Abstract
Leptin regulates appetite and metabolism but also immunity and inflammation. Although functional leptin receptors (LepR) are expressed on hematopoietic cells, the role of these receptors in regulating immune function in vivo remains controversial. To clarify this issue, we performed bone marrow (BM) transplantation between obese db/db mice, lacking LepR, and wild-type (WT) mice. Results indicate that expression of LepR on BM-derived cells directly, though partially, regulates spleen and thymus cellularity, although the environment of db mice contributes to maintaining reduced cellularity of these organs. Selective expression of LepR on BM-derived cells also modulates leptin and adiponectin levels, with induction of a more favorable adipokine environment in the WT→db/db group. However, LepR signaling in BM-derived cells is not involved in regulation of body weight (BW) and composition, glycemia, hepatosteatosis or adipose tissue inflammation, although it modulates expression of interleukin (IL)-1β in the brain. Finally, data indicate that db mice have an increased susceptibility to irradiation compared to WT mice in terms of BW loss and recovery of leukocyte counts in peripheral blood. Therefore, interpretation of results obtained using BM chimeras between WT and db mice should take into account the difference in radiation sensitivity between the two types of animals.
INTRODUCTION
Leptin, a pleiotropic molecule secreted predominately by white adipocytes, regulates appetite and metabolism, but also a variety of physiological and pathological processes, including immunity and inflammation (1). Leptin exerts its biological activities by binding to the long form of its receptor, leptin receptors (LepR), a member of class I cytokine receptors (2). LepR is highly expressed in the hypothalamus and other areas of the brain, but is also found in several peripheral tissues, including immune cells (3), suggesting that leptin can influence a variety of cell types and body functions. For more specific details on leptin signaling, the reader is referred to reviews on this topic (1,4,5).
Despite extensive demonstration that leptin can act on several types of peripheral cells in vitro, the exact function of peripheral LepR expression has not been clearly demarcated in vivo (1). The question of the respective roles of central vs. peripheral LepR expression in mediating leptin’s effects in vivo as well as the role of LepR expression in different peripheral tissues has been investigated by two main approaches: genetic manipulation and bone marrow transfer (BMT).
Selective neuronal deficiency of LepR reproduces the phenotype of db mice in terms of obesity, diabetes, and hepatic steatosis, whereas mice selectively deficient for LepR in hepatocytes have a normal phenotype (6), thus demonstrating that most, if not all, of the anorexigenic and metabolic effects of leptin are secondary to the activity of this adipokine on neurons. However, studies using these genetically engineered animals did not evaluate the effect of LepR deficiency on immune and inflammatory function.
A second set of studies employed generation of bone marrow (BM) chimeras between wild-type (WT) and db mice to evaluate the role of LepR expression on BM-derived cells in the regulation of thymus cellularity, which is dramatically reduced in db mice, and other immune parameters (7,8). Both reports using this approach demonstrated that transplantation of db BM into WT mice (db→WT) does not affect thymus (or lymph node) cellularity compared to its respective control BMT (WT→WT) (9,10). However, whereas results from Palmer et al. showed that transplantation of WT BM into db mice (WT→db) reproduces the thymus and lymph node atrophy of db mice (9), data from Trotter-Mayo and Roberts indicated a significant, though partial, reconstitution of thymus cellularity in the WT→db group. Therefore, although the environment of db mice (obesity, diabetes, high-corticosterone levels, lack of LepR expression on non-BM cells, etc.) likely contributes to the reduced cellularity of immune organs in db mice, consensus on the specific role of LepR expression on BM cells has not been reached.
Because the two reports did not reach consensus and also did not include some of the control groups (db→db BMT was missing in ref. (9) and WT→WT was missing in ref. (10)), we performed a study including all four groups of BMT mice, namely WT→WT, db→WT, db→db, and WT→db, as well as their nonirradiated counterparts to investigate the role of LepR expression on BM-derived cells while controlling for the possible unspecific effects of irradiation. Furthermore, we evaluated the effect of BMT on body weight (BW) and composition, glucose and adipokine levels as well as expression of pro- and anti-inflammatory cytokines in white adipose tissue (WAT) and brain.
METHODS AND PROCEDURES
Mice
Animal protocols were approved by the Animal Care and Use Committee at the University of Illinois at Chicago, IL. Lean WT, LepR-deficient obese db mice, as well as B6.SJL-Ptprca Pep3b/BoyJ (B6.SJL) congenic mice were obtained from The Jackson Laboratories (Bar Harbor, ME). Mice used in these experiments were male, 5 weeks of age at the beginning of the experiment and on a C57BL6 background. The animals were housed under conventional conditions and provided water and standard laboratory chow ad libitum.
BM isolation
In a sterile environment, BM cells were isolated from the femurs and tibias of 12-week-old B6.SJL and db mice by flushing the bones with 5 ml of Hanks’ balanced salt solution (Invitrogen, Carlsbad, CA) into a sterile 15 ml tube. Samples were passed through a 100 μm strainer to eliminate debris and break up cell clumps. After centrifugation cells were resuspended in PBS.
Irradiation and BMT
Recipient db and WT mice, which express the CD45.2 allele on their leukocytes, and B6.SJL mice, which express CD45.1, were lethally irradiated at the split dose of 10 gy γ-irradiation with a 3-h interval between doses (5 gy/dose) using a 137Cs irradiator source in well-ventilated vinyl containers without anesthesia. The following groups were included: B6.SJL BM→WT mice (WT→WT), db BM→B6.SJL mice (db→WT), db BM→db mice (db→db), and B6.SJL BM→db mice (WT→db). Mice were reconstituted 24-h postirradiation with 5 × 106 BM cells by intravenous injection in the tail vein. After irradiation, mice were kept on drinking water supplemented with 2 mg/ml of neomycin sulfate (Phoenix Pharmaceuticals, St Joseph, MO) for 10 days before returning to regular drinking water for the remaining of the 4-week experiment. Control WT and db mice underwent the same procedures and manipulations as irradiated mice without being exposed to irradiation or injection of BM cells. Mice were euthanized 4 weeks after BMT or sham-irradiation following a 4-h fasting period. This time point was chosen based on previously reported data on the time course of hematopoietic reconstitution after BMT in C57BL6 mice (11).
Food intake, BW, and body composition
BW and food intake were evaluated immediately before irradiation, on days 1, 3, and 7 postirradiation and weekly thereafter. Body composition was evaluated by dual-energy X-ray absorptiometry scanning of the whole mouse, excluding the head, at the completion of the 4-week experiment.
Spleen and thymus cellularity and flow cytometry
Single-cell suspensions were prepared from thymocytes and splenocytes obtained from mice in each experimental group at the end of the experimental period and total cellularity evaluated by counting on a hemocytometer. To evaluate the efficiency of BMT, splenocytes and thymocytes were stained with fluorescein isothiocyanate-conjugated anti-CD45.2 and phycoerythrin-conjugated CD45.1 to differentiate between donor and recipient cells. The cellular composition of the thymus was evaluated by flow cytometry using fluorescein isothiocyanate-conjugated anti-CD8 and phycoerythrin-conjugated anti-CD4 antibodies (BD Biosciences, San Jose, CA). Rat immunoglobulin G conjugated with fluorescein isothiocyanate and phycoerythrin served as negative control. Cells were analyzed using a FACSCalibur (Becton Dickinson, San Diego, CA).
Hematologic parameters
Peripheral blood was obtained from the retro-orbital plexus under isoflurane anesthesia at the end of the experimental period and analyzed for total white blood cells (WBC), red blood cells (RBC), and hemoglobin (Hgb) using the Coulter ACT analyzer (Beckman Coulter, Brea, CA).
Glucose and adipokine levels
Blood glucose levels were measured in 4-h fasted mice using the OneTouch glucose monitoring system (LifeScan, Milpitas, CA). Serum leptin and adiponectin concentrations were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
Quantitative reverse transcription PCR
Total RNA was extracted from adipose tissue and brain and reverse transcribed. Quantitative reverse transcription PCR was performed using primers specific for murine cytokines obtained from Applied Biosystems (Foster City, CA) and the TaqMan system (Applied Biosystems, Foster City, CA). Data normalized to GAPDH were compared using the ΔΔct method and expressed as fold-induction of gene expression over nonirradiated WT mice.
Statistical analysis
Data are presented as means ± s.e.m. Statistical analyses were performed using XLStat software (Addinsoft, Paris, France). Significance of differences was determined by factorial ANOVA (Fischer’s pair-wise comparison) and paired Student’s t-test. Differences were considered significant at P < 0.05.
RESULTS
Efficiency of BMT and evaluation of peripheral hematologic parameters
The experimental design of the study allowed for evaluation of the efficiency of BMT in three of the four groups of BM chimeras, namely, WT→WT, db→WT, and WT→db. Evaluation of CD45.1 and CD45.2 expression on thymocytes 30 days after BMT indicated that donor cells represented 99.3 ± 0.2, 97.8 ± 1.3, and 92.2 ± 3.9% in WT→WT, db→WT, and WT→db, respectively (mean ± s.e.m., n = 5; P > 0.05). Therefore, the efficiency of BMT was not significantly different in the three groups when evaluated by percentage of donor-derived cells in the thymus, indicating that, under the conditions used in the present study, lack of LepR and obesity does not influence the efficiency of BMT in terms of elimination of host hematopoietic cells by irradiation. Due to the unavailability of congenic CD45.1-expressing db mice, BMT efficiency could not be evaluated in db→db mice. However, because in db→db BMT both donor and recipient cells lack LepR, evaluation of BMT efficiency in this experimental group is not critical to data evaluation.
Total number of leukocytes (WBC) and erythrocytes (RBC) as well as Hgb levels were measured in peripheral blood in the various experimental groups to evaluate the efficiency of recovery from irradiation and BMT as well as to study the role of LepR expression on BM-derived cells on hematologic parameters. As shown in Figure 1a, at 4 weeks post-BMT, total WBC counts were not significantly different between nonirradiated WT, WT→WT, and db→WT mice, indicating complete recovery from irradiation in the peripheral leukocyte compartment of WT recipients. In agreement with previous data (9), total WBC counts were comparable between nonirradiated WT and db mice. In contrast with what observed in WT recipients of BMT, WBC counts were significantly decreased in both db→db and WT→db mice compared to nonirradiated db mice, indicating incomplete recovery of peripheral blood leukocyte counts in irradiated db mice irrespective of the origin of the donor BM cells.
Figure 1.
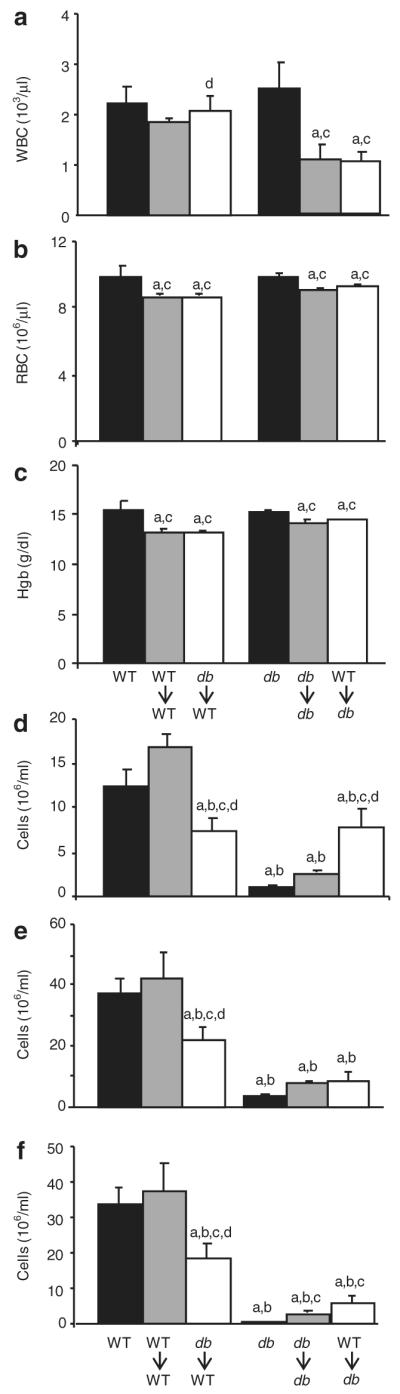
Hematologic parameters and cellularity of spleen and thymus. Peripheral blood was analyzed for (a) leukocyte and (b) erythrocytes counts as well as (c) hemoglobin levels. Total cellularity of (d) spleen and (e) thymus as well as the number of (f) double positive CD4 CD8 thymocytes were evaluated 30 days after irradiation in BMT groups or after sham-irradiation in nonirradiated groups (WT and db). Data are expressed as mean ± s.e.m., n = 5 mice. aP < 0.05 vs. WT mice; bP < 0.05 vs. WT→WT mice; cP < 0.05 vs. db mice; dP < 0.05 vs. db mice. BMT, bone marrow transfer; Hgb, hemoglobin; RBC, red blood cell; WBC, white blood cell; WT, wild type.
As shown in Figure 1b and c, there was no significant difference in RBC or Hgb levels between nonirradiated WT and db mice. Irradiation and BMT lead to a minor but significant reduction in RBC (Figure 1b) and Hgb levels (Figure 1c) in each group, regardless of type of BMT or genotype of the recipient animal, suggesting a persistent deficit in the erythrocyte compartment up to 1 month after irradiation in each BMT recipient.
These data indicate that, despite efficient engraftment of BM cells in both WT and db recipients, as indicated by the percentage of donor cells present in the thymus, db mice had an inefficient recovery from irradiation in terms of WBC counts compared to WT recipients, whereas the deficit in terms of RBC and Hgb was comparable between WT and db recipients of BMT. Thus, the obese, diabetic, and LepR-deficient environment of db mice leads to inefficient recovery of WBC counts in peripheral blood following BMT irrespective of whether WBC do (WT→db) or do not (db→db) express LepR. Because WBC counts in nonirradiated db mice were comparable to those of WT mice, inefficient recovery of the WBC compartment is likely secondary to an increased susceptibility to irradiation in db mice, in agreement with results from Palmer et al. reporting lethality in db but not in WT mice following irradiation (9). Future studies will be aimed at evaluating which specific aspect of the db phenotype hampers recovery from irradiation in terms of reconstitution of WBC, an issue of potential clinical relevance in obese and/or diabetic patients receiving BMT. Our data also suggest that expression of LepR on BM-derived cells does not significantly alter WBC, RBC, or Hgb in peripheral blood, because WT→WT and db→WT as well as WT→db and db→db groups had comparable levels of WBC, RBC, or Hgb.
Effect of BMT on spleen and thymus cellularity and immature CD4+CD8+ thymocytes
To determine whether the direct leptin signaling on BM-derived cells is required to regulate spleen and thymic cellularity, the total number of cells present in the spleen and thymus was evaluated in the different experimental groups. Cellularity of the spleen (Figure 1d) and thymus (Figure 1e) was not significantly different between nonirradiated WT and WT→WT mice, indicating complete recovery from irradiation and efficient reseeding of immune organs. In contrast, a significant reduction in both spleen and thymus cellularity was observed in db→WT mice compared with both WT and WT→WT mice, indicating reduced efficiency of LepR-deficient cells in repopulating immune organs in a WT environment.
Immature CD4+CD8+ thymocytes (double positive (DP) thymocytes) represent 80–90% of cells in the thymus of WT mice but only 20–30% in db/db mice (12). As shown in Figure 1f, db→WT BMT lead to a significant reduction in the number of DP cells compared to both WT and WT→WT mice. These data indicate that expression of LepR on BM cells in the context of a WT environment is sufficient to maintain low spleen and thymus cellularity and reduced number of DP thymocytes. The overall picture of immune cellularity in the db→WT group reproduced that of nonirradiated db mice, i.e., normal WBC counts in peripheral blood in the presence of reduced spleen and thymus cellularity.
Our data are at variance with results reported in both of the two previous BMT studies that demonstrated lack of effect of db→WT BMT in modifying thymus cellularity (9,10). Possible reasons for this difference include the different time of evaluation x 8–12 weeks in refs. (9,10)), lack of one of the irradiated control groups (WT→WT) and lower efficiency of db→WT BMT (80% in ref. (10) vs. 92% in our study), as well as use of heterozygote db/+ mice as BM donors in the WT→WT group in ref. (9).
As expected (7,12–14), a profoundly reduced cellularity of both spleen (Figure 1d) and thymus (Figure 1e) as well as significantly reduced numbers of DP thymocytes (Figure 1f) were present in nonirradiated db mice compared with WT mice. Reduced spleen and thymus cellularity was also observed in the db→db group. However, transfer of WT cells into db mice (WT→db) lead to a significant increase in spleen cellularity compared to db and db→db mice. In fact, spleen cellularity in WT→db mice was comparable to that of db→WT animals. Unlike the spleen, thymocyte counts in WT→db mice remained low.
Finally, despite the presence of a significantly higher number of DP cells in the thymus of both db→db and WT→db mice compared to nonirradiated db mice, in agreement with data reported in ref. (10), the number of DP thymocytes in each of the groups of db recipients remained significantly lower compared to each of the groups of WT recipients. Comparable results were obtained when data were analyzed in terms of percentage of DP cells instead of total numbers (data not shown).
Data obtained in db recipients indicate that selective expression of LepR on BM cells is able to restore spleen, but not thymus cellularity, in the obese and diabetic environment of db mice. However, it is important to note that db mice had an inefficient recovery from irradiation in terms of WBC counts (and BW, as shown below). Therefore, lack of effect of WT→db BMT in terms of recovery of thymus cellularity is likely an unspecific result of the increased sensitivity of db mice to irradiation. In fact, the WT→db group had a significant, though partial, recovery in the spleen, an organ that is relatively less radiosensitive compared with the thymus. Trotter-Mayo and Roberts, who analyzed the thymus of WT→db mice 7–8 weeks after BMT, a time when a more efficient recovery may have occurred compared to the 4 weeks in our study, found a significantly increased thymus cellularity when WT cells were transplanted into db mice (10).
In conclusion, our data, together with those of (9,10),suggest that reduced cellularity of immune organs in db mice is partially caused by lack of direct leptin’s effects on immune cells but also secondary to the presence of an hormonal and metabolic milieu that promotes lymphocyte apoptosis (2,15).
Effect of BMT on BW, food intake, body composition, and glycemia
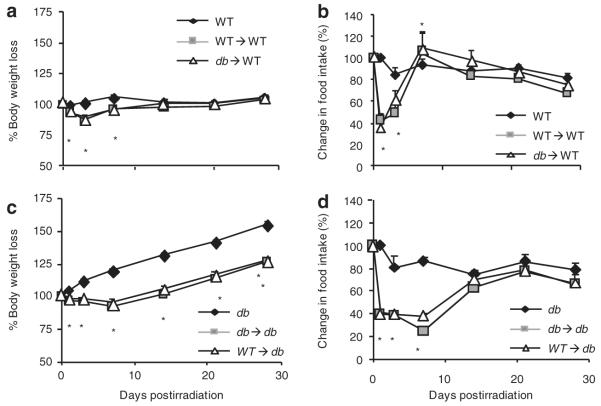
Previous data indicated that db mice have an increased susceptibility to irradiation despite BMT, with reduced survival rates at irradiation doses that are not lethal in WT mice if followed by BMT (9). To evaluate the recovery from irradiation in db mice, we measured BW and food intake at various times postirradiation. No lethality from irradiation was observed in any of the groups. As shown in Figure 2a, both WT→WT and db→WT mice lost a significant amount of weight compared to their nonirradiated WT counterpart during the first 7 days postirradiation and were completely recovered in terms of BW by day 14. Final BW at 4 weeks postirradiation was comparable in the three groups (Table 1). BW loss was accompanied by a significant decrease in food intake in WT→WT and db→WT mice at days 1 and 3 postirradiation compared to WT mice (Figure 2b). At day 7 postirradiation both WT→WT and db→WT mice had a significant increase in food intake compared to WT mice, likely as a compensatory response to the anorexia of the previous days. Food intake in WT, WT→WT, and db→WT mice groups was not significantly different at each subsequent time point. Body composition and glycemia (Table 1) at 4 weeks postirradiation were comparable in nonirradiated WT, WT→WT, and db→WT mice. Collectively, these results are in agreement with data reported in ref. (9).
Figure 2.
Body weight and food intake in the 4 weeks postirradiation. Body weight and food intake were recorded immediately before irradiation and at the indicated times postirradiation. (a) Body weight and (b) food intake in nonirradiated WT (black diamonds), WT→WT (gray squares) and db→WT (white triangles) mice. (c) Body weight and (d) food intake in nonirradiated db (black diamonds), db→db (grey squares), and WT→db (white triangles) mice. Data expressed as mean ± s.e.m., n = 10. *P < 0.05 WT→WT or db→WT vs. WT (a and b) and db→db and WT→db vs. db (c and d). WT, wild type.
table 1.
Effect of irradiation and BMT on glycemia, BW, and body composition in WT and db/db mice
| Initial BW | Final BW | % Lean mass | % Fat mass | Glycemia | |
|---|---|---|---|---|---|
| WT | 16.3 ± 0.2 | 17.1 ± 0.3 | 47.7 ± 5.0 | 9.0 ± 3.0 | 150.5 ± 10.4 |
| WT→WT | 15.8 ± 0.6 | 16.4 ± 0.9 | 47.8 ± 1.5 | 11.8 ± 1.8 | 151.9 ± 10.3 |
| db→WT | 14.7 ± 0.3 | 15.6 ± 0.2 | 43.9 ± 5.6 | 9.8 ± 2.5 | 141.9 ± 16.2 |
| db | 26.5 ± 0.5a | 41.2 ± 0.7a | 14.2 ± 1.3a | 68.9 ± 1.4a | 315.8 ± 38.3a |
| db → db | 27.7 ± 0.5a | 35.7 ± 0.4a,b | 16.0 ± 0.7a | 66.1 ± 0.5a | 369.0 ± 55.3a |
| WT→db | 26.7 ± 0.7a | 34.2 ± 0.9a,b | 14.5 ± 0.7a | 66.8 ± 1.1a | 342.0 ± 25.8a |
Initial BW (immediately before irradiation), final BW (4 weeks after irradiation and BMT), as well as lean and fat mass and glycemia evaluated 4 weeks after irradiation in BMT groups or after sham-irradiation (WT and db). Data are expressed as mean ± s.e.m., n = 5 mice.
BMT, bone marrow transfer; BW, body weight; WT, wild type.
P < 0.001 vs. WT, WT→WT or db→WT.
P < 0.005 vs. db.
In contrast with the minor reduction in BW and food intake observed in WT recipients of BMT, db mice receiving irradiation and BMT displayed a more profound and prolonged response compared to nonirradiated db mice. In fact, in db→db and WT→db mice BW was significantly decreased compared to nonirradiated db mice beginning at day 1 postirradiation and for the remaining of the whole 4 weeks (Figure 2c). Final BW at 4 weeks postirradiation was significantly lower in db→db and WT→db compared to nonirradiated db mice, although glycemia and body composition were comparable in the three groups (Table 1). Food intake followed a similar trend, with a significant reduction observed for the first 7 days postirradiation in db→db and WT→db mice compared to nonirradiated db mice (Figure 2d). Severe liver steatosis was present in each db group and absent from each WT group and the degree of steatosis was not affected by irradiation or BMT (data not shown).
These data indicate that the presence of LepR on hematopoietic cells is not necessary to initiate leptin’s anorexigenic and metabolic effects, in agreement with previous data (6,9,16). Furthermore, the severe sickness behavior observed in irradiated db mice demonstrates leptin binding to LepR does not mediate irradiation-induced anorexia and suggest that leptin might actually play a protective role under these conditions.
Data evaluating food intake and BW over the course of 4 weeks suggest an increased sensitivity to the deleterious effects of irradiation in db compared to WT mice. Decreased BW secondary to reduced adiposity, accompanied by hepatomegaly and reduced insulin sensitivity, has been reported in ob/ob mice receiving lethal irradiation followed by BMT (irrespective of origin of BM cells) as well as nonlethal abdominal irradiation, although these alterations occurred months after exposure to radiation (17,18). Irradiation and BMT (again irrespective of origin of BM cells) also resulted in reduced growth rates and altered atherosclerotic lesion size and composition in low-density lipoprotein receptor knockout mice (19), indicating that the effect is not restricted to ob/ob and db mice. Together with our data, these results indicate that pathologies such as obesity, diabetes, and dysregulated lipid metabolism are associated with an altered response to irradiation. Further studies are warranted to investigate the biological bases for this effect and its possible implications in humans, particularly in light of the reported increased incidence of metabolic syndrome in long-term survivors of BMT and in subjects receiving total body or abdominal/chest cancer radiation therapy (20,21).
Effect of BMT on serum adipokine levels
We examined serum leptin and adiponectin to determine whether BMT altered levels of these two adipokines. As shown in Figure 3a, both WT→WT and db→WT mice had significantly higher leptin levels compared to nonirradiated WT mice. However, there was no difference in leptin levels between WT→WT and db→WT mice, suggesting that the increase in leptin was the result of irradiation and not due to the type of BMT. Importantly, higher levels of leptin in WT→WT and db→WT mice were not associated with an increase in BW or increase in fat mass (see Table 1), indicating a dissociation between leptin and adiposity in response to irradiation.
Figure 3.
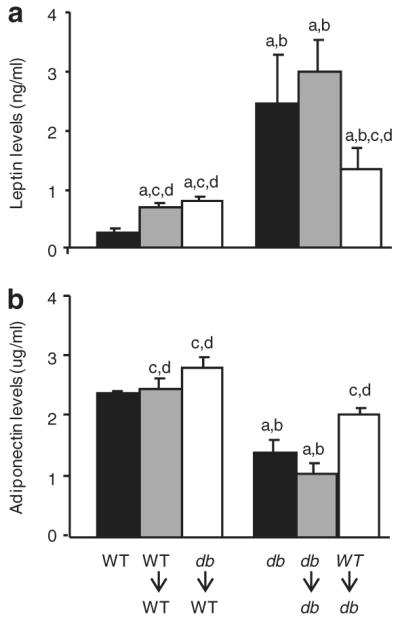
Leptin and adiponectin levels. (a) Serum leptin and (b) adiponectin were measured by ELISA 30 days after irradiation in BMT groups or after sham-irradiation in nonirradiated groups (WT and db). Data are expressed as mean ± s.e.m., n = 5 mice. aP < 0.05 vs. WT mice; bP < 0.05 vs. WT→WT or WT→db mice; cP < 0.05 vs. db mice; dP < 0.05 vs. db→db mice. BMT, bone marrow transfer; ELISA, enzyme-linked immunosorbent assay; WT, wild type.
Nonirradiated db mice had significantly higher leptin levels compared to WT mice, in agreement with previous findings (22). Irradiation and db→db BMT did not significantly alter leptin levels compared to nonirradiated db mice. However, WT→db BMT had significantly lower leptin levels compared with either db or db→db mice.
There was no significant difference in adiponectin levels between WT, WT→WT, and db→WT mice (Figure 3b). Nonirradiated db mice had significantly lower adiponectin levels compared to WT mice, consistent with observations in obese and hyperglycemic individuals (23). Adiponectin levels were significantly higher in WT→db mice compared to nonirradiated db and db→db mice. Thus, the selective presence of a functional LepR on BM-derived cells (WT→db group) is able to ameliorate the imbalance between leptin and adiponectin levels of db mice, leading to reduced leptin and enhanced adiponectin concentrations in serum. Increased leptin levels in db mice represent a compensatory mechanism due to perceived lack of leptin production secondary to defective leptin signaling. Our results indicate that restoration of leptin signaling selectively in BMT-derived cells (WT→db group) is able to attenuate this effect, suggesting that hematopoietic cells may be part of the leptin-sensing armamentarium of the body and can send signals to adipocytes leading to reduction in leptin output. We hypothesize the increase in adiponectin levels in WT→db mice to be secondary to normalization of leptin levels.
Effect of BMT on cytokine expression in adipose tissue (WAT) and brain
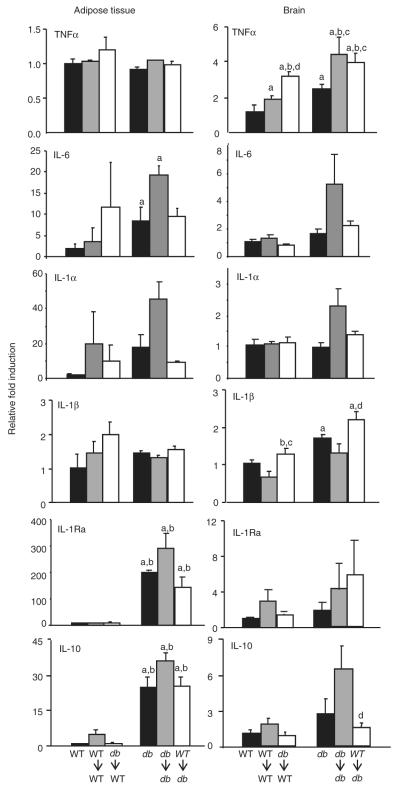
Obesity is associated with chronic low-grade inflammation of WAT, leading to increased expression of several cytokines and other mediators, which likely contribute to development of insulin resistance (24). Inflammation, including high expression of cytokines and activation of NFκB, has also been reported in the brain—particularly the hypothalamus—of obese animals, where it contributes to development of leptin resistance (25,26). However, the mechanisms linking leptin signaling to cytokine expression in WAT and brain have not been elucidated so far. To evaluate whether LepR signaling on BM-derived cells alters cytokine expression in WAT and brain, we compared expression of the proinflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1α, IL-1β, and the anti-inflammatory cytokines IL-1Ra and IL-10 in WAT and brain in the different experimental groups at the end of the 4-week experimental period. As shown in the left-hand panels of Figure 4, significantly higher levels of IL-6, IL-1Ra, and IL-10 mRNA were observed in WAT obtained from nonirradiated db compared to WT mice, in agreement with previous reports demonstrating high levels of these cytokines in obese animals and human subjects (24,27,28). However, at variance with previous data, expression TNF-α, IL-1α, or IL-1β did not significantly differ between WT and db mice (24). A possible reason for the discrepancy between our data and previous reports is that mice used in our study were relatively small compared with animals used in studies demonstrating high expression of TNF-α in adipose tissue of db mice (for example, db mice were on average 41 g in our study, compared with ~60 g in ref. (29)). Irradiation and BMT did not significantly alter expression of any of the cytokines measured in the various experimental groups, indicating that the alterations in expression of pro- and anti-inflammatory cytokines in WAT of db mice are not caused by direct leptin signaling in BM-derived cells but are likely secondary to the presence of an expanded adipose mass. These data also indicate that any possible acute inflammatory effect of irradiation on expression of WAT cytokines is resolved by 4 weeks postexposure.
Figure 4.
Cytokine expression in adipose tissue and brain. Expression of TNF-α, IL-6, IL-1α, IL-1β, IL-1Ra, and IL-10 were determined by real-time PCR in adipose tissue and brain. Data are expressed as mean ± s.e.m., n = 5 mice per group. aP < 0.05 vs. WT mice; bP < 0.05 vs. WT→WT mice; cP < 0.05 vs. db mice; dP < 0.05 vs. db→db. IL, interleukin; TNF-α, tumor necrosis factor-α; WT, wild type.
In the brain, TNF-α and IL-1β were the only two cytokines to be significantly elevated in nonirradiated db compared to WT mice (Figure 4, right-hand panels). Although previous results indicated reduced expression of IL-1β in the hypothalamus of fa/fa rats with defective Ob-R signaling (30,31), expression of cytokines in the whole brain of WT and db mice has not been previously compared. Whether elevated expression of these two proinflammatory cytokines in the brain of db mice may be at least in part responsible for the phenotypic and behavioral alterations of these animals remains to be investigated. Irradiation and BMT increased brain TNF-α expression in both WT and db recipients, irrespective of the origin of BMT cells, suggesting it results from an unspecific, long-lasting effect of irradiation. Recent data indicate that TNF-α blockade might be an effective intervention to prevent acute astrogliosis and blood–brain barrier damage induced by radiation (32). Whether anti-TNF-α therapy may also be effective in protecting against long-term effects of irradiation and might need to be administered for a prolonged period after exposure is an issue worth investigating.
In contrast to the unspecificity of the TNF-α response, brain IL-1β mRNA expression was significantly increased in db→WT compared to WT→WT as well as in WT→db compared with db→db mice, suggesting that activation of LepR on BM-derived cells may be implicated in regulating the IL-1β system in the brain, as suggested by studies performed in fa/fa rats (30,31,33). Irradiation and BMT did not significantly and consistently alter expression of IL-6, IL-1α, IL-1Ra, or IL-10 in the brain of either WT or db recipients. The long-term effects of total body irradiation on expression of cytokines in the brain is an area of critical importance given the amply demonstrated long-term consequence of irradiation in long-term survivors of childhood cancers (20,21).
DISCUSSION
In conclusion, the present report indicates that expression of LepR on BM-derived cells participates in regulation of immune cellularity, although the obese and diabetic environment of db mice also contributes to modulate the composition and cellularity of spleen and thymus. Selective expression of leptin signaling in BM-derived cells results in regulation of leptin and adiponectin levels, with induction of a more favorable adipokine environment (low leptin, high adiponectin) in db mice receiving WT BMT. These results warrant further investigation for their potential implication in modulating adipokine levels in obese and diabetic individuals. However, LepR signaling in BM cells is not involved in regulation of metabolic parameters or WAT inflammation, although it may be involved in modulating expression of IL-1β in the brain. However, our data also indicate that db mice have an increased susceptibility to irradiation, with longer and perhaps incomplete recovery. Therefore, interpretation of results obtained using BM chimeras between WT and db mice should take into account the difference in radiation sensitivity between the two types of animals.
ACKNOWLEDGMENTS
We thank Joseph A. Sennello for expert technical assistance. This work was supported by NIH grant DK061483 (to G.F.).
Footnotes
DISCLOSURE The authors declared no conflict of interest.
REFERENCES
- 1.Fantuzzi G. Three questions about leptin and immunity. Brain Behav Immun. 2009;23:405–410. doi: 10.1016/j.bbi.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 3.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 4.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 5.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes G, Handwerger BS, Yunis EJ, Brown DM. Immune response in the mutant diabetic C57BL/Ks-dt+ mouse. Discrepancies between in vitro and in vivo immunological assays. J Clin Invest. 1978;61:243–250. doi: 10.1172/JCI108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dardenne M, Savino W, Gastinel LN, Nabarra B, Bach JF. Thymic dysfunction in the mutant diabetic (db/db) mouse. J Immunol. 1983;130:1195–1199. [PubMed] [Google Scholar]
- 9.Palmer G, Aurrand-Lions M, Contassot E, et al. Indirect effects of leptin receptor deficiency on lymphocyte populations and immune response in db/db mice. J Immunol. 2006;177:2899–2907. doi: 10.4049/jimmunol.177.5.2899. [DOI] [PubMed] [Google Scholar]
- 10.Trotter-Mayo RN, Roberts MR. Leptin acts in the periphery to protect thymocytes from glucocorticoid-mediated apoptosis in the absence of weight loss. Endocrinology. 2008;149:5209–5218. doi: 10.1210/en.2008-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frasca D, Guidi F, Arbitrio M, et al. Hematopoietic reconstitution after lethal irradiation and bone marrow transplantation: effects of different hematopoietic cytokines on the recovery of thymus, spleen and blood cells. Bone Marrow Transplant. 2000;25:427–433. doi: 10.1038/sj.bmt.1702169. [DOI] [PubMed] [Google Scholar]
- 12.Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 14.Faggioni R, Jones-Carson J, Reed DA, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor α and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansour E, Pereira FG, Araújo EP, et al. Leptin inhibits apoptosis in thymus through a janus kinase-2-independent, insulin receptor substrate-1/phosphatidylinositol-3 kinase-dependent pathway. Endocrinology. 2006;147:5470–5479. doi: 10.1210/en.2006-0223. [DOI] [PubMed] [Google Scholar]
- 16.Leiter EH, Prochazka M, Shultz LD. Effect of immunodeficiency on diabetogenesis in genetically diabetic (db/db) mice. J Immunol. 1987;138:3224–3229. [PubMed] [Google Scholar]
- 17.Ablamunits V, Weisberg SP, Lemieux JE, Combs TP, Klebanov S. Reduced adiposity in ob/ob mice following total body irradiation and bone marrow transplantation. Obesity (Silver Spring) 2007;15:1419–1429. doi: 10.1038/oby.2007.170. [DOI] [PubMed] [Google Scholar]
- 18.Okada S, Kobayashi K, Ishikawa M, et al. Abdominal Irradiation Ameliorates Obesity in ob/ob Mice. J Clin Biochem Nutr. 2007;40:123–130. doi: 10.3164/jcbn.40.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiller NK, Kubo N, Boisvert WA, Curtiss LK. Effect of gamma-irradiation and bone marrow transplantation on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1674–1680. doi: 10.1161/hq1001.096724. [DOI] [PubMed] [Google Scholar]
- 20.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 21.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer–a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr. Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 24.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 25.Posey KA, Clegg DJ, Printz RL, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zhang G, Zhang H, et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juge-Aubry CE, Somm E, Giusti V, et al. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes. 2003;52:1104–1110. doi: 10.2337/diabetes.52.5.1104. [DOI] [PubMed] [Google Scholar]
- 28.Juge-Aubry CE, Somm E, Pernin A, et al. Adipose tissue is a regulated source of interleukin-10. Cytokine. 2005;29:270–274. doi: 10.1016/j.cyto.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto M, Arai H, Tamura Y, et al. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009;204:388–394. doi: 10.1016/j.atherosclerosis.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Ilyin SE, Plata-Salamán CR. Molecular regulation of the brain interleukin-1β system in obese (fa/fa) and lean (Fa/Fa) Zucker rats. Brain Res Mol Brain Res. 1996;43:209–218. doi: 10.1016/s0169-328x(96)00178-7. [DOI] [PubMed] [Google Scholar]
- 31.Wisse BE, Ogimoto K, Morton GJ, et al. Physiological regulation of hypothalamic IL-1β gene expression by leptin and glucocorticoids: implications for energy homeostasis. Am J Physiol Endocrinol Metab. 2004;287:E1107–E1113. doi: 10.1152/ajpendo.00038.2004. [DOI] [PubMed] [Google Scholar]
- 32.Wilson CM, Gaber MW, Sabek OM, Zawaski JA, Merchant TE. Radiation-induced astrogliosis and blood-brain barrier damage can be abrogated using anti-TNF treatment. Int J Radiat Oncol Biol Phys. 2009;74:934–941. doi: 10.1016/j.ijrobp.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Plata-Salamán CR, Ilyin SE. Interleukin-1β (IL-1β)-induced modulation of the hypothalamic IL-1β system, tumor necrosis factor-α, and transforming growth factor-β1 mRNAs in obese (fa/fa) and lean (Fa/Fa) Zucker rats: implications to IL-1β feedback systems and cytokine-cytokine interactions. J Neurosci Res. 1997;49:541–550. doi: 10.1002/(SICI)1097-4547(19970901)49:5<541::AID-JNR4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]