fetal abnormality on initial ultrasound examination. The study was approved by the Colorado Institutional Review Board. Mothers were compensated for bringing their infants to the laboratory for recording.
After written informed consent, including the assent of the father when available, women completed a 7-day placebo lead-in. Subjects whose compliance exceeded 70% of these capsules by medicine vial counts were randomized to identical appearing placebo or drug early in second trimester. Mothers received standard prenatal care, including standard folate and multivitamin supplements and high resolution ultrasounds during pregnancy. Parents’ psychiatric histories were elicited via the Structured Clinical Interview for DSM-IV Axis I Disorders. Principal diagnoses were major depressive disorder, panic disorder, agoraphobia, generalized anxiety disorder, obsessive compulsive disorder, and post traumatic stress disorder. Current psychiatric symptoms were defined as a mother having sufficient symptoms to meet criteria for an anxiety or depression diagnosis during her pregnancy or an illness with onset prior to pregnancy with continued symptoms causing impairment during pregnancy.
Mothers were visited at least 3 times by the study nurse during pregnancy to inquire about compliance with the study medication and prenatal care. Compliance was not otherwise verified. Ten of the mothers were discovered at these visits to have smoked during pregnancy, despite abstinence at enrollment. Because smoking was a pre-specified exclusion, their infants’ electrophysiological data were analyzed separately, but they were included in the safety assessments. Clinical data were acquired by the study nurse or recorded from the hospital records. At 6 months of ages, the infants’ general development was checked with the Mullen Scales of Early Learning.29 The Scales assess five developmental domains: 1) gross motor skills, 2) visual perception and discrimination, 3) fine motor planning and control, 4) receptive language, and 5) expressive language. An Early Learning Composite score, derived from four of the scales excluding the gross motor subscale, estimates cognitive ability.
To assess longer term sequelae of abnormal neonatal cerebral inhibition, a cohort of 93 infants recruited from the same population with the same inclusion and exclusion criteria as the infants in the clinical trial were studied. They had been recruited 4 years earlier and received P50 recordings in the newborn period.28 At a mean age of 41 months, 50 of the 93 subjects (54%) were administered the Child Behavioral Checklist, a parental report of symptom.
Choline administration as phosphatidylcholine
Choline is catabolized by intestinal flora to trimethylurea, which has an obnoxious odor. Phosphatidylcholine cannot be catabolized, is well-absorbed and is interchangeably metabolized with choline; oral phosphatidylcholine intake increases serum choline levels.24 Doses of 3600 mg q am and 2700 mg q pm were administered from 17.2 ± 2.1 weeks after mother’s last menstrual period up to delivery. Phosphatidylcholine is 13–15% choline; thus this dose should provide approximately 900 mg of choline per day, about the same amount as in 3 large eggs. All mothers, regardless of treatment assignment, were fully advised of dietary practices that would result in similar levels of choline intake. After birth, infants received 100 mg of phosphatidylcholine in an oral suspension once daily or placebo, the same treatment as in utero. Choline supplementation continued for the infant to 52 weeks post last menstrual period (approximately 3 months of age) as estimated by first trimester ultrasound. Postmortem studies show continually increasing KCC2 expression over this same period.30 The Institute of Medicine recommendation for infant daily adequate choline intake is 125 mg per day.31
Statistical analysis
The ratio of P50 amplitudes discriminates patients with schizophrenia from normal subjects with larger effect size than the amplitude of either the first or second response alone or their difference.32 However, ratios have more variance than the underlying amplitude measurements. Therefore, non-parametric analyses less affected by this variance have been used to detect genetic and treatment effects. The statistical plan established before the trial specified values ≥ 0.5 of the P50 ratio as abnormal. Values ≥ 0.5 for the P50 ratio have been previously associated with variants in the CHRNA7 gene complex, including transmission of alleles that segregate with schizophrenia.33 Analysis of the P50 ratios recorded in this study showed a significant admixture of two components, a lower one with mean value of 0.24 ± 0.12 (SD) and a higher one with mean value of 0.57 ± 0.19; χ2 = 8.50, df 3, P = 0.037. P50 ratios ≥ 0.5 in this study are above the 0.98 probability distribution of the lower component, the same criterion that was used to specify an affected phenotype for previous genetic studies.
Analyses of infants’ other outcomes, including height, weight, and head circumference, were adjusted for gestation, with estimated due dates based on an early-pregnancy ultrasound.
χ2, Fisher’s exact test and t-tests were used to compare the treatment groups. For all analyses α = 0.05 (two-tailed) was the criterion for significance. A post hoc power analysis showed that the groups entered into the one-month analysis had 85% power for determining the observed difference in primary outcome. The power to observe rare side effects was too small to be meaningful.
Results
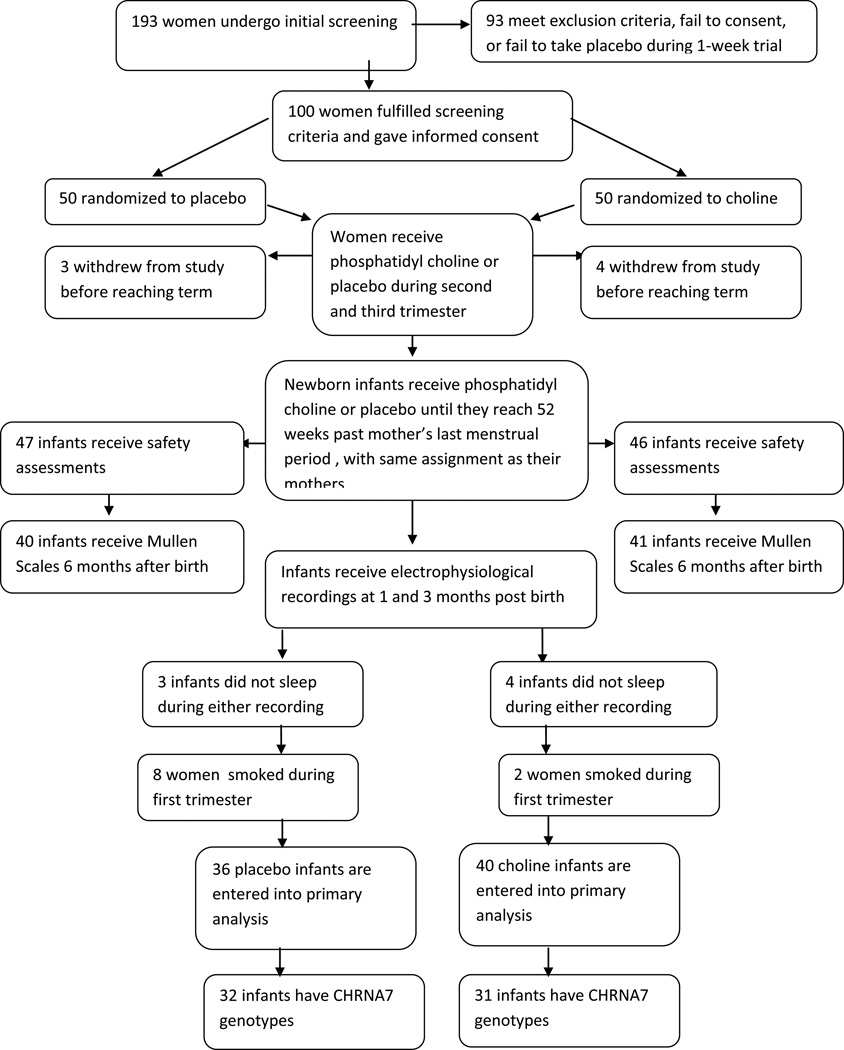
One hundred ninety three women were screened and 100 women were randomized to choline or placebo. Seven withdrew from the study or were lost to follow-up prior to the infant electrophysiological recording (Figure 1). Ten were excluded from the primary outcome analysis because of smoking detected subsequent to randomization.
Figure 1.
Patient recruitment, treatment, and study assessments.
Safety and tolerability of choline treatment
There were no significant differences in maternal outcome during pregnancy and delivery in the 46 women who received choline, compared to the 47 who received placebo (Table 1). Maternal side effects from choline treatment were not significantly different in frequency between treatment groups. All pregnancies resulted in live births. Seven placebo-treated and 4 choline-treated women delivered prior to 37 weeks post their last menstrual period. There were no overall differences in Apgar scores, weight, length, head circumference, and perinatal complications (Table 2). At one month of age, the two infant groups continued to be not significantly different in measurements or possible side effects from the choline treatment. At 6 months, there were no differences in performance on the Mullen Scales of Early Learning.
Table 1.
Effects of placebo and choline on pregnancy and delivery
| Placebo (N = 47) |
Choline (N = 46) |
|||
|---|---|---|---|---|
| Number or Mean |
Standard Deviation |
Number or Mean |
Standard Deviation |
|
| Weight gain (lbs) | 32.6 | 14.8 | 32.7 | 18 |
| Pregnancy-related hypertension | 3 | 11 | ||
| Proteinuria | 8 | 8 | ||
| Edema | 7 | 15 | ||
| Blurred vision or scotoma | 4 | 5 | ||
| Gestational Diabetes | 2 | 3 | ||
| Premature labor | 7 | 9 | ||
| Bleeding | 7 | 4 | ||
| Polyhydraminos | 2 | 2 | ||
| Oligohydraminos Infant small for gestational age | 2 | 4 | ||
| For women <160 cm | 0/14 | 3/9 | ||
| For women ≥160 cm | 1/33 | 4/37 | ||
| Infant large for gestational age | 1 | 2 | ||
| Cesarian delivery | 11 | 15 | ||
| Forceps | 1 | 1 | ||
| Vacuum | 4 | 4 | ||
| Breech | 5 | 4 | ||
| Meconium fluid | 4 | 4 | ||
| Abnormal | 4 | 4 | ||
| fetal monitor | ||||
| Nuchal cord | 8 | 11 | ||
| Rh incompatible | 5 | 11 | ||
| Gestational age < 259 days | 7 | 4 | ||
| Meconium aspiration | 7 | 6 | ||
| Symptoms: | ||||
| Nausea | 15 | 14 | ||
| Back pain | 15 | 11 | ||
| Headache | 10 | 15 | ||
| Diarrhea | 14 | 10 | ||
| Vomiting | 10 | 8 | ||
| Shortness of breath | 8 | 7 | ||
| Abdominal pain | 6 | 8 | ||
| Dizziness | 8 | 6 | ||
| Fatigue | 5 | 7 | ||
| Flatulence | 3 | 6 | ||
| Anxiety | 6 | 1 | ||
| Limb pain | 5 | 2 | ||
| Urinary urgency | 3 | 3 | ||
| Palpitations | 3 | 3 | ||
| Parasthesia | 4 | 1 | ||
| Anorexia | 2 | 2 | ||
| Chest pain | 3 | 1 | ||
| Rash | 4 | 0 | ||
| Body odor | 0 | 0 |
No values are significantly different between placebo and choline treated groups.
Table 2.
Effects of placebo and choline on the newborn infant
| Placebo | Choline | |||
|---|---|---|---|---|
| (N = 47) | (N = 46) | |||
| Number or Mean |
Standard Deviation |
Number or Mean |
Standard Deviation |
|
| Status at Delivery: | ||||
| Duration of Gestation (days) | 271 | 14 | 273 | 17 |
| Sex (male) | 22 | 26 | ||
| Birth weight (g) | 3193 | 540 | 3114 | 636 |
| Birth length (cm) | 50.0 | 2.7 | 49.0 | 2.2 |
| Head circumference | 34.2 | 1.9 | 34.0 | 5.5 |
| APGAR: 1 min | 7.7 | 1.3 | 7.5 | 2.0 |
| APGAR: 5 min | 8.7 | 0.7 | 8.7 | 1.0 |
| Jaundice | 25 | 15 | ||
| Respiratory distress | 1 | 2 | ||
| Status at adjusted age of 4 weeks | ||||
| Weight (g) | 4580 | 575 | 4342 | 868 |
| Head circumference (cm) | 37.5 | 1.4 | 36.7 | 5.7 |
| Length (cm) | 55.0 | 2.3 | 53.3 | 8.4 |
| Status at adjusted age of 12 weeks | ||||
| Weight (g) | 6211 | 744 | 5764 | 1125 |
| Head circumference (cm) | 41.0 | 3.3 | 39.6 | 6.3 |
| Length (cm) | 60.4 | 4.2 | 58.7 | 9.4 |
| Below 3 sd in measurements for age for weight, head circumference, or length at any time point | 0 | 0 | ||
| Adverse events reported for at least 4 infants | ||||
| Diarrhea | 14 | 12 | ||
| Flatulence | 8 | 6 | ||
| Constipation | 5 | 7 | ||
| Cough/runny nose | 6 | 5 | ||
| Reflux | 4 | 4 | ||
| Fever | 3 | 4 | ||
| Vomiting | 3 | 3 | ||
| Thrush/yeast infection | 2 | 3 | ||
| Fishy body odor | 0 | 2 | ||
| Mullen Scales of Early Learning at 6 months (T scores) | ||||
| Gross Motor | 47.9 | 6.7 | 47.1 | 7.5 |
| Fine Motor | 51.2 | 6.2 | 51.8 | 6.8 |
| Visual Reception | 50.2 | 9.1 | 48.0 | 6.6 |
| Receptive Language | 48.9 | 7.4 | 46.6 | 5.9 |
| Expressive Language | 41.7 | 4.4 | 41.7 | 7.6 |
| Early Learning Composite | 96.7 | 6.8 | 93.5 | 9.6 |
No values are significantly different between placebo and choline treated groups. Mullen scores were obtained on 40 placebo and 41 choline-treated infants.
Electrophysiological assessment of infants’ inhibitory brain function
Electrophysiological recordings were obtained from 93 infants. Seven did not sleep or had poor quality recordings. Ten women were discovered to have smoked during the first trimester and some were observed smoking during nursing visits subsequently during the study; their infants’ recordings were not considered in the primary analysis. Two recordings were performed, one at a mean gestation-adjusted age of 33 days, and a second with a mean adjusted age of 89 days at the termination of choline administration. Fifty-two infants received both recordings; data from all infants whether recorded on one or both days were analyzed. There was no overall difference between the two groups or those who could not be entered into the primary analysis in maternal age, parental socioeconomic status or psychiatric illnesses, gestational age at birth, or infant’s sex (Table S1). Week of treatment initiation was not significantly associated with the primary outcome (r = −0.17, ns).
The primary variable was the P50 inhibition ratio, the amplitude of the P50 response to the second of paired auditory stimuli divided by the amplitude of the response to the first stimulus (Figure 2A). A smaller ratio indicates an inhibition of the cerebral response to the repeated stimulus. The mean P50 ratio at age 33 days was 0.45 ± 0.24 (SD) and at age 89 days was 0.39 ± 0.24 (SD), similar to values in a previous study of 83 infants of parents with no mental illness and no tobacco exposure.19 The a priori data analysis plan dichotomized P50 ratios into < 0.5, considered intact cerebral inhibition and ≥ 0.5, considered diminished inhibition (Figure 2B). P50 ratios < 0.5 were observed in 43% of the infants treated with placebo and 76% of the infants treated with choline at the first recording (χ2 = 6.90, df 1, P = 0.009, d = 0.7). At the second recording, 72% of the placebo-treated infants and 76% of the choline-treated infants had P50 ratios < 0.5 (Table S2).
Figure 2.
A. Recordings of P50 averaged evoked potentials from infants. The infant on choline is 30 days gestation-adjusted age. The infant on placebo is 29 days old. For each infant, the 2 auditory stimuli were delivered 0.5 sec apart. The diminished amplitude of the second response, relative to the first, demonstrates cerebral inhibition, quantified as the P50 ratio. Positive potential is upward going; amplitudes were measured from the preceding negative potential, both indicated by tick marks. B. Histogram of P50 ratio at mean adjusted age 33 days. The dashed line demarcates normal level of P50 inhibition, ratio < 0.5. More choline than placebo-treated infants were in this normal adult range (χ2 = 6.90, df 1, P = 0.009).
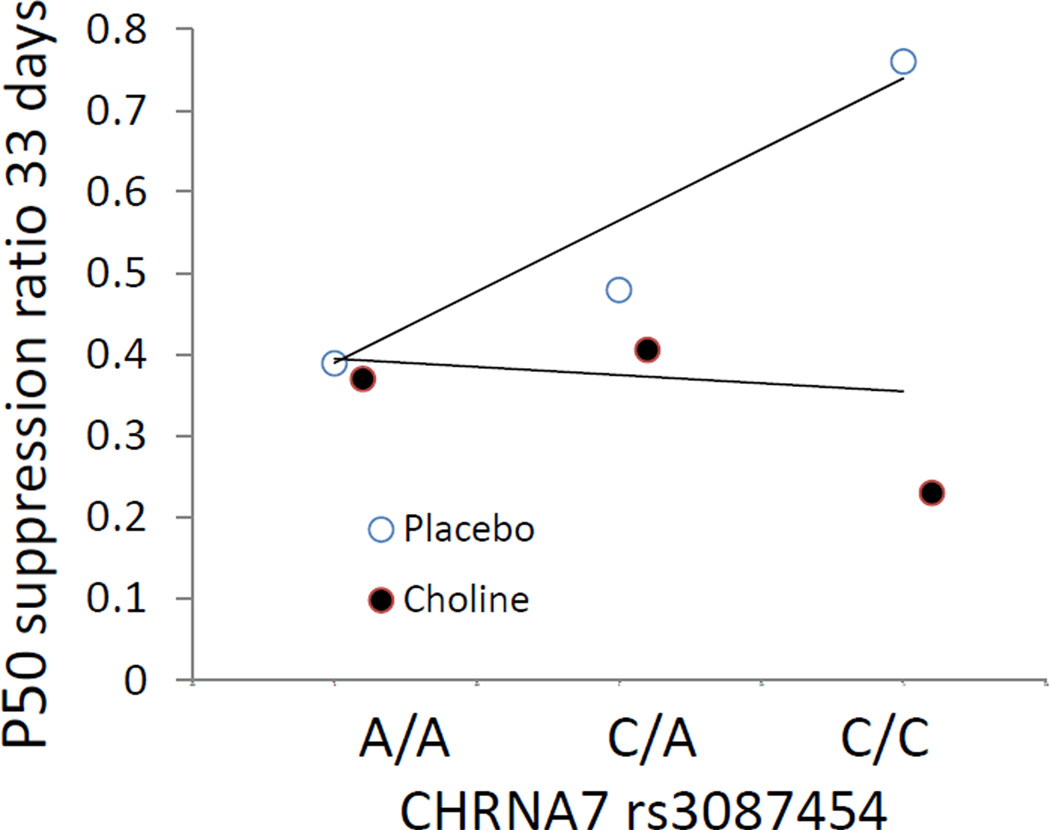
CHRNA7 rs3087454 infant genotype was correlated with P50 inhibition at 33 days in the placebo group (rs = 0.38, df 30, P = 0.032), whereas the choline-treated group showed no effect (rs = −0.05, df 28, ns; Figures 3, S3).
Figure 3.
Regression of P50 ratio on infant CHRNA7 genotype. Mean values are shown; individual values are shown in Figure S3. The placebo-treated infants showed a significant correlation of P50 ratio with rs3087454 (rs = 0.38, df 30, P = 0.032, dashed line). There was no significant correlation for the choline-treated infants (solid line).
Other than the ratio between the first and second P50 responses, the latencies and amplitudes of the individual P50 responses did not differ between treatment groups. P50 ratios correlated with the amplitudes of the P50 responses to the second stimulus (first time point r = 0.69, P < 0.001; second time point r = 0.78, P < 0.001), but not to the first stimulus (first time point r = 0.11, P = 0.375; second time point r = −0.09, P = 0.5).
Of 10 infants whose mothers smoked during pregnancy, 8 received placebo and 2 choline. Postnatal smoking was not regulated. Nine of the 10 tobacco-exposed infants were successfully recorded at the first time point; 7 of the 10 at the second time point. Five of the 7 placebo-treated infants and both the choline-treated infants had P50 ratios < 0.5 at the first recording. However, at the second recording, 4 of the 5 placebo-treated infants and 1 of 2 choline-treated infants had P50 ratios ≥ 0.5. Other subgroup analyses are shown in Table S3.
Sequelae of diminished infant P50 inhibition in later childhood
Twenty-four children recorded four years before initiation of the choline trial with deficient P50 suppression in infancy had increased attentional symptoms assessed by their parents’ report on the Child Behavior Checklist at 3.5 years, 2.75 ± 1.51 items compared to 1.46 ± 1.39 items for 26 children with intact P50 suppression as infants (Wilcoxon rank-sum P = 0.004).
Discussion
Choline treatment of mothers during the last two trimesters of pregnancy and their infants during the first 12 weeks of postnatal life was safely tolerated by both mother and infant. By approximately 5 postnatal weeks adjusted for duration of gestation, infants treated with choline were significantly more likely to have normal cerebral inhibition. The selection of inhibition of the auditory P50 cerebral evoked response as the primary outcome reflects issues in the assessment of developmental risk for schizophrenia. The primary measurement must (1) reflect a developmental process whose outcome in measurable in newborns, (2) that occurs in the time window of observation and treatment, (3) that is likely to be directly affected by the treatment, and (4) that has a longer term relationship to later mental illnesses. All four requirements were addressed by the inhibition of the auditory P50 cerebral evoked response. The measurement of P50 inhibition was shown to be feasible in human newborns,28 the switch of GABA from excitatory to inhibitory is a well-established fetal developmental milestone,17,30 the mouse model experiment showed that a similar mouse inhibition is affected by perinatal choline treatment,26 and family and genetic studies showed that P50 inhibition in adults and newborns is related to transmission of risk for schizophrenia.19,33
Transient developmental delay and risk for later schizophrenia
Other evidence of early developmental delay associated with later schizophrenia has been observed. A landmark behavioral observation showed dystonia and poor coordination in home films of infants who would later develop schizophrenia.2 Recent structural magnetic resonance imaging has shown differences in brain volume in males who have parents with schizophrenia.34 Children who later develop schizophrenia have delayed development of attentional control as well as delayed motor, verbal, social, and general cognitive development.1–9 The apparent normalization of cerebral inhibition by 13 weeks in the placebo group is similar to the transient nature of other developmental delays previously observed in infants, children, and adolescents vulnerable to later psychosis.1–9 The apparent normalization may reflect compensatory mechanisms. Mouse Chrna7 null mutants eventually develop GABA-mediated inhibition.17 Maternal oxytocin released during labor, which also promotes the change in polarity of GABA receptor activation, may be one contributor to this compensatory, if delayed, developmental process.35 Although developmental delays may be transient, the risk for later psychosis persists into adult life. In regard to cerebral inhibition, postmortem brain tissue from persons with schizophrenia continues to show only partial conversion of chloride transporters to the adult KCC2 type.30
Use of surrogate markers in the prevention of illness
Diminished neonatal P50 inhibition as target for a treatment intended to prevent schizophrenia is an example of increasing efforts that are directed towards early treatment of surrogate markers for later illness. The Food and Drug Administration is now authorized to recognize such efforts, because early treatments directed to a surrogate marker may be the only possible strategy to prevent an illness like schizophrenia that will not appear until decades after the critical period of brain development when preventive treatment is possible.36 The strategy has had notable successes and failures for other illnesses. Treatment of the surrogate marker high serum cholesterol successfully prevents later heart disease. In contrast, piaglitazone treatment of the surrogate marker glucose instability in type 2 diabetes mellitus effectively decreases later heart disease, but rosiglitazone, another drug in this same class that also treats glucose instability, unexpectedly increases heart disease.37 Both drugs bind to peroxisome proliferator–activated receptor gamma (PPARG), but their mixture of PPARG activation and inhibition was found to differ subtly. Relevant to this study, both nicotine and choline activate α7-nicotinic receptors, but nicotine also profoundly desensitizes nicotinic receptors and choline does not.23 Maternal smoking is associated with poorer neonatal cerebral inhibition and later childhood behavioral abnormalities, whereas choline appears to have benefits.14,19
Three lessons are widely acknowledged: (1) the mechanism that links the treatment to the surrogate marker and then to the illness itself must be understood, (2) diligent efforts to understand all effects of the treatment must be made, and (3) there needs to be a continuing commitment to assess whether normalization of the surrogate indeed has its intended positive clinical effects on the later development of illness. 36
Mechanism of the treatment
Sensory gating deficiency assessed by P50 auditory evoked potential response to repeated stimuli was initially developed as an endophenotype to assess transmission of genetic risk, which is most often through unaffected parents. P50 ratios ≥ 0.50 were found in 91% unrelated patients with schizophrenia compared to 6% of normal controls. Generally one of two unaffected parents and about half of unaffected siblings of schizophrenia probands were also abnormal, and transmission of the phenotype through clinically unaffected family members to an ill proband was genetically linked with the CHRNA7 genotype.33 A second group of investigators replicated the genetic association between P50 gating and CHRNA7 genotype.38 Associations with other known schizophrenia risk genes, COMT and NRG1, have also been found.39–40
The mechanism of action of choline was examined in the DBA/2 mouse model by breeding a Chrna7 null mutation on the DBA/2 background. The wildtype mice, which have naturally occurring Chrna7 promoter variants responsible for partially decreased α7-nicotinic receptor expression, respond to perinatal choline treatment by developing normal hippocampal auditory evoked response inhibition. The homozygote null mutants have no α7-nicotinic receptors and fail to respond to perinatal choline, demonstrating that α7-nicotinic receptor activation is the critical mechanism.41 Unlike the DBA/2 mice, the infants were not selected for CHRNA7 variants, but the genetic analysis indicates a similar genotypic effect of a CHRNA7 promoter variant on the development of cerebral inhibition in the placebo group. The placebo subjects homozygous for the minor allele associated with schizophrenia (C/C) have the highest mean ratios, and those homozygous for the more common allele (A/A) have the lowest, and the heterozygotes (A/C) are intermediate (Figure 3). As with the DBA/2 wildtype mice, perinatal choline treatment mitigates this genetic effect and allows infants to develop cerebral inhibition regardless of CHRNA7 genotype.
Other actions of choline
Choline also participates in membrane biosynthesis and one carbon metabolic pathways, including those involved in DNA methylation.42 However, stimulation of α7-nicotinic receptors requires higher levels of choline (KM = 300 µM) than activation by choline kinase required to synthesize membrane sphingomyelin (KM = 17 µM) or by choline dehydrogenase required for its role as a methyl donor (KM = 5.7 µM).23,43,44 The implication is that agonism at α7-nicotinic receptors is much more affected by supplemental dietary intake, particularly if the infant has a genetically determined receptor expression deficit.
Longer term clinical effect
A surrogate marker has never been used a target for the prevention of a brain illness. Alzheimers disease with its development in adults over a decade has been considered, but the development of schizophrenia over several decades that include development from fetus to early adulthood is more daunting. Therefore, the commitment to look for clinical effects is critical. P50 abnormalities appear to predict early childhood inattentiveness as shown in the cohort initiated 4 years ago, but more such efforts, including the longer term follow up of children who have received supplemental choline will be needed.
Limitations
This is the first randomized controlled trial of choline supplementation in pregnancy for any reason. There are obvious limitations. Because of the small sample in this initial trial, the frequency of most side effects cannot be determined. Nor does the trial establish that this supplementation will actually prevent later mental illness in the offspring. The choline dose approximated recommended dietary levels, which would increase choline intake to twice normal levels if the mother ate an adequate diet. In the mouse experiment we supplemented at five times dietary levels. Plasma levels were not measured, because they primarily reflect the most recent meal and do not reach a fasting steady state. Thus, we do not know the optimal human dose.
Conclusion
Many perinatal interventions, such as folic acid to prevent neural tube deficits, did not have their effects definitively established until they were applied population wide.45 The safety profile of phosphatidylcholine would support such administration. Schizophrenia with its low 10% parent to child transmission and the high population allele frequencies of most risk genes, including CHRNA7, merits population-wide prevention, but schizophrenia presents a more formidable problem for determining the ultimate outcome of the choline intervention, because the illness itself does not appear until decades later. Changes in biomarkers such as P50 inhibition in the newborn period in studies such as this one are thus the only available indicator of the putative success of intervention in the most vulnerable developmental period.
Supplementary Material
Acknowledgements
Supported by the Institute for Children’s Mental Disorders, the Anschutz Family Foundation, and National Institutes of Health grants P50MH086383 and R01MH056539. The authors thank Drs. Steve Zeisel and Gary O. Zerbe for their help in study design and Drs. William Hay, Henry Galan, Brian Stafford, and Kim Kelsay for their involvement in the Data Safety Monitoring Board. Clinical Trials Registration: NCT00332124.
References
- 1.Fish B. The detection of schizophrenia in infancy: a preliminary report. J Nervous Mental Dis. 1957;124:1–24. doi: 10.1097/00005053-195701000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Walker EF, Savoie T, David D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- 3.Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Dev Psychopath. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- 4.Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder. Results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 5.Sørensen HJ, Mortensen EL, Schiffman J, Reinisch JM, Maeda J, Mednick SA. Early developmental milestones and risk of schizophrenia: A 45-year follow-up of the Copenhagen Perinatal Cohort. Schiz Res. 2010;118:41–47. doi: 10.1016/j.schres.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones P, Murray R, Jones P, Rodgers B, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 7.Done DJ, Crow TJ, Johnstone EC, Sacker A. Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ. 1994;309:9–17. doi: 10.1136/bmj.309.6956.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke MC, Tanskanen A, Huttunen M, Leon DA, Murray RM, Jones PB, Cannon M. Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am J Psychiatry. 2011;168:1295–1302. doi: 10.1176/appi.ajp.2011.11010011. [DOI] [PubMed] [Google Scholar]
- 9.Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psych Med. 2012;42:743–755. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- 10.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1, ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki P, Riekki T, Miettunen J, Isohanni M, Jones PB, Murray GK, Veijola J. Schizophrenia in the offspring of antenatally depressed mothers in the Northern Finland 1966 birth cohort: relationship to family history of psychosis. Am J Psychiatry. 2010;167:70–77. doi: 10.1176/appi.ajp.2009.09010133. [DOI] [PubMed] [Google Scholar]
- 13.McGrath JJ, Eyles DW, Pedersen CB, Anderson C, Ko P, Burne TH, Norgaard-Pedersen B, Hougaard DM, Mortensen PB. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry. 2010;67:889–894. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- 14.Ekblad M, Gissler M, Lehtonen L, Korkeila J. Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch Gen Psychiatry. 2010;67:841–849. doi: 10.1001/archgenpsychiatry.2010.92. [DOI] [PubMed] [Google Scholar]
- 15.Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 16.Curley A, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- 18.Ross RG, Stevens KE, Proctor WR, Leonard S, Kisley MA, Hunter SK, Freedman R, Adams CE. Research review: Cholinergic mechanisms, early brain development, and risk for schizophrenia. J Child Psychol Psychiatry. 2010;51:535–549. doi: 10.1111/j.1469-7610.2009.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull. 2011;37:1200–1208. doi: 10.1093/schbul/sbq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Court JA, Lloyd S, Johnson M, Griffiths M, Birdsall NJ, Piggott MA, Oakley AE, Ince PG, Perry EK, Perry RH. Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Brain Res Dev Brain Res. 1997;101:93–105. doi: 10.1016/s0165-3806(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 21.Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 22.Descarries L, Aznavour N, Hamel E. The acetylcholine innervation of cerebral cortex: New data on its normal development and its fate in the hAPP(SW,IND) mouse model of Alzheimer’s disease. J Neural Trans. 2008;112:149–162. doi: 10.1007/s00702-004-0186-z. [DOI] [PubMed] [Google Scholar]
- 23.Papke RL, McCormack TJ, Jack BA, Wang D, Bugaj-Gaweda B, Schiff HC, Buhr JD, Waber AJ, Stokes C. Rhesus monkey α7 nicotinic acetylcholine receptors: Comparisons to human α7 receptors expressed in Xenopus oocytes. European J Pharmacol. 2005;524:11–18. doi: 10.1016/j.ejphar.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 24.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Ann Rev Nutrition. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Zhang H, Ju G, Zhang X, Xu Q, Liu S, Yu Y, Shi J, Boyle S, Wang Z, Shen Y, Wei J. A study of the PEMT gene in schizophrenia. Neuroscience Letters. 2007;424:203–206. doi: 10.1016/j.neulet.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Stevens KE, Adams C, Yonchek J, Hickel C, Danielson J, Kisley M. Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacol. 2008;198:413–420. doi: 10.1007/s00213-008-1170-3. [DOI] [PubMed] [Google Scholar]
- 27.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Developmental Brain Research. 1999;118:51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 28.Hunter SK, Corral N, Ponicsan H, Ross RG. Reliability of P50 auditory sensory gating measures in infants. Neuroreport. 2008;19:79–82. doi: 10.1097/WNR.0b013e3282f35823. [DOI] [PubMed] [Google Scholar]
- 29.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 30.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. Journal of Neuroscience. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute of Medicine and National Academy of Sciences USA. Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Washington DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 32.Cheng W-P, Arfken CL, Sangal MP, Boutros NN. Probing the relative contribution of the first and second responses to sensory gating indices, a meta-analysis. Psychophysiol. 2011;48:980–992. doi: 10.1111/j.1469-8986.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 33.Freedman R, Coon H, Myles-Worsley M, Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilmore JH, Kang C, Evans DD, Wolfe HM, Smith JK, Lieberman JA, Lin W, Hamer RM, Styner M, Gerig G. Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am J Psychiatry. 2010;167:1083–1091. doi: 10.1176/appi.ajp.2010.09101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyzio R, Cossart R, Khalilov H, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 36.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx. 2004;1:189–195. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen CJ. Revisiting the rosiglitazone story — lessons learned. N Engl J Med. 2010;363:803–806. doi: 10.1056/NEJMp1008233. [DOI] [PubMed] [Google Scholar]
- 38.Houy E, Raux G, Thibaut F, Belmont A, Demily C, Allio G. The promoter-194C polymorphism of the nicotinic α 7 receptor gene has a protective effect against the P50 sensory gating deficit. Mol Psychiatry. 2004;9:320–322. doi: 10.1038/sj.mp.4001443. [DOI] [PubMed] [Google Scholar]
- 39.Lu BY, Martin KE, Edgar JC, Smith AK, Lewis SF, Escamilla MA, Miller GA, Canive JM. Effect of catechol O-methyltransferase val(158)met polymorphism on the p50 gating endophenotype in schizophrenia. Biol Psychiatry. 2007;62:822–825. doi: 10.1016/j.biopsych.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 40.Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman R, Choo KS, Stevens KE. Choline enhances perinatal development of sensory inhibition though alpha7-nicotinic receptors. Society of Biological Psychiatry Abstracts. 2012:1010. [Google Scholar]
- 42.Yan J, Jiang X, Perry CA, Malysheva OV, Devapatla S, Presman E, Vermeylen F, Stabler SP, Allen RH, Caudill MA. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012;95:1060–1071. doi: 10.3945/ajcn.111.022772. [DOI] [PubMed] [Google Scholar]
- 43.Ross BM, Moszczynska A, Blusztajn JK, Sherwin A, Lozano A, Kish SJ. Phospholipid biosynthetic enzymes in human brain. Lipids. 1997;32:351–358. doi: 10.1007/s11745-997-0044-x. [DOI] [PubMed] [Google Scholar]
- 44.Haubrich DR, Gerber NH. Choline dehydrogenase: assay, properties, and inhibitors. Biochem Pharmacol. 1981;30:2993–3000. doi: 10.1016/0006-2952(81)90265-3. [DOI] [PubMed] [Google Scholar]
- 45.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.